Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagent
2.2. Methods
2.3. Characterization
3. Results and Discussion
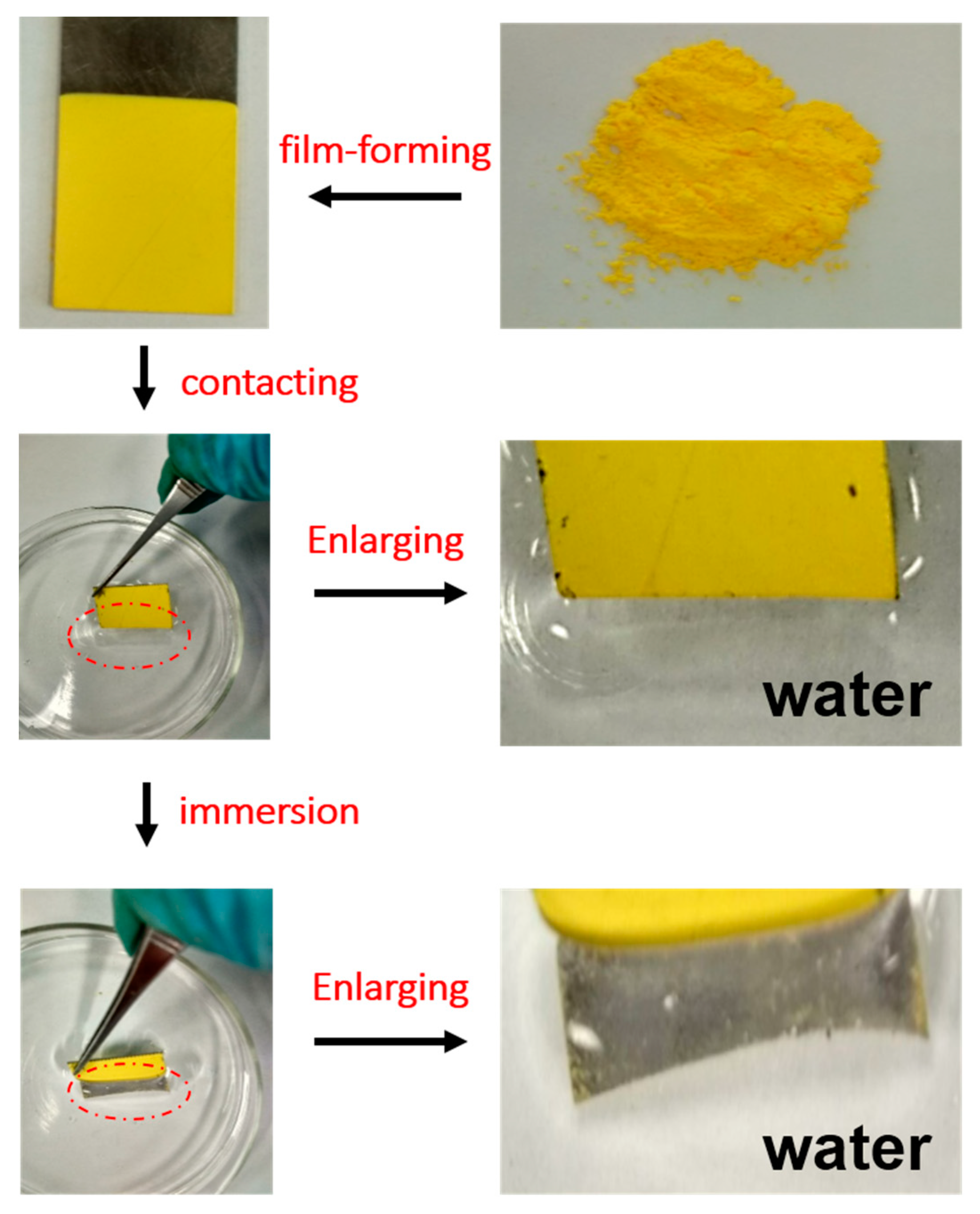
3.1. Microstructure and Surface Morphology
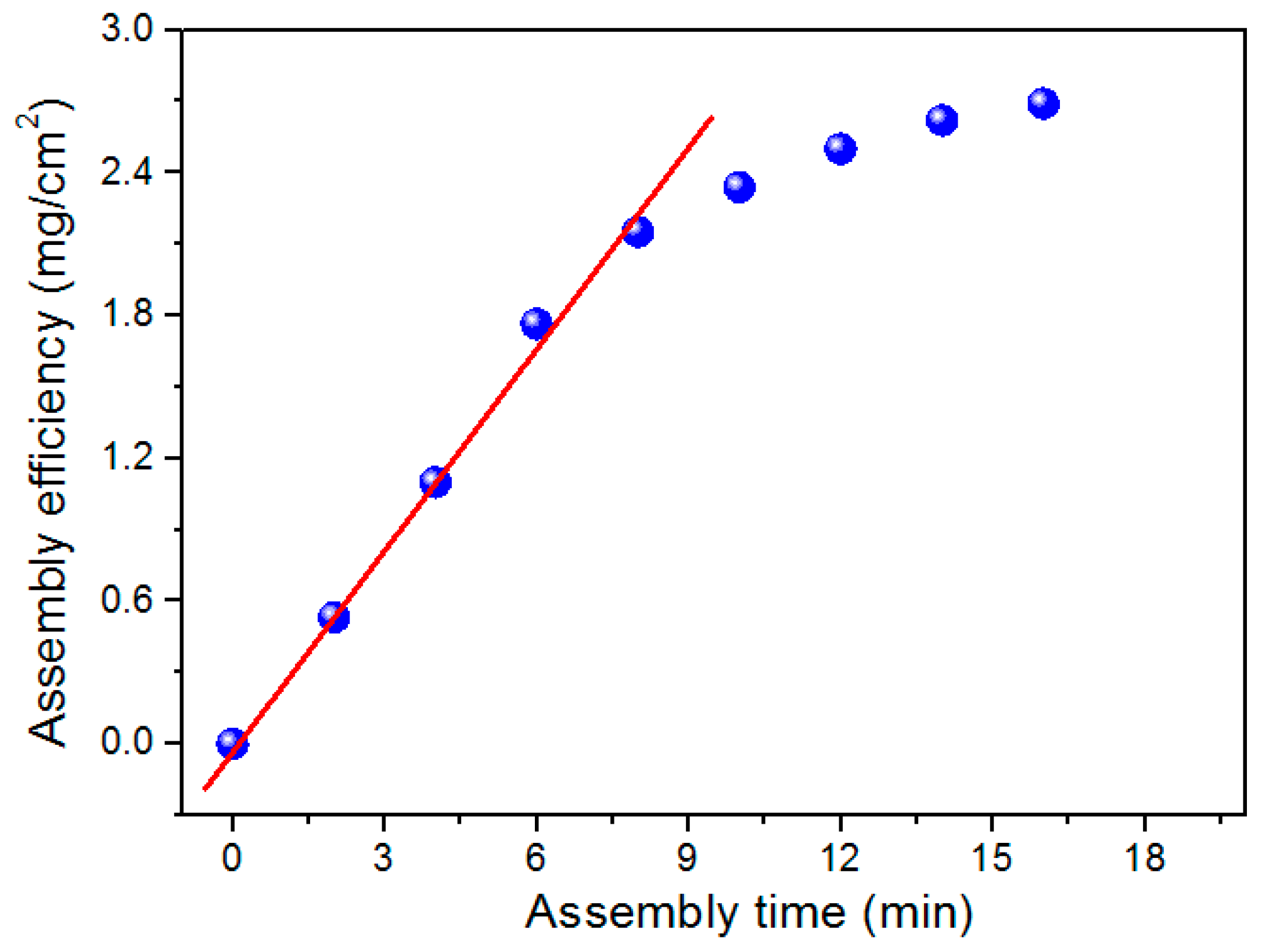
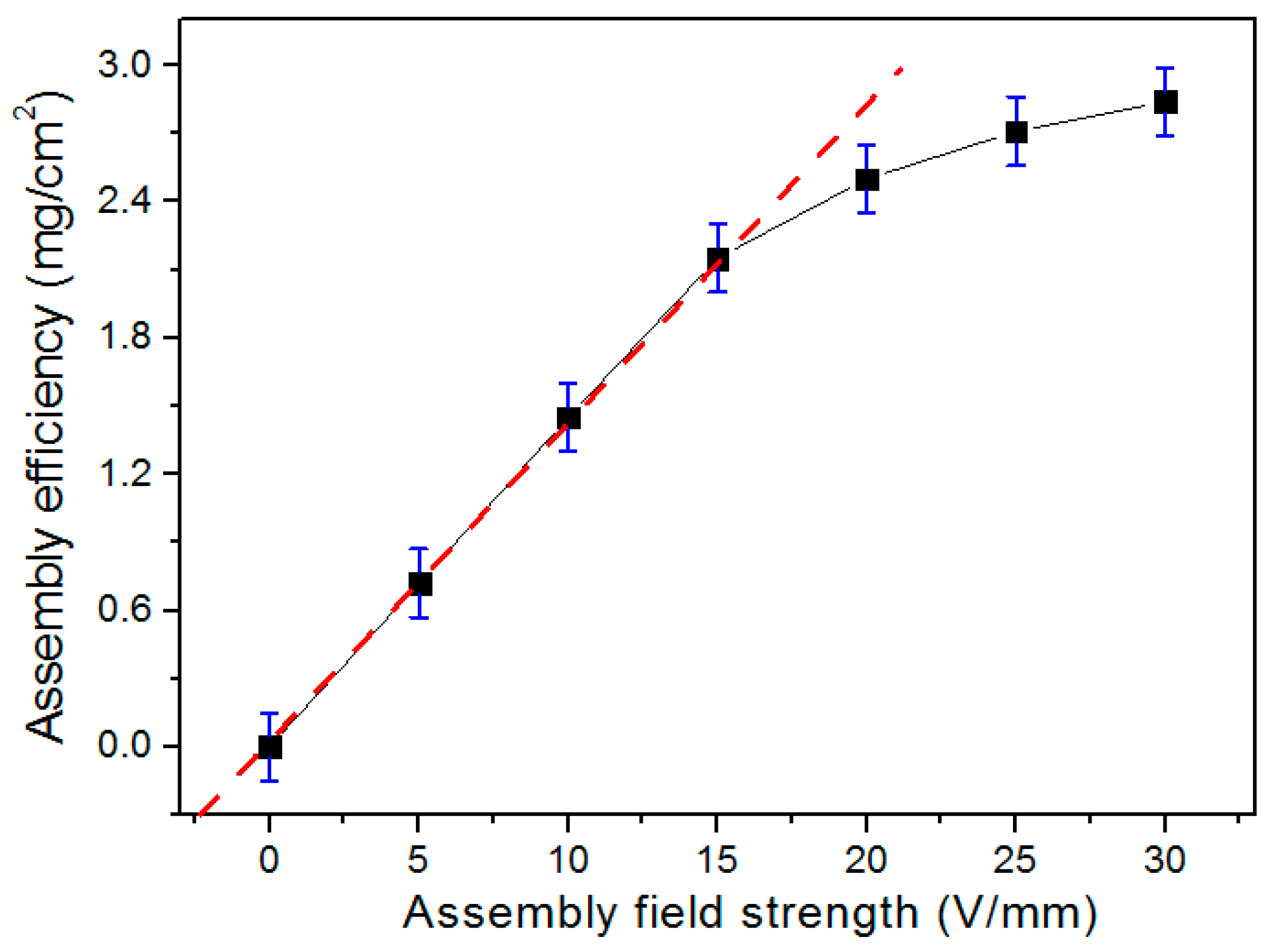
3.2. EAD Dynamics
3.3. Hydrophobic Studies
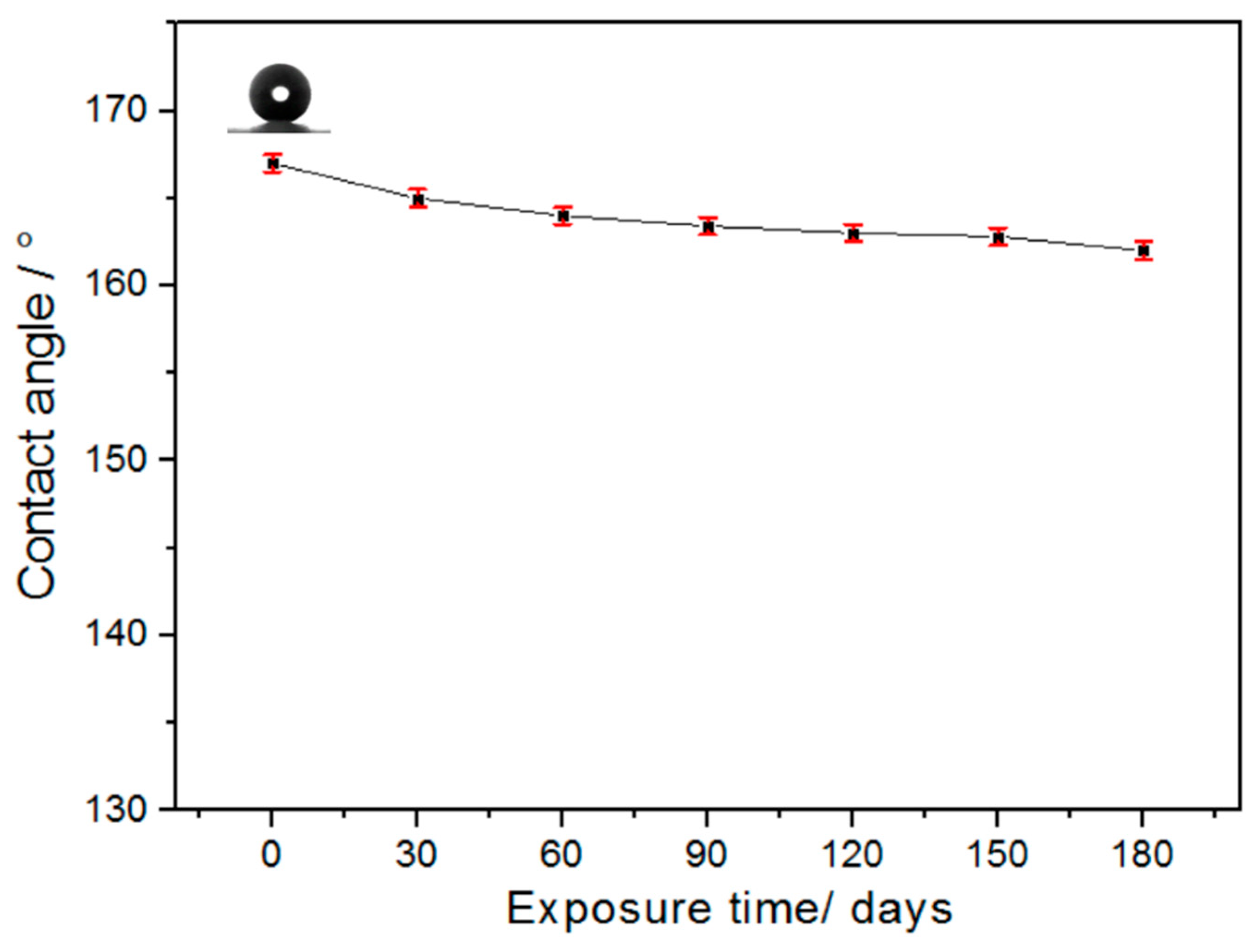
3.4. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Switzer, J.A.; Shunsky, M.G.; Bohannan, E.W. Electrodeposited ceramic single crystals. Science 1999, 284, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Hu, X.L.; Yang, H.L.; Sun, Y.M.; Hu, C.C.; Huang, Y.H. Flexible asymmetric micro-supercapacitors based on Bi2O3 and MnO2 nanoflowers: Larger areal mass promises higher energy density. Adv. Energy Mater. 2015, 5, 1401882–1401889. [Google Scholar] [CrossRef]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Qi, J.; Wu, X.H. Photocatalytic property of Cu2+-doped Bi2O3 films under visible light prepared by the solegel method. Vacuum 2014, 107, 204–207. [Google Scholar] [CrossRef]
- Liu, G.; Li, S.; Lu, Y.Y.; Zhang, J.; Feng, Z.C.; Li, C. Controllable synthesis of α-Bi2O3 and γ-Bi2O3 with high photocatalytic activity by α-Bi2O3→γ-Bi2O3→α-Bi2O3 transformation in a facile precipitation method. J. Alloys Compd. 2016, 689, 787–799. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Jung, J.I.; Luo, J. Thermal runaway, flash sintering and asymmetrical microstructural development of ZnO and ZnO-Bi2O3 under direct currents. Acta Mater. 2015, 94, 87–100. [Google Scholar] [CrossRef]
- Sopha, H.; Podzemna, V.; Hromadko, L.; Macak, J.M. Preparation of porcupine-like Bi2O3 needle bundles by anodic oxidation of bismuth. Electrochem. Commun. 2017, 84, 6–9. [Google Scholar] [CrossRef]
- Gao, X.M.; Shang, Y.Y.; Liu, L.B.; Fu, F. Chemisorption-enhanced photocatalytic nitrogen fixation via 2D ultrathin p-n heterojunction AgCl/δ-Bi2O3 nanosheets. J. Catal. 2019, 371, 71–80. [Google Scholar] [CrossRef]
- Ke, J.; Liu, J.; Sun, H.Q.; Zhang, H.Y.; Duan, X.G.; Liang, P.; Li, X.Y.; Tade, M.O.; Liu, S.M.; Wang, S.B. Facile assembly of Bi2O3/Bi2S3/MoS2 n-p heterojunction with layered n-Bi2O3 and p-MoS2 for enhanced photocatalytic water oxidation and pollutant degradation. Appl. Catal. B Environ. 2017, 200, 47–55. [Google Scholar] [CrossRef]
- Wang, W.Y.; Wang, X.W.; Cao, Z.Q.; Li, Q.C.; Feng, G.; Zhang, R.B. Enhancing photooxidative performance with Bi2O3 nanoparticle modified BiVO4 heterostructural flowers. J. Phys. Chem. C 2018, 122, 23582–23590. [Google Scholar] [CrossRef]
- Coronado-Castañeda, R.R.S.; Maya-Treviño, M.L.; Garza-González, E.; Peral, J.; Villanueva-Rodríguez, M.; Hernández-Ramírez, A. Photocatalytic degradation and toxicity reduction of isoniazid using β-Bi2O3 in real wastewater. Catal. Today 2019, in press. [Google Scholar]
- Que, Q.S.; Xing, Y.L.; He, Z.L.; Yang, Y.W.; Yin, X.T.; Que, W.X. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017, 209, 220–223. [Google Scholar] [CrossRef]
- Bai, J.W.; Li, Y.; Wei, P.K.; Liu, J.D.; Chen, W.; Liu, L. Enhancement of photocatalytic activity of Bi2 O3-BiOI composite nanosheets through vacancy engineering. Small 2019, 1900020–1900028. [Google Scholar] [CrossRef]
- Guo, X.G.; Liang, T.T.; Rager, M.; Cui, X. Low-temperature controlled synthesis of novel bismuth oxide (Bi2O3) with microrods and microflowers with great photocatalytic activities. Mater. Lett. 2018, 228, 427–430. [Google Scholar] [CrossRef]
- Qiu, Y.F.; Liu, D.F.; Yang, J.H.; Yang, S.H. Controlled synthesis of bismuth oxide nanowires by an oxidative metal vapor transport deposition technique. Adv. Mater. 2006, 18, 2604–2608. [Google Scholar] [CrossRef]
- Zou, Q.; Li, H.; Yang, Y.P.; Miao, Y.C.; Huo, Y.N. Bi2O3/TiO2 photocatalytic film coated on floated glass balls for efficient removal of organic pollutant. Appl. Surf. Sci. 2019, 467–468, 354–360. [Google Scholar] [CrossRef]
- Guan, Z.C.; Wang, H.P.; Wang, X.; Hu, J.; Du, R.G. Fabrication of heterostructured β-Bi2O3-TiO2 nanotube array composite film for photoelectrochemical cathodic protection applications. Corros. Sci. 2018, 136, 60–69. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Triana, C.; Lee, S.W. Ink-jet Bi2O3 films and powders for CO2 capture and self-cleaning applications. Thin Solid Films 2019, 677, 83–89. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kwak, N.W.; Byeon, P.; Chung, S.Y.; Jung, W.C. Conductive nature of grain boundaries in nanocrystalline stabilized Bi2O3 thin-film electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 6269–6275. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.J.; Tang, M.F.; Hong, G.H.; Gao, J.M.; Chen, Y. Preparation of robust superhydrophobic halloysite clay nanotubes via mussel-inspired surface modification. Appl. Sci. 2017, 7, 1129. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avci, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Tembely, M.; Dolatabadi, A. Supercooled water droplet impacting superhydrophobic surfaces in the presence of cold air flow. Appl. Sci. 2017, 7, 130. [Google Scholar] [CrossRef]
- Ravi, V.K.; Scheidt, R.A.; DuBose, J.; Kamat, P.V. Hierarchical arrays of cesium lead halide perovskite nanocrystals through electrophoretic deposition. J. Am. Chem. Soc. 2018, 140, 8887–8894. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.G.; Li, X.M.; Wei, Z.B.; Li, X.L.; Niu, L.D. Rapid fabrication and characterization of superhydrophobic tri-dimensional Ni/Al coatings. Appl. Surf. Sci. 2016, 387, 8–15. [Google Scholar] [CrossRef]
- Fritz, P.A.; Lange, S.C.; Giesbers, M.; Zuilhof, H.; Boom, R.M.; Schroen, C.G.P.H. Simultaneous silicon oxide growth and electrophoretic deposition of graphene oxide. Langmuir 2019, 35, 3717–3723. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.G.; Lai, C.; Jiang, X.; Mi, W.H.; Yin, Y.J.; Li, X.M.; Shu, Y.J. Remarkably facile fabrication of extremely superhydrophobic high-energy binary composite with ultralong lifespan. Chem. Eng. J. 2018, 335, 843–854. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of dispersions of colloidal alumina particles in aqueous suspensions. J. Colloid Interface. Sci. 2015, 291, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.G.; Li, X.M.; Li, H.R.; Zhang, D.X.; Lai, C.; Li, W.L. A comprehensive investigation on the electrophoretic deposition (EPD) of nano-Al/Ni energetic composite coatings for the combustion application. Surf. Coat. Technol. 2015, 265, 83–91. [Google Scholar] [CrossRef]
- Fang, J.; Shan, X.-Q.; Wen, B.; Lin, J.-M.; Owens, G. Stability of titania nanoparticles in soil suspensions and transport in saturated homogeneous soil columns. Environ. Pollut. 2009, 157, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.G.; Li, X.M.; Lai, C.; Jiang, X.; Li, X.L.; Shu, Y.J. Facile approach to the green synthesis of novel ternary composites with excellent superhydrophobic and thermal stability property: An expanding horizon. Chem. Eng. J. 2017, 309, 240–248. [Google Scholar] [CrossRef]
- Lu, Y.; Sathasivam, S.; Song, J.L.; Crick, C.R.; Carmalt, C.J.; Parkldn, I.P. Robust self-cleaning surfaces that function when exposed to either air or oil. Science 2015, 347, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Mammen, L.; Butt, H.J.; Vollmer, D. Candle soot as a template for a transparent robust superamphiphobic coating. Science 2012, 335, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Leu, I.C.; Hou, M.H. Kinetics of electrophoretic deposition for nanocrystalline zinc oxide coatings. J. Am. Ceram. Soc. 2004, 87, 84–88. [Google Scholar] [CrossRef]
- Larmour, I.A.; Bell, S.E.J.; Saunders, G.C. Remarkably simple fabrication of superhydrophobic surfaces using electroless galvanic deposition. Angew. Chem. Int. Ed. 2007, 119, 1740–1742. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, T.-t.; Guo, X.-g. Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings. Appl. Sci. 2019, 9, 2653. https://doi.org/10.3390/app9132653
Liang T-t, Guo X-g. Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings. Applied Sciences. 2019; 9(13):2653. https://doi.org/10.3390/app9132653
Chicago/Turabian StyleLiang, Tao-tao, and Xiao-gang Guo. 2019. "Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings" Applied Sciences 9, no. 13: 2653. https://doi.org/10.3390/app9132653
APA StyleLiang, T.-t., & Guo, X.-g. (2019). Remarkably Facile Preparation of Superhydrophobic Functionalized Bismuth Trioxide (Bi2O3) Coatings. Applied Sciences, 9(13), 2653. https://doi.org/10.3390/app9132653



