Solid Phase Assembly of Fully Protected Trinucleotide Building Blocks for Codon-Based Gene Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
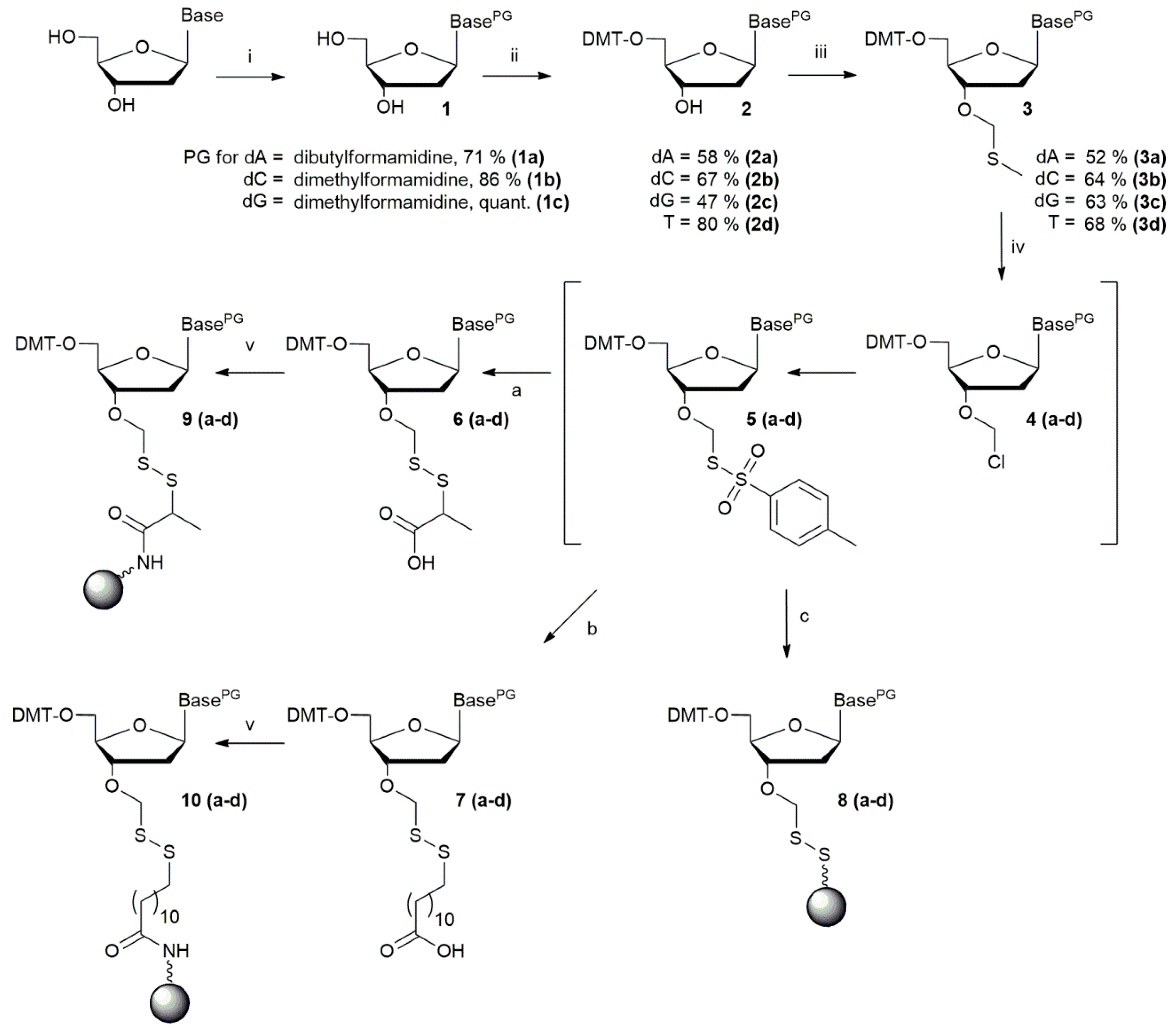
2.2. Synthesis of 5′-O-Dimethoxytrityl-3′-O-Methylthiomethyl-2′-Deoxynucleosides
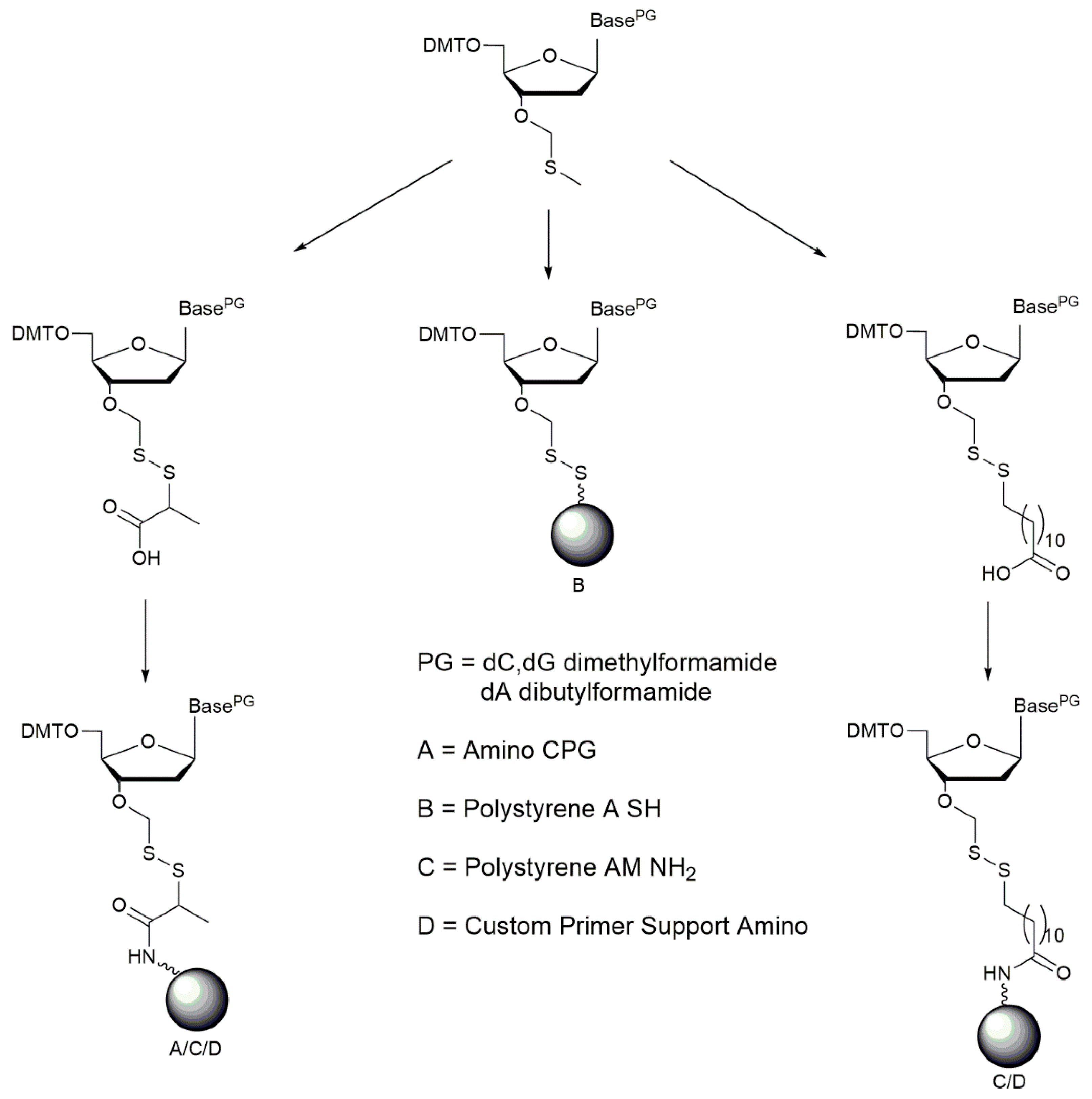
2.3. Loading of Support
2.3.1. Thymidine as Starting Nucleoside
Loading of Polystyrene A SH (B)
Loading of Amino-Functionalized Supports (A, C, D)
Capping of Unreacted Amino or Thiol Groups on Solid Supports A, B, C, and D
2.3.2. Loading of 2′-Deoxyguanosine on Solid Support D
2.3.3. Loading of 2′-Deoxyadenosine on Solid Support D
2.3.4. Loading of 2′-Deoxycytidine on Solid Support D
2.3.5. Capping of Unreacted Amino Groups on Solid Supports D
2.4. Cleavage from Support
Choice of Cleavage Reagent
2.5. Synthesis and Purification of Trinucleotides
2.5.1. Trinucleotide Cleavage from Solid Support
2.5.2. Precipitation of Trinucleotides
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morley, K.L.; Kazlauskas, R.J. Improving enzyme properties: When are closer mutations better? Trends Biotechnol. 2005, 23, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Zhu, H.; Ma, X.-Y.; Zhang, T.; Ma, Y.-S.; Wei, D.-Z. Mutant Library Construction in Directed Molecular Evolution: Casting a Wider Net. Mol. Biotechnol. 2006, 34, 55–68. [Google Scholar] [CrossRef]
- Zhao, H. Directed evolution of novel protein functions. Biotechnol. Bioeng. 2007, 98, 313–317. [Google Scholar] [CrossRef]
- Shivange, A.V.; Marienhagen, J.; Mundhada, H.; Schenk, A.; Schwaneberg, U. Advances in generating functional diversity for directed protein evolution. Chem. Boil. 2009, 13, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Neylon, C. Chemical and biochemical strategies for the randomization of protein encoding DNA sequences: Library construction methods for directed evolution. Nucleic Acids Res. 2004, 32, 1448–1459. [Google Scholar] [CrossRef]
- Janczyk, M.; Appel, B.; Springstubbe, D.; Fritz, H.-J.; Müller, S. A new and convenient approach for the preparation of β-cyanoethyl protected trinucleotide phosphoramidites. Org. Biomol. Chem. 2012, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Raetz, R.; Appel, B.; Müller, S. Preparation of trinucleotide synthons for the synthesis of gene libraries. Chim. Oggi-Chem. Today 2016, 34, Xiv–Xvii. [Google Scholar]
- Kungurtsev, V.; Lönnberg, H.; Virta, P. Synthesis of protected 2′-O-deoxyribonucleotides on a precipitative soluble support: A useful procedure for the preparation of trimer phosphoramidites. RSC Adv. 2016, 6, 105428–105432. [Google Scholar] [CrossRef]
- Suchsland, R.; Appel, B.; Müller, S. Preparation of trinucleotide phosphoramidites as synthons for gene library synthesis. Beilstein J. Org. Chem. 2018, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Suchsland, R.; Appel, B.; Müller, S. Synthesis of Trinucleotide Building Blocks in Solution and on Solid Phase. Curr. Protoc. Nucleic Acid Chem. 2018, 75, e60. [Google Scholar] [CrossRef]
- Tang, K.; Fu, D.; Kötter, S.; Cotter, R.J.; Cantor, C.R.; Köster, H. Matrix-assisted laser desorption/ionization mass spectrometry of immobilized duplex DNA probes. Nucleic Acids Res. 1995, 23, 3126–3131. [Google Scholar] [CrossRef] [PubMed]
- Bordwell, F.G.; Pitt, B.M. The Formation of α-Chloro Sulfides from Sulfides and from Sulfoxides. J. Am. Chem. Soc. 1955, 77, 572–577. [Google Scholar] [CrossRef]
- Wu, E.; Carlson, R.M. Thiolsulfonate functionalized polystyrene resin: Preparation and application in the isolation and identification of electrophilic mutagens. J. Environ. Sci. 2007, 19, 1520–1527. [Google Scholar]
- Bannwarth, W.; Knörr, R. Formation of carboxamides with N,N,N′,N′-tetramethyl (succinimido) uronium tetrafluoroborate in aqueous/organic solvent systems. Tetrahedron Lett. 1991, 32, 1157–1160. [Google Scholar] [CrossRef]
- Adinolfi, M.; Iadonisi, A.; Schiattarella, M. An approach to the highly stereocontrolled synthesis of α-glycosides. Compatible use of the very acid labile dimethoxytrityl protecting group with Yb(OTf)3-promoted glycosidation. Tetrahedron Lett. 2003, 44, 6479–6482. [Google Scholar] [CrossRef]
- Schulhof, J.; Molko, D.; Teoule, R. The final deprotection step in oligonucleotide synthesis is reduced to a mild and rapid ammonia treatment by using labile base-protecting groups. Nucleic Acids Res. 1987, 15, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Andrus, A.; Beaucage, S.L. 2-mercaptobenzothiazole—An improved reagent for the removal of methyl phosphate protecting groups from oligodeoxynucleotide phosphotriesters. Tetrahedron Lett. 1988, 29, 5479–5482. [Google Scholar] [CrossRef]
- Semenyuk, A.; Földesi, A.; Johansson, T.; Estmer-Nilsson, C.; Blomgren, P.; Brännvall, M.; Kirsebom, L.A.; Kwiatkowski, M. Synthesis of RNA Using 2‘-O-DTM Protection. J. Am. Chem. Soc. 2006, 128, 12356–12357. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Phillips, W.G. Pummerer rearrangements of sulfonium salts. J. Am. Chem. Soc. 1969, 91, 682–687. [Google Scholar] [CrossRef]
- Bates, D.K.; Winters, R.T.; Picard, J.A. Interrupted Pummerer Rearrangement: Capture of Tricoordinate Sulfur Species Generated under Pummerer Rearrangement Conditions. J. Org. Chem. 1992, 57, 3094–3097. [Google Scholar] [CrossRef]
- Han, J.; Han, G. A Procedure for Quantitative Determination of Tris(2-Carboxyethyl)phosphine, an Odorless Reducing Agent More Stable and Effective Than Dithiothreitol. Anal. Biochem. 1994, 220, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Seio, K.; Sekine, M. Improved synthesis of oligonucleotides containing 2-thiouridine derivatives by use of diluted iodine solution. Tetrahedron Lett. 2006, 47, 583–585. [Google Scholar] [CrossRef]
- Jabgunde, A.M.; Molina, A.G.; Virta, P.; Lönnberg, H.; Flitsch, S. Preparation of a disulfide-linked precipitative soluble support for solution-phase synthesis of trimeric oligodeoxyribonucleotide 3′-(2-chlorophenylphosphate) building blocks. Beilstein J. Org. Chem. 2015, 11, 1553–1560. [Google Scholar] [CrossRef] [PubMed]

| Entry # | Solid Support | Linker | Starting Nucleoside | Loading [µg/mol] | Recovery of Trinucleotide |
|---|---|---|---|---|---|
| 1 | A Amino CPG | 12-mercaptododecanoic acid | Thymidine | 7.9 | quantitative |
| 2 | A Amino CPG | 2-mercaptopropanoic acid | Thymidine | 10.5 | quantitative |
| 3 | B Thiol PS | - | Thymidine | 238.4 | <10% |
| 4 | C Amino PS | 12-mercaptododecanoic acid | Thymidine | 245.0 | <10% |
| 5 | C Amino PS | 2-mercaptopropanoic acid | Thymidine | 57.8 | <10% |
| 6 | D Amino PS (hcl) | 12-mercaptododecanoic acid | Thymidine | 89.3 | quantitative |
| 7 | D Amino PS (hcl) | 2-mercaptopropanoic acid | Thymidine | 58.9 | quantitative |
| Deoxyguanosine | 51.6 | quantitative | |||
| Deoxycytidine | 58.8 | quantitative | |||
| Deoxyadenosine | 113.0 | quantitative |
| Phosphate Protecting Group | Trinucleotide | Mass Calculated | Mass Found | Cleavage Reagent |
|---|---|---|---|---|
| Me | ATT | 1260.14 | 1284.84 (+Na) | TCEP |
| Me | AAT | 1339.24 | 1385.82 (+2Na) | TCEP |
| Me | TTT | 1180.34 | 1180.30 | TCEP |
| Me | AAA | 1402.50 | 1448.22 (+2Na) | TCEP |
| Me | GGA | 1449.49 | 1472.49 (+Na) | DTT |
| Me | GGC | 1459.47 | 1482.34 (+Na) | DTT |
| Me | GGT | 1370.44 | 1393.38 (+Na) | DTT |
| β-cyanoethyl | TTT | 1258.37 | 1281.29 (+Na) | DTT |
| β-cyanoethyl | TTA | 1337.42 | 1360.34 (+Na) | DTT |
| β-cyanoethyl | TTC | 1347.39 | 1370.38 (+Na) | DTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchsland, R.; Appel, B.; Janczyk, M.; Müller, S. Solid Phase Assembly of Fully Protected Trinucleotide Building Blocks for Codon-Based Gene Synthesis. Appl. Sci. 2019, 9, 2199. https://doi.org/10.3390/app9112199
Suchsland R, Appel B, Janczyk M, Müller S. Solid Phase Assembly of Fully Protected Trinucleotide Building Blocks for Codon-Based Gene Synthesis. Applied Sciences. 2019; 9(11):2199. https://doi.org/10.3390/app9112199
Chicago/Turabian StyleSuchsland, Ruth, Bettina Appel, Matthäus Janczyk, and Sabine Müller. 2019. "Solid Phase Assembly of Fully Protected Trinucleotide Building Blocks for Codon-Based Gene Synthesis" Applied Sciences 9, no. 11: 2199. https://doi.org/10.3390/app9112199
APA StyleSuchsland, R., Appel, B., Janczyk, M., & Müller, S. (2019). Solid Phase Assembly of Fully Protected Trinucleotide Building Blocks for Codon-Based Gene Synthesis. Applied Sciences, 9(11), 2199. https://doi.org/10.3390/app9112199





