Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar
Abstract
:1. Introduction
2. The Experiment
2.1. Materials and Mix Design
2.2. Sample Preparation
2.3. Testing Methods
3. Results and Analysis
3.1. Flow Properties
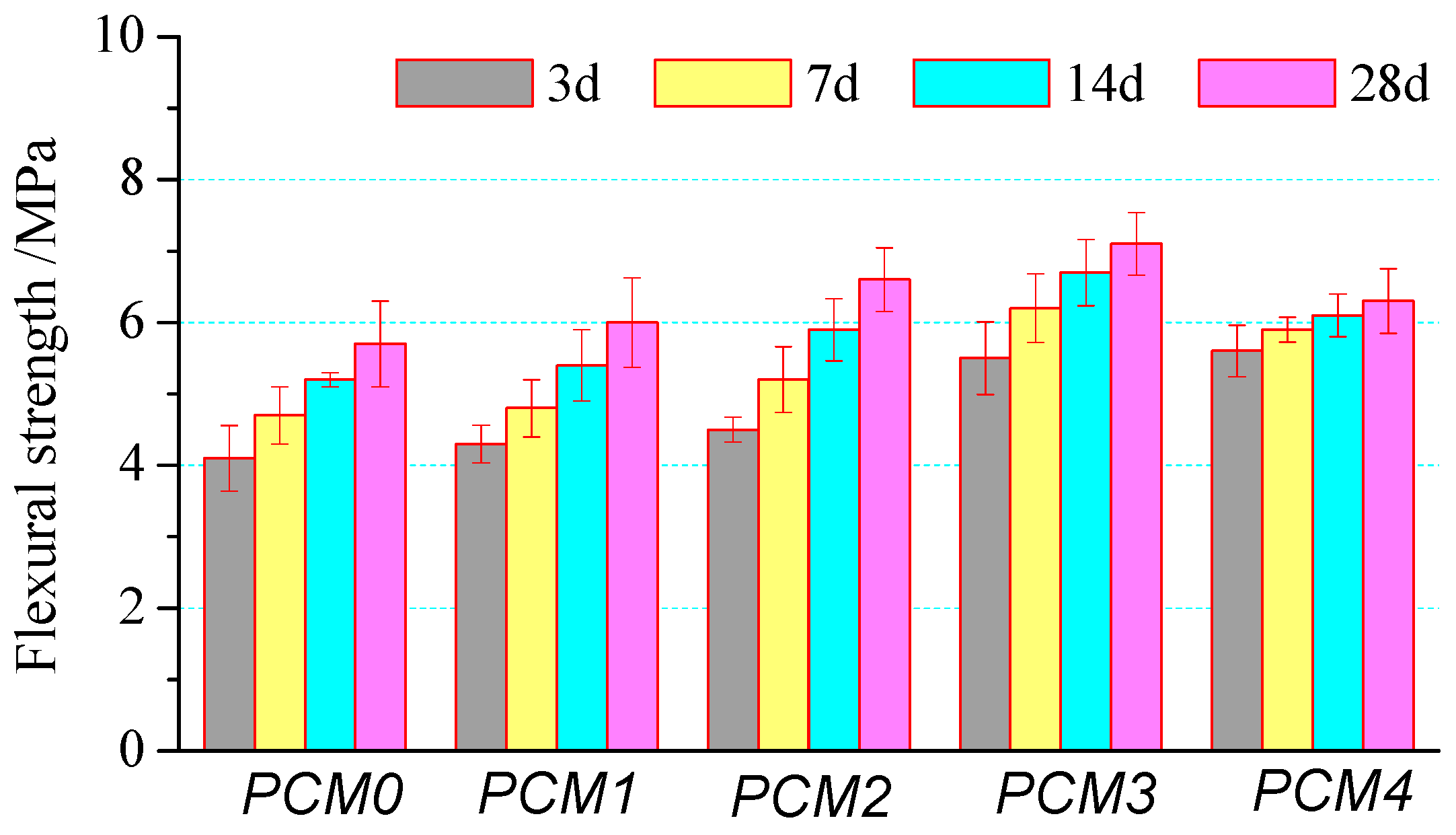
3.2. Flexural Strength
3.3. Compressive Strength
3.4. Volume Density
3.5. Capillary Water Absorption
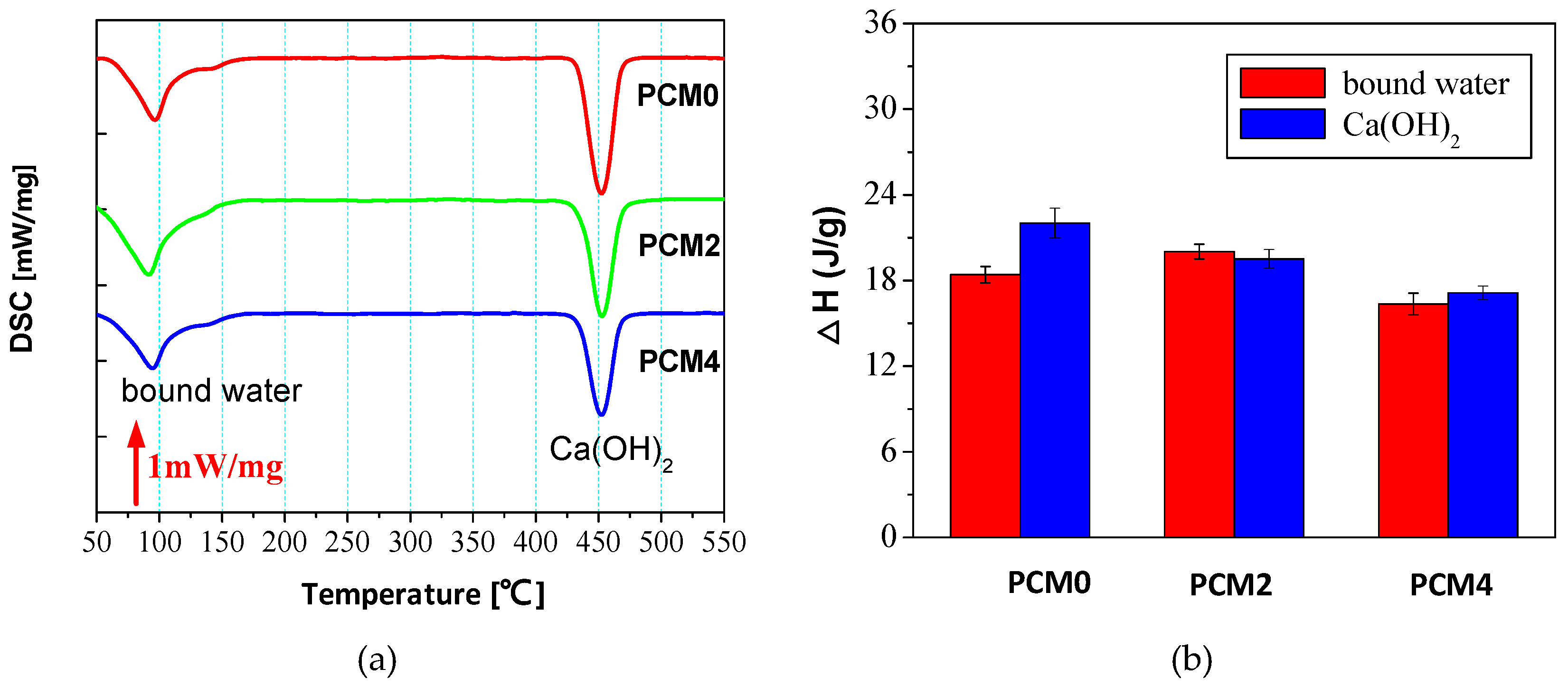
3.6. Thermal Analysis
3.7. FTIR Spectroscopy
3.8. SEM Investigation
4. Conclusions
- (1)
- PVA has a strong cohesiveness and water retention, and its incorporation reduces the fluidity of cement mortar. The incorporation of 0.2%, 0.6%, 1.0%, and 2.0% PVA reduced the slump value by 13.9%, 31.5%, 41.1%, and 50.5% as compared to the control one, respectively.
- (2)
- The mechanical properties of cement mortar show a significant increase at first and then decrease with the increase of PVA content. Cement mortar containing 0.6% PVA has the highest compressive strength, its 28-day compressive strength is 12.1% higher than that of the control one. The incorporating 1.0% PVA increased the flexural strength greatly, its 28-day flexural strength is about 24.8% higher than that of the control one.
- (3)
- The bulk density and water absorption test results show that the incorporation of 0.6% PVA increases the bulk density of cement mortar by 5.90%, and the corresponding capillary water absorption decreases by 60% as compared to the control mortar, respectively.
- (4)
- SEM tests show that three-dimensional PVA networks are formed in cement mortar. The crack-bridging effect of PVA film can be observed in the SEM images. When 2.0% PVA was incorporated, PVA formed heavy films, coating cement particles and preventing them from hydration.
- (5)
- DCS and FTIR analyses results manifest the adding of 0.6% PVA accelerates the hydration of the cement matrix, while the incorporation of 2.0% PVA impacts the hydration rate of cement in a negative way.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, Z.; Li, W.; Wang, K.; Shah, S.P. Research progress in advanced nanomechanical characterization of cement-based materials. Cem. Concr. Compos. 2018, 94, 277–295. [Google Scholar] [CrossRef]
- Sang, G.; Zhu, Y.; Yang, G. Mechanical properties of high porosity cement-based foam materials modified by EVA. Constr. Build. Mater. 2016, 112, 648–653. [Google Scholar] [CrossRef]
- Yang, H.; Cui, H.; Tang, W.; Li, Z.; Han, N.; Xing, F. A critical review on research progress of graphene/cement-based composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 273–296. [Google Scholar] [CrossRef]
- Ohama, Y. Handbook of Polymer-Modified Concrete and Mortars; Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Sakai, E.; Sugita, J. Composite mechanism of polymer modified cement. Cem. Concr. Res. 1995, 25, 127–135. [Google Scholar] [CrossRef]
- Park, D.; Ahn, J.; Oh, S.; Song, H.; Noguchi, T. Drying effect of polymer-modified cement for patch-repaired mortar on constraint stress. Constr. Build. Mater. 2009, 23, 434–447. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Rong, C.; Wang, Z. Properties of polymer modified steel fiber-reinforced cement concretes. Constr. Build. Mater. 2010, 24, 1201–1206. [Google Scholar] [CrossRef]
- Mirza, J.; Mirza, M.S.; Lapointe, R. Laboratory and field performance of polymer-modified cement-based repair mortars in cold climates. Constr. Build. Mater. 2002, 16, 365–374. [Google Scholar] [CrossRef]
- Assaad, J.J. Development and use of polymer-modified cement for adhesive and repair applications. Constr. Build. Mater. 2018, 163, 139–148. [Google Scholar] [CrossRef]
- Shaker, F.A.; El-Dieb, A.S.; Reda, M.M. Durability of Styrene Butadiene latexmodified concrete. Cem. Concr. Res. 1997, 27, 711–720. [Google Scholar] [CrossRef]
- Almeida, A.E.D.S.; Sichieri, E.P. Mineralogical study of polymer modified mortar with silica fume. Constr. Build. Mater. 2006, 20, 882–887. [Google Scholar] [CrossRef]
- Xu, F.; Zhou, M.; Chen, J.; Ruan, S. Mechanical performance evaluation of polyester fiber and SBR latex compound-modified cement concrete road overlay material. Constr. Build. Mater. 2014, 63, 142–149. [Google Scholar] [CrossRef]
- Yahya, G.O.; Ali, S.A.; Al-Naafa, M.A.; Hamad, E.Z. Preparation and viscosity behavior of hydrophobically modified poly(vinyl alcohol) (PVA). J. Appl. Polym. Sci. 2010, 57, 343–352. [Google Scholar] [CrossRef]
- Rim, I.W.; Robertson, R.E.; Robert, Z. Effects of Some Nonionic Polymeric Additives on the Crystallization of Calcium Carbonate. Cryst. Growth Des. 2005, 5, 513–522. [Google Scholar]
- Wang, S.; Ren, J.; Li, W.; Sun, R.; Liu, S. Properties of polyvinyl alcohol/xylan composite films with citric acid. Carbohydr. Polym. 2014, 103, 94–99. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Panyam, J.; Prabha, S.; Labhasetwar, V. Residual polyvinyl alcohol associated with poly (d,l-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Control. Release 2002, 82, 105–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, L.; Cui, M.; Yang, B.; Li, J.; Sun, H.; Yao, F. Physically crosslinked poly(vinyl alcohol)–carrageenan composite hydrogels: Pore structure stability and cell adhesive ability. RSC Adv. 2015, 5, 78180–78191. [Google Scholar] [CrossRef]
- Chopra, P.; Nayak, D.; Nanda, A.; Ashe, S.; Rauta, P.R.; Nayak, B. Fabrication of poly(vinyl alcohol)-Carrageenan scaffolds for cryopreservation: Effect of composition on cell viability. Carbohydr. Polym. 2016, 147, 509–516. [Google Scholar] [CrossRef]
- Ma, Y.; Bai, T.; Wang, F. The physical and chemical properties of the polyvinylalcohol/polyvinyl-pyrrolidone/hydroxyapatite composite hydrogel. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 59, 948. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, X.; Wang, Y.; Xu, H.; Chen, X. Performance and characterization of irradiated poly(vinyl alcohol)/polyvinylpyrrolidone composite hydrogels used as cartilages replacement. J. Appl. Polym. Sci. 2009, 113, 736–741. [Google Scholar] [CrossRef]
- Singh, N.B.; Rai, S. Effect of polyvinyl alcohol on the hydration of cement with rice husk ash. Cem. Concr. Res. 2001, 31, 239–243. [Google Scholar] [CrossRef]
- Knapen, E.; Van Gemert, D. Polymer film formation in cement mortars modified with water-soluble polymers. Cem. Concr. Compos. 2015, 58, 23–28. [Google Scholar] [CrossRef]
- Kim, J.H.; Robertson, R.E. Effects of Polyvinyl Alcohol on Aggregate-Paste Bond Strength and the Interfacial Transition Zone. Adv. Cem. Based Mater. 1998, 8, 66–76. [Google Scholar] [CrossRef]
- Test Method for Fluidity of Cement Mortar, China; GB/T 2419-2005; Architecture & Building Press: Beijing, China, 2005. (In Chinese)
- Test Methods for Bulk Density, Moisture and Water Absorption of Aerated Concrete, China; GB/T 11970-1997; Architecture & Building Press: Beijing, China, 1997. (In Chinese)
- Determination of The Water Absorption Coefficient of Construction Materials; DIN 52617; German Institute for Standardisation: Berlin, Germany, 1987.
- Method of Testing Cements-Determination of Strength, China; GB/T 17671-1999; Architecture and Building Press: Beijing, China, 2003. (In Chinese)











| P.O 42.5 R | Raw Material (%) | ||||||||
| SiO2 | Al2O3 | CaO | MgO | Na2O | K2O | Fe2O3 | SO3 | Loss | |
| 18.3 | 4.5 | 62.4 | 2.1 | 0.3 | 1.5 | 2.3 | 3.5 | 2.6 | |
| PVA-124 AR | Molecular Weight (Mw) | Degree of Hydrolysis (mole %) | PH | Volatile Content (%) | Ash Content (%) |
| 105,000 | 97 | 5~7 | 5.0 | 0.7 |
| Standard Sand | SiO2 Content(%) | Mud Content(%) | Ignition Loss(%) | Particle Size (mm) |
| >96 | <0.2 | <0.4 | 0.08~2 |
| Code | Ratio of Material Mass to Cement Mass (%) | ||||
|---|---|---|---|---|---|
| Cement | Sand | Water | PVA | Defoamer | |
| PCM0 | 100 | 150 | 40.2 | 0 | 0.14 |
| PCM1 | 100 | 150 | 40.24 | 0.2 | 0.14 |
| PCM2 | 100 | 150 | 40.32 | 0.6 | 0.14 |
| PCM3 | 100 | 150 | 40.4 | 1.0 | 0.14 |
| PCM4 | 100 | 150 | 40.5 | 2.0 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Li, G.; Deng, S.; Wang, Z. Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar. Appl. Sci. 2019, 9, 2178. https://doi.org/10.3390/app9112178
Fan J, Li G, Deng S, Wang Z. Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar. Applied Sciences. 2019; 9(11):2178. https://doi.org/10.3390/app9112178
Chicago/Turabian StyleFan, Jie, Gengying Li, Sijie Deng, and Zhongkun Wang. 2019. "Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar" Applied Sciences 9, no. 11: 2178. https://doi.org/10.3390/app9112178
APA StyleFan, J., Li, G., Deng, S., & Wang, Z. (2019). Mechanical Properties and Microstructure of Polyvinyl Alcohol (PVA) Modified Cement Mortar. Applied Sciences, 9(11), 2178. https://doi.org/10.3390/app9112178





