Plasma-Activation of Larger Liquid Volumes by an Inductively-Limited Discharge for Antimicrobial Purposes
Abstract
:1. Introduction
2. Materials and Methods
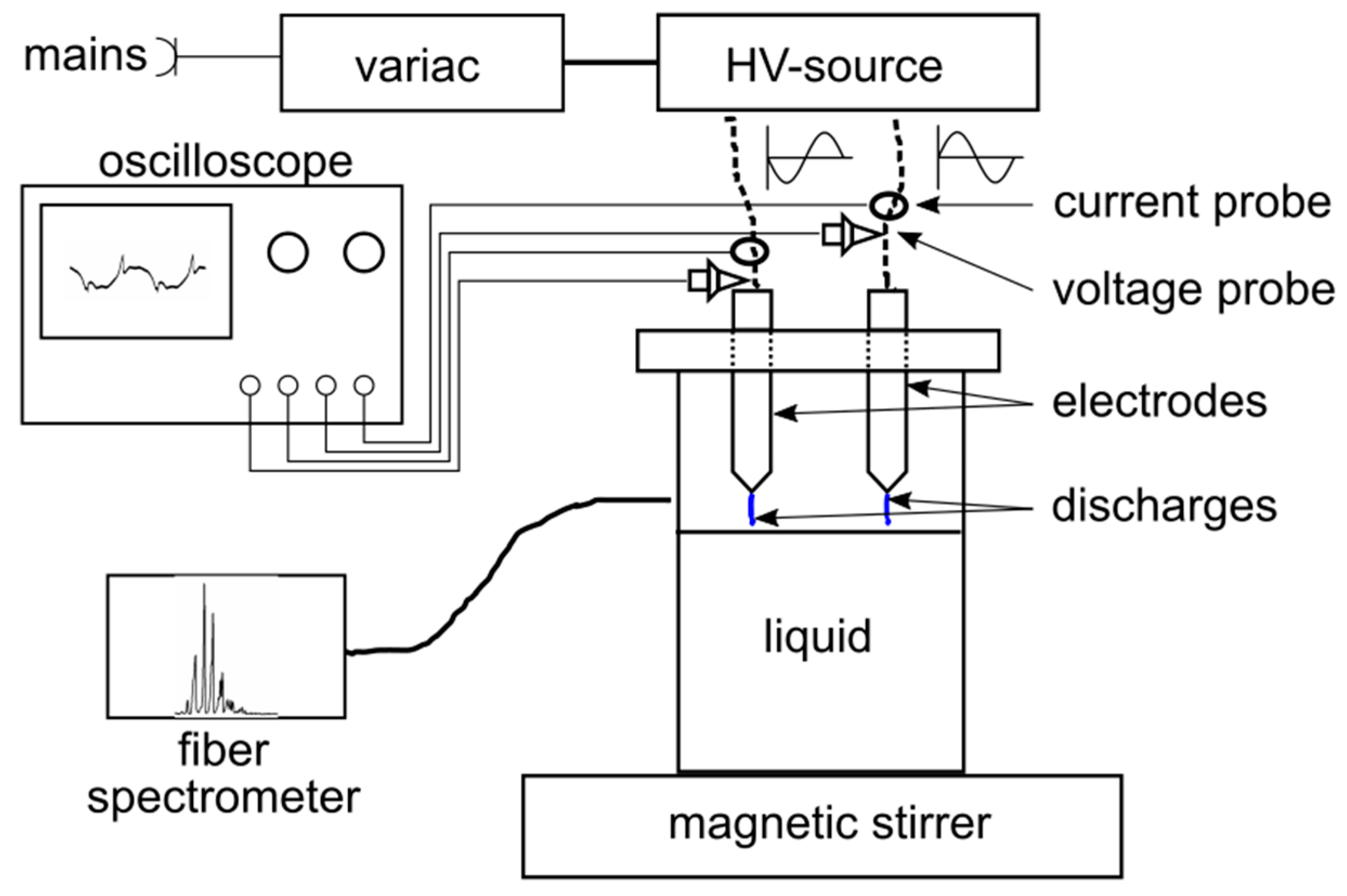
2.1. Electrical and Optical Investigations
2.2. Investigation of Properties of the Treated Water
2.3. Microorganisms and Culture Conditions
2.4. Determination of the Antimicrobial Effect of Plasma-Treated Liquids
3. Results and Discussion
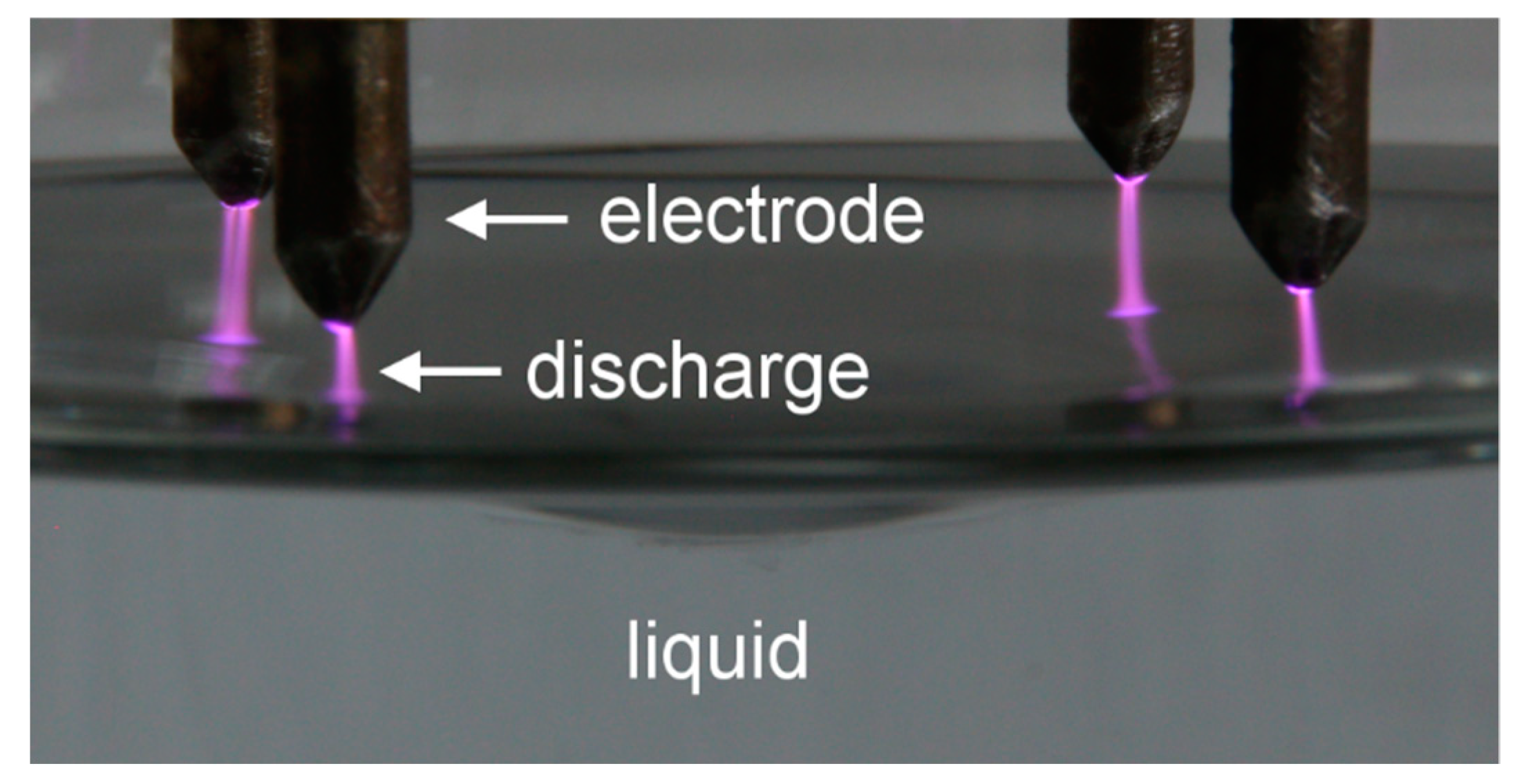
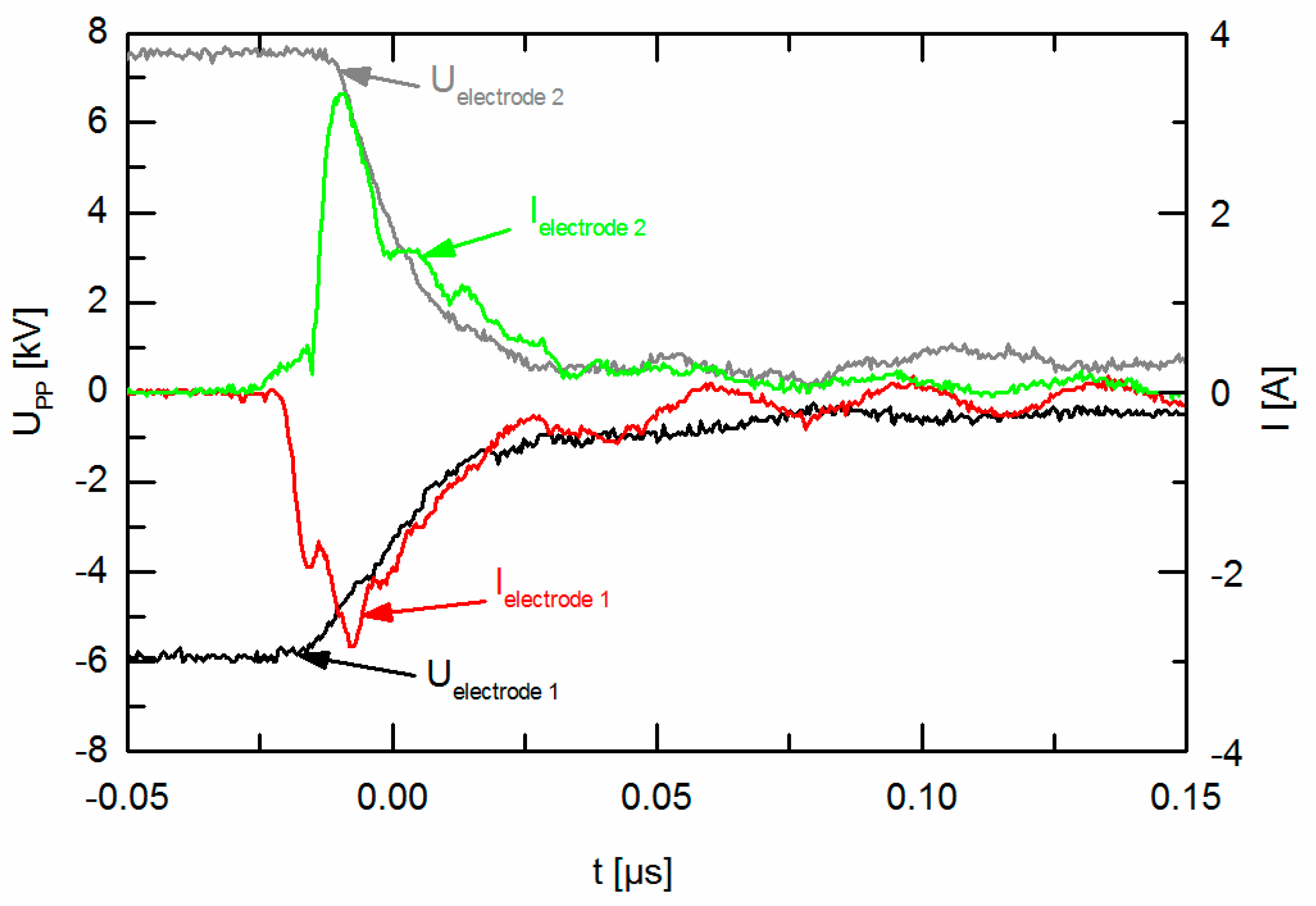
3.1. Electrical Characterization of the Discharge
3.2. Optical Characterization of the Discharge
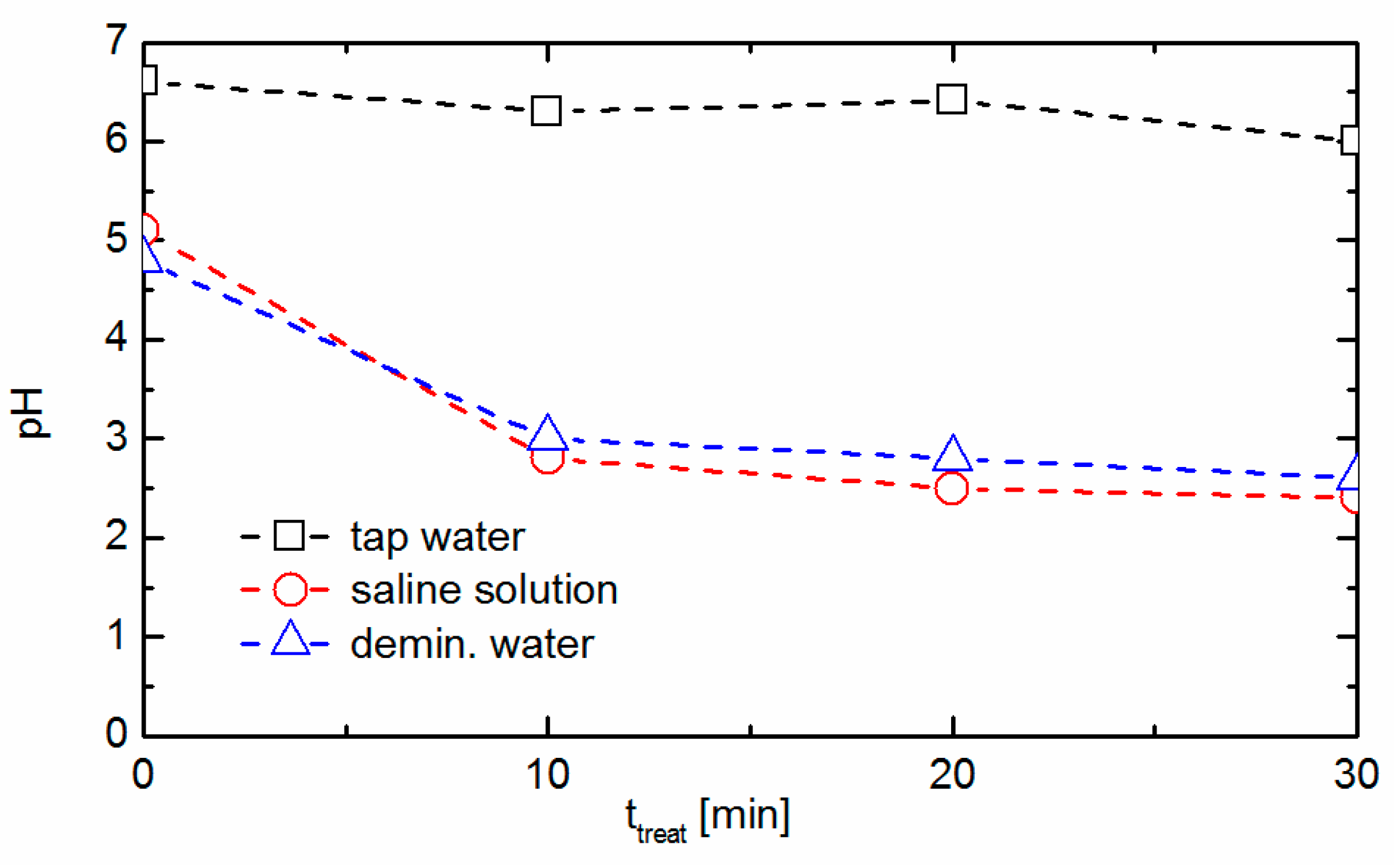
3.3. Chemical Characterization of the Liquids
3.4. Antimicrobial Properties of the Plasma-Treated Liquids
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Vanraes, P.; Bogaerts, A. Plasma physics of liquids—A focused review. Appl. Phys. Rev. 2018, 5, 031103. [Google Scholar] [CrossRef]
- Kolb, J.F.; Joshi, R.P.; Xiao, S.; Schoenbach, K.H. Streamer in water and other dielectric liquids. J. Phys. D Appl. Phys. 2008, 41, 234007. [Google Scholar] [CrossRef]
- Bruggeman, P.; Ribezl, E.; Maslani, A.; Degroote, J.; Malesevic, A.; Rego, R.; Vierendeels, J.; Leys, C. Characteristics of atmospheric pressure air discharges with a liquid cathode and a metal anode. Plasma Sources Sci. Technol. 2008, 17, 025012. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, H.-M.; Chang, M.B. Inactivation of Aquatic Microorganisms by Low-Frequency AC Discharges. IEEE Trans. Plasma Sci. 2008, 36, 215–219. [Google Scholar] [CrossRef]
- Kovačević, V.V.; Dojčinović, B.P.; Jović, M.; Roglić, G.M.; Obradović, B.M.; Kuraica, M.M. Measurement of reactive species generated by dielectric barrier discharge in direct contact with water in different atmospheres. J. Phys. D Appl. Phys. 2017, 50, 155205. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hähnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.-D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Burlica, R.; Kirkpatrick, M.J.; Locke, B. Formation of reactive species in gliding arc discharges with liquid water. J. Electrostat. 2006, 64, 35–43. [Google Scholar] [CrossRef]
- Liu, F.; Sun, P.; Bai, N.; Tian, Y.; Zhou, H.; Wei, S.; Zhou, Y.; Zhang, J.; Zhu, W.; Becker, K.; Fang, J. Inactivation of Bacteria in an Aqueous Environment by a Direct-Current, Cold-Atmospheric-Pressure Air Plasma Microjet. Plasma Process. Polym. 2010, 7, 231–236. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of organic dyes in water by electrical discharges. Plasma Chem. Plasma Process. 2007, 27, 589–598. [Google Scholar] [CrossRef]
- Machala, Z.; Chládeková, L.; Pelach, M. Plasma agents in bio-decontamination by dc discharges in atmospheric air. J. Phys. D Appl. Phys. 2010, 43, 222001. [Google Scholar] [CrossRef]
- Lu, X.P.; Laroussi, M. Ignition phase and steady-state structures of a non-thermal air plasma. J. Phys. D Appl. Phys. 2003, 36, 661–665. [Google Scholar] [CrossRef]
- Satoh, K.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Fouracre, R.A. Pulsed-Plasma Disinfection of Water Containing Escherichia coli. Jpn. J. Appl. Phys. 2007, 46, 1137–1141. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Pawłat, J. Electrical Discharges in Humid Environments: Generators, Effects, Application; Lublin University of Technology: Lublin, Poland, 2013. [Google Scholar]
- Kojtari, A.; Ercan, U.E.; Smith, J.; Friedman, G.; Sensenig, R.B.; Tyagi, S.; Joshi, S.G.; Ji, H.-F.; Brooks, A.D. Chemistry for Antimicrobial Properties of Water Treated with non-equilibrium plasma. J. Nanomed. Biother. Discov. 2013, 4, 120. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order postdischarge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Hänsch, M.A.C.; Mann, M.; Weltmann, K.-D.; von Woedtke, T. Analysis for antibacterial efficacy of plasma-treated sodium chloride solutions. J. Phys. D Appl. Phys. 2015, 48, 454001. [Google Scholar] [CrossRef]
- Trizio, I.; Sardella, E.; Francioso, E.; Dilecce, G.; Rizzi, V.; Cosma, P.; Schmidt, M.; Hänsch, M.; von Woedtke, T.; Favia, P.; et al. Investigation of air-DBD effects on biological liquids for in vitro studies on eukaryotic cells. Clin. Plasma Med. 2015, 3, 62–71. [Google Scholar] [CrossRef]
- Wang, B. A Novel Dielectric-Barrier-Discharge Loop Reactor for Cyanide Water Treatment. Plasma Chem. Plasma Process. 2017, 37, 1121–1131. [Google Scholar] [CrossRef]
- Banaschik, R.; Lukes, P.; Jablonowski, H.; Hammer, M.U.; Weltmann, K.-D.; Kolb, J.F. Potential of pulsed corona discharges generated in water for the degradation of persistent pharmaceutical residues. Water Res. 2015, 84, 127–135. [Google Scholar] [CrossRef]
- Malik, M.A. Water Purification by Plasmas: Which Reactors are Most Energy Efficient? Plasma Chem. Plasma Process. 2010, 30, 21–31. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.-D.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.D.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef]
- Jablonowski, H.; von Woedtke, T. Research on plasma medicine-relevant plasma-liquid interaction: What happened in the past five years? Clin. Plasma Med. 2015, 3, 42–52. [Google Scholar] [CrossRef]
- von Woedtke, T.; Haertel, B.; Weltmann, K.-D.; Lindequist, U. Plasma pharmacy—Physical plasma in pharmaceutical applications. Pharmazie 2013, 68, 492–498. [Google Scholar] [CrossRef]
- Joslin, J.M.; McCall, J.R.; Bzdek, J.P.; Johnson, D.C.; Hybertson, B.M. Aqueous Plasma Pharmacy: Preparation Methods, Chemistry, and Therapeutic Applications. Plasma Med. 2016, 6, 135–177. [Google Scholar] [CrossRef] [Green Version]
- Hahn, V.; Dikyol, C.; Altrock, B.; Schmidt, M.; Wende, K.; Ercan, U.K.; Weltmann, K.-D.; von Woedtke, T. Plasma-mediated inactivation of E. coli: Influence of protein on wet surface and liquid medium. Plasma Process. Polym. 2019, 16, e1800164. [Google Scholar] [CrossRef]
- Gilchrist, J.E.; Campbell, J.E.; Donnelly, C.B.; Peeler, J.T.; Delaney, J.M. Spiral plate method for bacterial determination. Appl. Microbiol. 1973, 25, 244–252. [Google Scholar]
- Schmidt, M.; Altrock, B.; Gerling, T.; Gerber, I.C.; Hahn, V.; Weltmann, K.-D.; von Woedtke, T. AC-driven pin-to-liquid discharge: Characterization and application. In Proceedings of the ISPC 23, Montreal, QC, Canada, 30 July–4 August 2017; Available online: https://www.ispc-conference.org/ispcproc/ispc23/190.pdf (accessed on 16 May 2019).
- Bruggeman, P.; Guns, P.; Degroote, J.; Vierendeels, J.; Leys, C. Influence of the water surface on the glow-to-spark transition in a metal-pin-to-water electrode system. Plasma Sources Sci. Technol. 2008, 17, 045014. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in Water Electro-Sprayed through Transient Spark Discharge in Air and their Bactericidal Effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Trinkwasseranalyse. Available online: http://www.sw-greifswald.de/Energie/Trinkwasser/Trinkwasseranalyse (accessed on 18 March 2019).
- Wasserhärte Verzeichnis. Available online: http://www.wasserhaerte.net/deutschland/index.html (accessed on 18 March 2019).
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1833. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Li, Y.F.; Heinlin, J.; Karrer, S.; Morfill, G.; Zimmermann, J.L. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS ONE 2012, 7, e34610. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Lackmann, J.W.; Narberhaus, F.; Bandow, J.E.; Denis, B.; Benedikt, J. Separation of VUV/UV photons and reactive particles in the effluent of a He/O2 atmospheric pressure plasma jet. J. Phys. D Appl. Phys. 2011, 44, 295201. [Google Scholar] [CrossRef]
- Ercan, U.K.; Smith, J.; Ji, H.F.; Brooks, A.D.; Joshi, S.G. Chemical changes in nonthermal plasma-treated N-acetylcysteine (NAC) solution and their contribution to bacterial inactivation. Sci. Rep. 2016, 6, 20365. [Google Scholar] [CrossRef]
- Marotta, E.; Ceriani, E.; Schiorlin, M.; Ceretta, C.; Paradisi, C. Comparison of the rates of phenol advanced oxidation in deionized and tap water within a dielectric barrier discharge reactor. Water Res. 2012, 46, 6239–6246. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, M.; Hahn, V.; Altrock, B.; Gerling, T.; Gerber, I.C.; Weltmann, K.-D.; von Woedtke, T. Plasma-Activation of Larger Liquid Volumes by an Inductively-Limited Discharge for Antimicrobial Purposes. Appl. Sci. 2019, 9, 2150. https://doi.org/10.3390/app9102150
Schmidt M, Hahn V, Altrock B, Gerling T, Gerber IC, Weltmann K-D, von Woedtke T. Plasma-Activation of Larger Liquid Volumes by an Inductively-Limited Discharge for Antimicrobial Purposes. Applied Sciences. 2019; 9(10):2150. https://doi.org/10.3390/app9102150
Chicago/Turabian StyleSchmidt, Michael, Veronika Hahn, Beke Altrock, Torsten Gerling, Ioana Cristina Gerber, Klaus-Dieter Weltmann, and Thomas von Woedtke. 2019. "Plasma-Activation of Larger Liquid Volumes by an Inductively-Limited Discharge for Antimicrobial Purposes" Applied Sciences 9, no. 10: 2150. https://doi.org/10.3390/app9102150




