Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
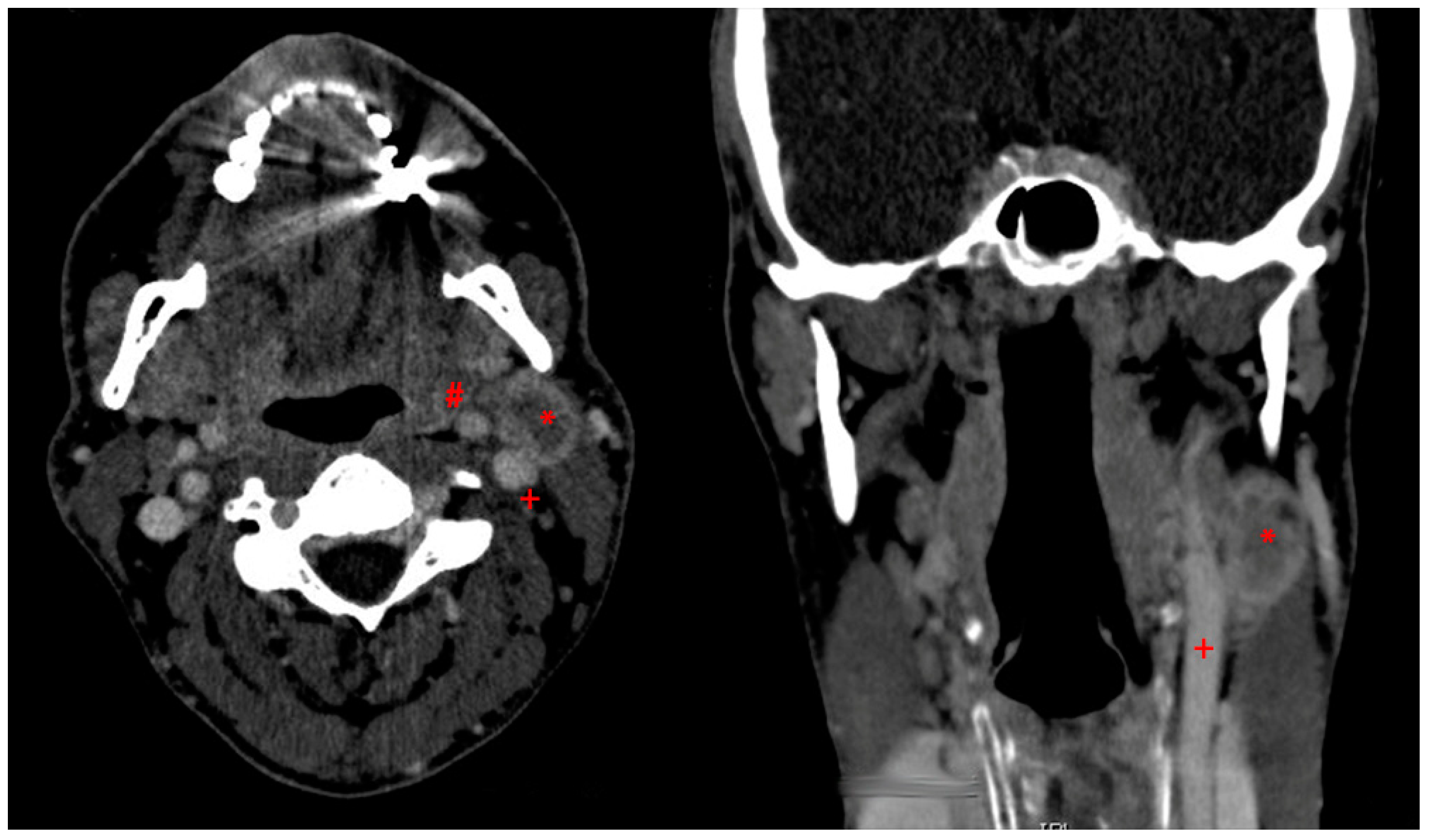
2.2. Tissue Samples
2.3. Plasma Source and Plasma Treatment
2.4. Cell Viability
2.5. Cell Cycle Analyses
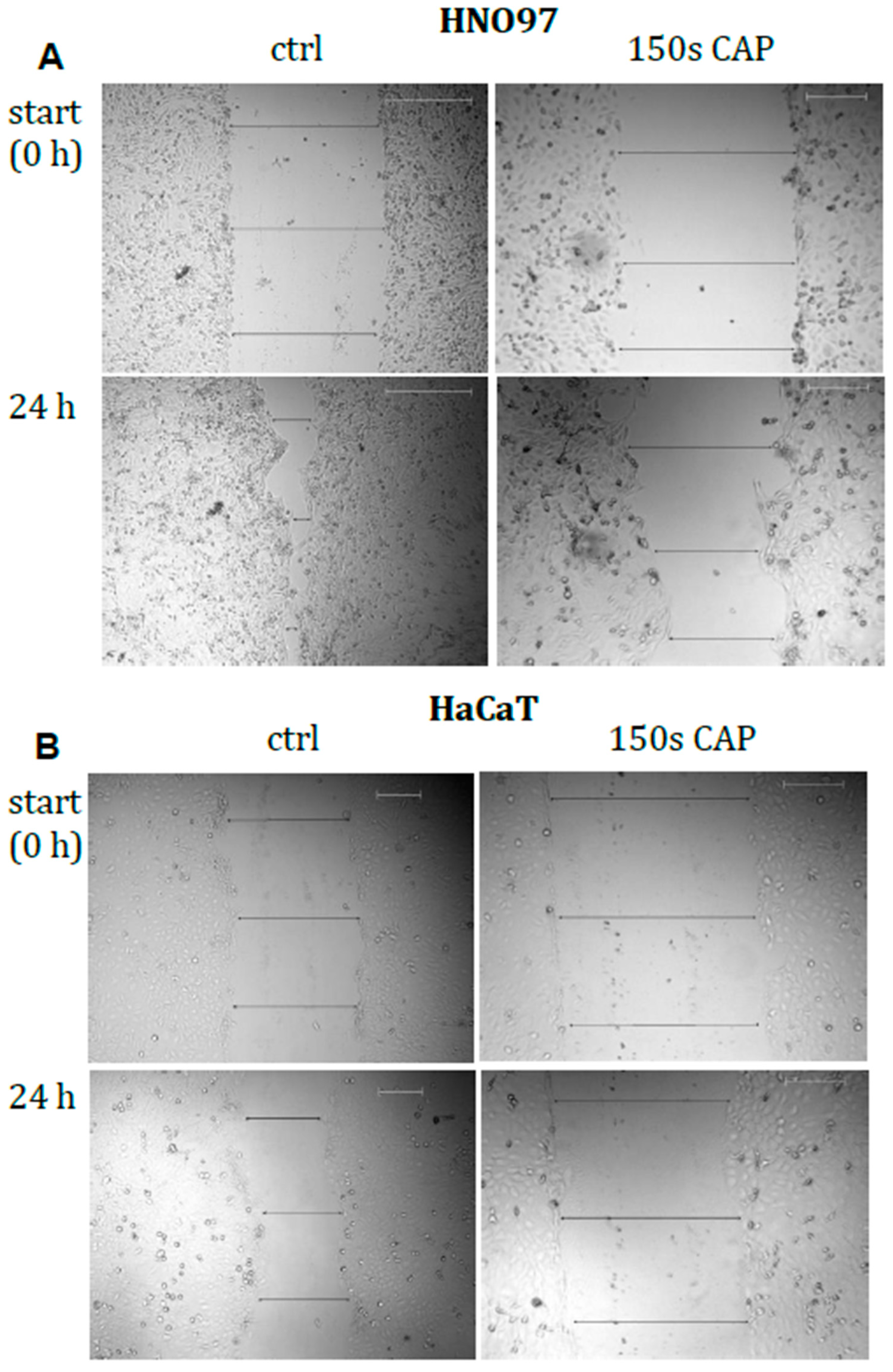
2.6. Cell Motility Assay
2.7. DNA Fragmentation
2.8. Cytochrome C Measurements
2.9. Cytokine Detection
2.10. Global Protein Expression
2.11. Statistics
3. Results
3.1. Examinations on Cultured Cells
3.1.1. CAP Reduced Viability in Normal and Tumour Cells
3.1.2. Cell Cycle Arrest after CAP Treatment in HaCaT Keratinocytes and in HNO97 Cells
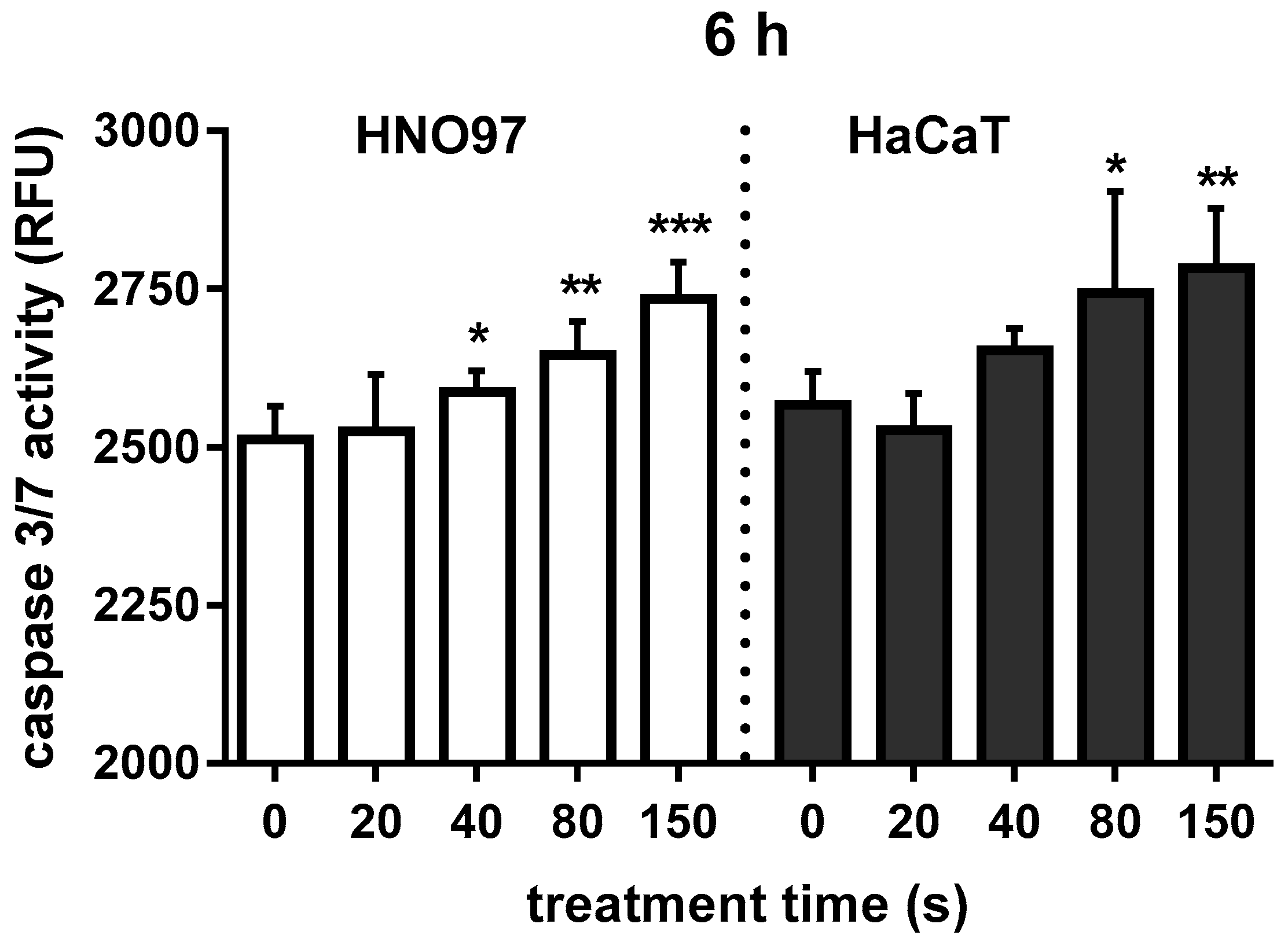
3.1.3. CAP Exposure Activated Caspase in HNO97 Cancer Cells and HaCaT Keratinocytes
3.1.4. Motility of Malignant Cells Was Significantly Impaired by CAP Treatment
3.1.5. Total Protein Expression Modulated by CAP Treatment Reflect Changes in Cell Motility
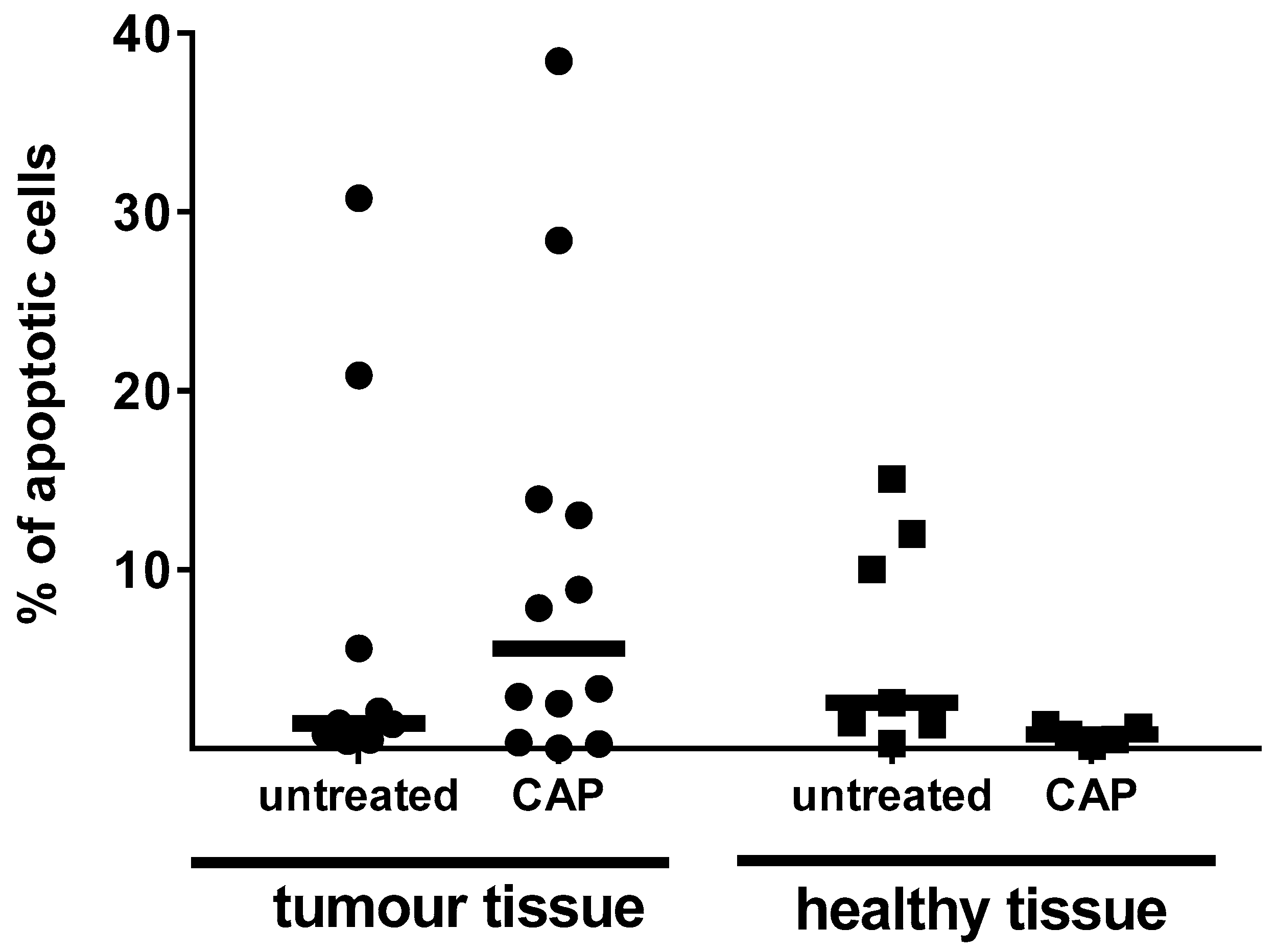
3.2. Examinations on Tissue Specimen
4. Discussion
5. Conclusions
- Controlled application of CAP may provide a means to kill malignant cells.
- CAP application may be a promising adjuvant treatment option to eliminate minimal residual cancer cells after radical surgery of carcinoma of the head and neck area.
- Investigations towards the underlying mechanism remain to be addressed under consideration of accompanying cell types.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Declarations
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [Green Version]
- Von Woedtke, T.; Reuter, S.; Masur, K.; Weltmann, K.D. Plasmas for medicine. Phys. Rep. 2013, 530, 291–320. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; Brandenburg, R.; Meyer, C.; Bussiahn, R.; Wilke, C.; von Woedtke, T. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib. Plasma Phys. 2009, 49, 631–640. [Google Scholar] [CrossRef]
- Isbary, G.; Shimizu, T.; Li, Y.F.; Stolz, W.; Thomas, H.M.; Morfill, G.E.; Zimmermann, J.L. Cold atmospheric plasma devices for medical issues. Expert Rev. Med. Dev. 2013, 10, 367–377. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Keidar, M.; Shashurin, A.; Volotskova, O.; Ann Stepp, M.; Srinivasan, P.; Sandler, A.; Trink, B. Cold atmospheric plasma in cancer therapy. Phys. Plasmas 2013, 20, 057101. [Google Scholar] [CrossRef]
- Schlegel, J.; Köritzer, J.; Boxhammer, V. Plasma in cancer treatment. Clin. Plasma Med. 2013, 1, 2–7. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio-Maxillo-Facial Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef] [Green Version]
- Heinlin, J.; Morfill, G.; Landthaler, M.; Stolz, W.; Isbary, G.; Zimmermann, J.L.; Shimizu, T.; Karrer, S. Plasma medicine: Possible applications in dermatology. J. Dtsch. Dermatol. Ges. 2010, 8, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Heinlin, J.; Schiffner-Rohe, J.; Schiffner, R.; Einsele-Kramer, B.; Landthaler, M.; Klein, A.; Zeman, F.; Stolz, W.; Karrer, S. A first prospective randomized controlled trial on the efficacy and safety of synchronous balneophototherapy vs. narrow-band UVB monotherapy for atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. JEADV 2011, 25, 765–773. [Google Scholar] [CrossRef]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.R.; Woedtke, T.; Bussiahn, R.; Weltmann, K.D.; Rieck, M.; Khalili, R.; Podmelle, F.; Waite, P. Experimental Recovery of CO2-Laser skin lesions by plasma stimulation. Am. J. Cosm. Surg. 2012, 29, 52–56. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin. Plasma Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Gorlitz, A.; Simon, D.; Schon, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (NCT01415622). J. Eur. Acad. Dermatol. Venereol. JEADV 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Nasruddin; Nakajima, Y.; Mukai, K.; Rahayu, H.S.E.; Nur, M.; Ishijima, T.; Enomoto, H.; Uesugi, Y.; Sugama, J.; Nakatani, T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin. Plasma Med. 2014, 2, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Daeschlein, G.; Scholz, S.; Ahmed, R.; von Woedtke, T.; Haase, H.; Niggemeier, M.; Kindel, E.; Brandenburg, R.; Weltmann, K.D.; Juenger, M. Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. J. Hosp. Infect. 2012, 81, 177–183. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Arnold, A.; von Podewils, S.; Haase, H.; Emmert, S.; von Woedtke, T.; Weltmann, K.-D.; Jünger, M. In Vitro Susceptibility of Important Skin and Wound Pathogens Against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process. Polym. 2012, 9, 380–389. [Google Scholar] [CrossRef]
- Hasse, S.; Duong Tran, T.; Hahn, O.; Kindler, S.; Metelmann, H.R.; von Woedtke, T.; Masur, K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clin. Exp. Dermatol. 2016, 41, 202–209. [Google Scholar] [CrossRef]
- Sensenig, R.; Kalghatgi, S.; Cerchar, E.; Fridman, G.; Shereshevsky, A.; Torabi, B.; Arjunan, K.P.; Podolsky, E.; Fridman, A.; Friedman, G.; et al. Non-thermal plasma induces apoptosis in melanoma cells via production of intracellular reactive oxygen species. Ann. Biomed. Eng. 2011, 39, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting Cancer Cells with Reactive Oxygen and Nitrogen Species Generated by Atmospheric-Pressure Air Plasma. PLoS ONE 2014, 9, 1. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Hasse, S.; Bodo, E.; Rose, C.; Funk, W.; Paus, R. Towards the development of a simplified long-term organ culture method for human scalp skin and its appendages under serum-free conditions. Exp. Dermatol. 2007, 16, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasse, S.; Hahn, O.; Kindler, S.; von Woedtke, T.; Metelmann, H.-R.; Masur, K. Atmospheric pressure plasma jet application on human oral mucosa modulates tissue regeneration. Plasma Med. 2014, 4, 431–438. [Google Scholar] [CrossRef]
- Wende, K.; Reuter, S.; von Woedtke, T.; Weltmann, K.-D.; Masur, K. Redox-Based Assay for Assessment of Biological Impact of Plasma Treatment. Plasma Process. Polym. 2014, 11, 655–663. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Graves, D.B. Oxy-nitroso shielding burst model of cold atmospheric plasma therapeutics. Clin. Plasma Med. 2014, 2, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.B. Reactive Species from Cold Atmospheric Plasma: Implications for Cancer Therapy. Plasma Process. Polym. 2014, 11, 1120–1127. [Google Scholar] [CrossRef]
- Yan, K.; Kanazawa, S.; Ohkubo, T.; Nomoto, Y. Oxidation and reduction processes during NO(x) removal with corona-induced nonthermal plasma. Plasma Chem. Plasma P 1999, 19, 421–443. [Google Scholar] [CrossRef]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’Connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug. Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Engel, R.H.; Evens, A.M. Oxidative stress and apoptosis: A new treatment paradigm in cancer. Front. Biosci. 2006, 11, 300–312. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug. Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Hay, J.; Shahzeidi, S.; Laurent, G. Mechanisms of bleomycin-induced lung damage. Arch. Toxicol. 1991, 65, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.-D.; Hackbarth, C.; Heidecke, C.-D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic Inhibition of Tumor Cell Proliferation by Cold Plasma and Gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Reuter, S.; Tresp, H.; Wende, K.; Hammer, M.U.; Winter, J.; Masur, K.; Schmidt-Bleker, A.; Weltmann, K.D. From RONS to ROS: Tailoring Plasma Jet Treatment of Skin Cells. IEEE Trans. Plasma Sci. 2012, 40, 2986–2993. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doring, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Hubner, N.O.; Gunther, C.; et al. Tissue Tolerable Plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef]
- Welz, C.; Emmert, S.; Canis, M.; Becker, S.; Baumeister, P.; Shimizu, T.; Morfill, G.E.; Harreus, U.; Zimmermann, J.L. Cold Atmospheric Plasma: A Promising Complementary Therapy for Squamous Head and Neck Cancer. PLoS ONE 2015, 10, e0141827. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Ha, C.S.; Hwang, S.W.; Lee, H.J.; Kim, G.C.; Lee, K.W.; Song, K. Non-Thermal Atmospheric Pressure Plasma Preferentially Induces Apoptosis in p53-Mutated Cancer Cells by Activating ROS Stress-Response Pathways. PLoS ONE 2014, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Carnelio, S.; Rao, N.; Rao, L. Expression of E-cadherin in primary oral squamous cell carcinoma and metastatic lymph nodes: An immunohistochemical study. Ind. J. Dent. Res. 2009, 20, 71–76. [Google Scholar]
- Haertel, B.; Wende, K.; von Woedtke, T.; Weltmann, K.D.; Lindequist, U. Non-thermal atmospheric-pressure plasma can influence cell adhesion molecules on HaCaT-keratinocytes. Exp. Dermatol. 2011, 20, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Beckner, M.E.; Jagannathan, S.; Peterson, V.A. Extracellular angio-associated migratory cell protein plays a positive role in angiogenesis and is regulated by astrocytes in coculture. Microvasc. Res. 2002, 63, 259–269. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef]
- Shashurin, A.; Stepp, M.A.; Hawley, T.S.; Pal-Ghosh, S.; Brieda, L.; Bronnikov, S.; Jurjus, R.A.; Keidar, M. Influence of Cold Plasma Atmospheric Jet on Surface Integrin Expression of Living Cells. Plasma Process. Polym. 2010, 7, 294–300. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; von Woedtke, T.; Hasse, S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin. Plasma Med. 2015, 3, 24–31. [Google Scholar] [CrossRef]
- Weiss, M.; Gumbel, D.; Hanschmann, E.M.; Mandelkow, R.; Gelbrich, N.; Zimmermann, U.; Walther, R.; Ekkernkamp, A.; Sckell, A.; Kramer, A.; et al. Cold Atmospheric Plasma Treatment Induces Anti-Proliferative Effects in Prostate Cancer Cells by Redox and Apoptotic Signaling Pathways. PLoS ONE 2015, 10, e0130350. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Modlin, R.L.; Moy, R.L.; Dubinett, S.M.; Mchugh, T.; Nickoloff, B.J.; Uyemura, K. Il-10 Production in Cutaneous Basal and Squamous-Cell Carcinomas—A Mechanism for Evading the Local T-Cell Immune-Response. J. Immunol. 1995, 155, 2240–2247. [Google Scholar]
- Konur, A.; Schulz, U.; Eissner, G.; Andreesen, R.; Holler, E. Interferon (IFN)-γ is a main mediator of keratinocyte (HaCaT) apoptosis and contributes to autocrine IFN-γ and tumour necrosis factor-α production. Br. J. Dermatol. 2005, 152, 1134–1142. [Google Scholar] [CrossRef] [PubMed]





 not changed.
not changed.
 not changed.
not changed.| Protein Name | Uniprot ID | HaCaT | HNO97 | Function |
|---|---|---|---|---|
| Ki67 | P46013 |  | →↑ | Proliferation |
| NQO1 | P15559 | →↑ |  | Nuclear factor erythroid 2-related factor 2 (NRF2) related signalling |
| TMX2 | Q9Y320 | ↑ |  | NRF2 related signalling |
| GSTM3 | P21266 | ↑ |  | NRF2 related signalling |
| PRDX1,2,4,6 | Q06830 |  |  | Redox regulation, elimination of peroxides |
| SBNO2 | Q9Y2G9 | ↑↑ | ↑ | Transcriptional co- regulation, interleukin signalling |
| ILKAP | Q9H0C8 | ↑↑ |  | Cell adhesion and growth factor signalling |
| AAMP | Q13685 | →↑ | ↓ | Cell migration, angiogenesis |
| ROCK2 | O75116 | →↑ | ↓↓ | Regulation of actin cytoskeleton, cell adhesion and motility |
| CTTB2 | Q8WZ74 | ↑↑ | →↑ | Cytoskeleton rearrangement, cell migration and motility |
| CFA20 | Q9Y6A4 | ↑↑ |  | Cell motility |
| SRGP2 | O75044 | n.d. | ↑ | Cell migration inhibition, actin dynamics |
| ELMO3 | Q96BJ8 |  | ↓ | Cell motility |
| CASP8/10 | Q14790/Q92851 |  | ↑ | Pro-apoptotic caspases |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasse, S.; Seebauer, C.; Wende, K.; Schmidt, A.; Metelmann, H.-R.; von Woedtke, T.; Bekeschus, S. Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study. Appl. Sci. 2019, 9, 2061. https://doi.org/10.3390/app9102061
Hasse S, Seebauer C, Wende K, Schmidt A, Metelmann H-R, von Woedtke T, Bekeschus S. Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study. Applied Sciences. 2019; 9(10):2061. https://doi.org/10.3390/app9102061
Chicago/Turabian StyleHasse, Sybille, Christian Seebauer, Kristian Wende, Anke Schmidt, Hans-Robert Metelmann, Thomas von Woedtke, and Sander Bekeschus. 2019. "Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study" Applied Sciences 9, no. 10: 2061. https://doi.org/10.3390/app9102061
APA StyleHasse, S., Seebauer, C., Wende, K., Schmidt, A., Metelmann, H. -R., von Woedtke, T., & Bekeschus, S. (2019). Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study. Applied Sciences, 9(10), 2061. https://doi.org/10.3390/app9102061






