Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Sb2O3 Sample
2.2. Materials Characterization
2.3. Preparation of Electrodes
2.4. Electrochemical Measurement
3. Results
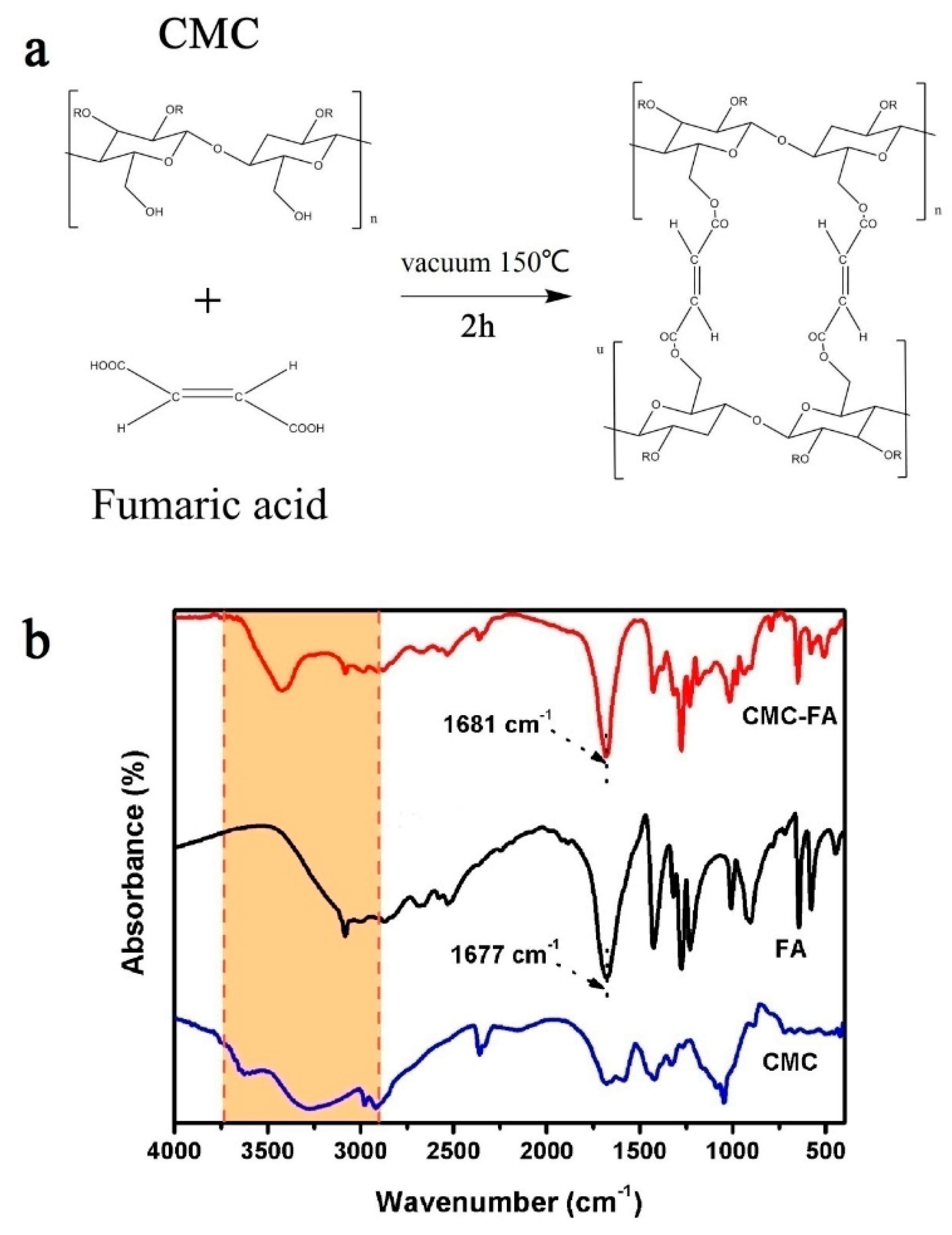
3.1. Material Characterization
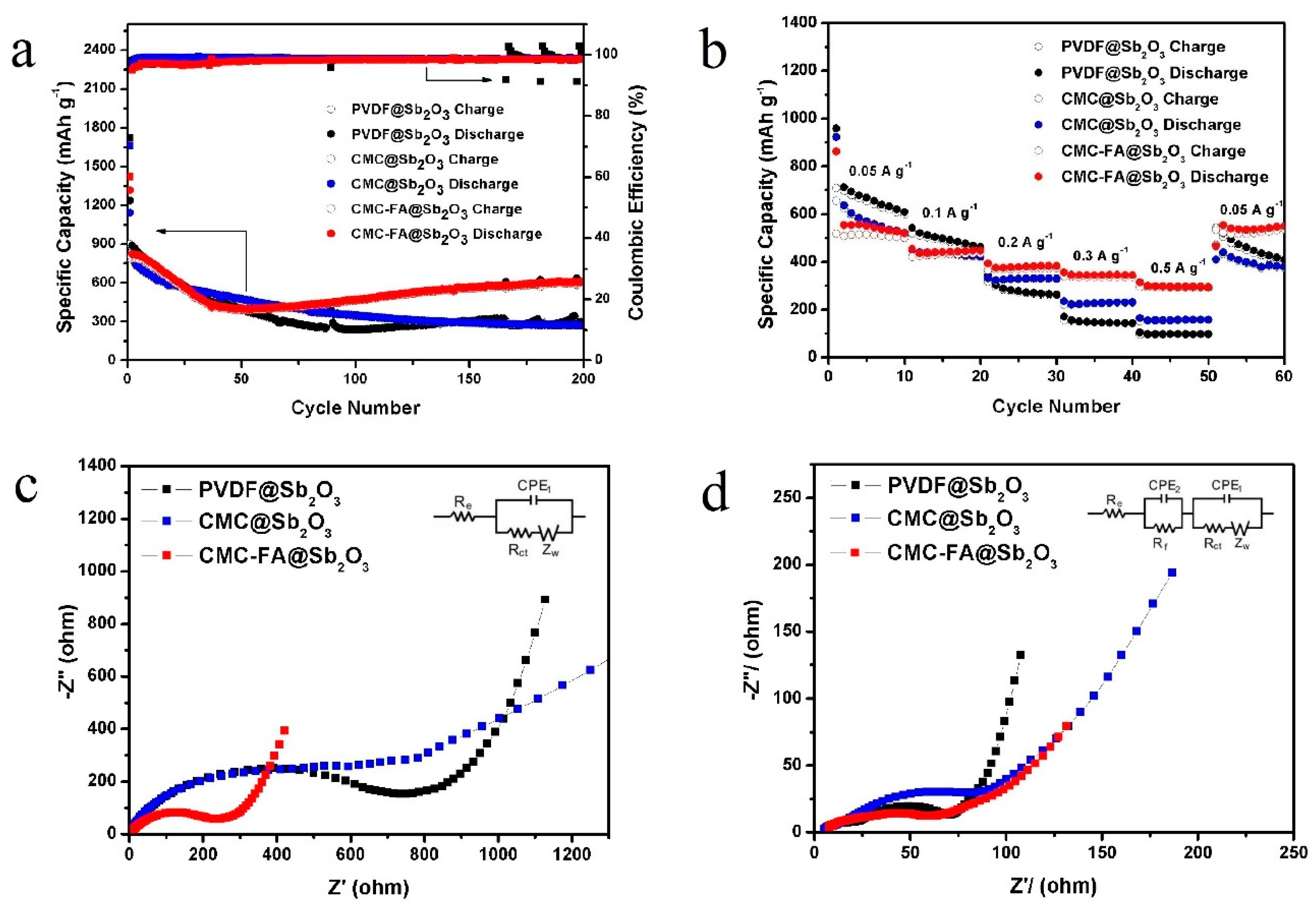
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhai, X.L.; Yang, K.K.; Wang, F.; Wei, H.J.; Zhang, W.H.; Ren, F.Z.; Pang, H. Mesoporous NH4NiPO4 center dot H2O for High-Performance Flexible All-Solid-State Asymmetric Supercapacitors. Front. Chem. 2019, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Suga, T.; Konishi, H.; Nishide, H. Photocrosslinked nitroxide polymer cathode-active materials for application in an organic-based paper battery. Chem. Commun. (Camb. Engl.) 2007, 2017, 1730–1732. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Li, W.; Ma, B.; Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 2015, 44, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Arico, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef]
- Wu, N.T.; Du, W.Z.; Gao, X.; Zhao, L.; Liu, G.L.; Liu, X.M.; Wu, H.; He, Y.B. Hollow SnO2 nanospheres with oxygen vacancies entrapped by a N-doped graphene network as robust anode materials for lithium-ion batteries. Nanoscale 2018, 10, 11460–11466. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Wang, Z.J.; Lei, D.N.; Lv, W.; Zhao, Q.; Ni, B.; Liu, Y.; Li, B.H.; Kang, F.Y.; He, Y.B. Spherical Li Deposited inside 3D Cu Skeleton as Anode with Ultrastable Performance. ACS Appl. Mater. Interfaces 2018, 10, 20244–20249. [Google Scholar] [CrossRef]
- Zheng, J.C.; Yang, Z.; He, Z.J.; Tong, H.; Yu, W.J.; Zhang, J.F. In situ formed LiNi0.8Co0.15Al0.05O2@Li4SiO4 composite cathode material with high rate capability and long cycling stability for lithium-ion batteries. Nano Energy 2018, 53, 613–621. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Recent progress in Li-rich layered oxides as cathode materials for Li-ion batteries. RSC Adv. 2014, 4, 63268–63284. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; van Aken, P.A.; Maier, J.; Yu, Y. Fast Li Storage in MoS2-Graphene-Carbon Nanotube Nanocomposites: Advantageous Functional Integration of 0D, 1D, and 2D Nanostructures. Adv. Energy Mater. 2015, 5, 1401170. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Li, J. Graphene and Graphene-like Layered Transition Metal Dichalcogenides in Energy Conversion and Storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wen, Y.; Wang, Y.; van Aken, P.A.; Maier, J.; Yu, Y. Carbon-Encapsulated Pyrite as Stable and Earth-Abundant High Energy Cathode Material for Rechargeable Lithium Batteries. Adv. Mater. 2014, 26, 6025–6030. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Q.; Wang, W.; An, Y.; Li, J.; Guo, L. Preparation of Flower-like SnO2 Nanostructures and Their Applications in Gas-Sensing and Lithium Storage. Cryst. Growth Des. 2011, 11, 2942–2947. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Rui, X.; Qi, D.; Luo, Y.; Leow, W.R.; Chen, S.; Guo, J.; Wei, J.; Li, W.; et al. Conductive Inks Based on a Lithium Titanate Nanotube Gel for High-Rate Lithium-Ion Batteries with Customized Configuration. Adv. Mater. 2016, 28, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Bai, Y.; Zhou, W.; An, Y.; Li, J.; Guo, L. The self-assembly of porous microspheres of tin dioxide octahedral nanoparticles for high performance lithium ion battery anode materials. J. Mater. Chem. 2011, 21, 10189–10194. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.J.; Wang, C.; Wang, F.; Wang, H.C.; Zhang, W.H.; Wang, X.F.; Yan, C.L.; Kim, B.H.; Ren, F.Z. Nitrogen-Doped Carbon Coated WS2 Nanosheets as Anode for High-Performance Sodium-Ion Batteries. Front. Chem. 2018, 6, 236. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Zhao, Y.F.; Wang, Y.; Wang, Z.J.; Zhang, W.H.; Ren, F.Z. Facile Synthesis of Two-Dimensional Porous MgCo2O4 Nanosheets as Anode for Lithium-Ion Batteries. Appl. Sci. 2018, 8, 22. [Google Scholar] [CrossRef]
- Liu, G.L.; Cui, J.; Luo, R.J.; Liu, Y.; Huang, X.X.; Wu, N.T.; Jin, X.Y.; Chen, H.P.; Tang, S.Y.; Kim, J.K.; et al. 2D MoS2 grown on biomass-based hollow carbon fibers for energy storage. Appl. Surf. Sci. 2019, 469, 854–863. [Google Scholar] [CrossRef]
- Liu, G.; Liu, X.; Li, X.; Jin, X.; Xiao, H.; Wu, N.; Guo, D.; Tang, S. Coordination Engineering Construction of Si@ZnS@N,S-Doped Reduced Graphene Oxide Nanocomposite as Anode Material with Enhanced Lithium Storage Performance. Energy Technol. 2019, 7, 1900186. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Lu, X.; Lv, X.; Ma, G.; Wang, Q.; Lei, Z. Sb2O3 Nanoparticles Anchored on Graphene Sheets via Alcohol Dissolution-Reprecipitation Method for Excellent Lithium-Storage Properties. ACS Appl. Mater. Interfaces 2017, 9, 34927–34936. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Li, W.; Wang, F.; Xia, Y.Y.; Asiri, A.M.; Zhao, D.Y. Controllable synthesis of SnO2@C yolk-shell nanospheres as a high-performance anode material for lithium ion batteries. Nanoscale 2014, 6, 3217–3222. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, L.; Chen, H.; Hou, Q.; Chen, X. Synthesis of a symmetric bundle-shaped Sb2O3 and its application for anode materials in lithium ion batteries. Mater. Lett. 2018, 212, 103–106. [Google Scholar] [CrossRef]
- Hong, K.S.; Nam, D.H.; Lim, S.J.; Sohn, D.; Kim, T.H.; Kwon, H. Electrochemically Synthesized Sb/Sb2O3 Composites as High-Capacity Anode Materials Utilizing a Reversible Conversion Reaction for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17264–17271. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.J.; Jiang, Y.Z.; Sun, W.P.; Wang, H.T.; Jin, C.H.; Yan, M. Reversible Conversion-Alloying of Sb2O3 as a High-Capacity, High-Rate, and Durable Anode for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 19449–19455. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Zhang, Z.F.; Wang, J.W.; Wang, Q.T.; Ma, G.F.; Lei, Z.Q. Sb2O4/reduced graphene oxide composite as high-performance anode material for lithium ion batteries. J. Alloys Compd. 2017, 699, 611–618. [Google Scholar] [CrossRef]
- Zhou, X.Z.; Zhang, Z.F.; Xu, X.H.; Yan, J.; Ma, G.F.; Lei, Z.Q. Anchoring Sb6O13 Nanocrystals on Graphene Sheets for Enhanced Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 35398–35406. [Google Scholar] [CrossRef]
- Deng, M.X.; Li, S.J.; Hong, W.W.; Jiang, Y.L.; Xu, W.; Shuai, H.L.; Zou, G.Q.; Hu, Y.C.; Hou, H.S.; Wang, W.L.; et al. Octahedral Sb2O3 as high-performance anode for lithium and sodium storage. Mater. Chem. Phys. 2019, 223, 46–52. [Google Scholar] [CrossRef]
- Gao, H.; Zhou, W.; Jang, J.-H.; Goodenough, J.B. Cross-Linked Chitosan as a Polymer Network Binder for an Antimony Anode in Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1502130. [Google Scholar] [CrossRef]
- Pan, J.; Wang, N.; Zhou, Y.; Yang, X.; Zhou, W.; Qian, Y.; Yang, J. Simple synthesis of a porous Sb/Sb2O3 nanocomposite for a high-capacity anode material in Na-ion batteries. Nano Res. 2017, 10, 1794–1803. [Google Scholar] [CrossRef]
- Nam, D.-H.; Hong, K.-S.; Lim, S.-J.; Kim, M.-J.; Kwon, H.-S. High-Performance Sb/Sb2O3 Anode Materials Using a Polypyrrole Nanowire Network for Na-Ion Batteries. Small 2015, 11, 2885–2892. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Cui, Y.; Li, J.; Xu, Z.; Yang, J.; Wang, R.; Cheng, Y.; Hang, J. A flexible Sb2O3/carbon cloth composite as a free-standing high performance anode for sodium ion batteries. Chem. Commun. 2017, 53, 13165–13167. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yana, D.; Ma, J.; Qin, W.; Zhang, X.; Lu, T.; Pan, L. One-step microwave-assisted synthesis of Sb2O3/reduced graphene oxide composites as advanced anode materials for sodium-ion batteries. Ceram. Int. 2016, 42, 15634–15642. [Google Scholar] [CrossRef]
- Liu, Y.; Tai, Z.; Zhou, T.; Sencadas, V.; Zhang, J.; Zhang, L.; Konstantinov, K.; Guo, Z.; Liu, H.K. An All-Integrated Anode via Interlinked Chemical Bonding between Double-Shelled-Yolk-Structured Silicon and Binder for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1703028. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Kim, H.; Cho, Y.; Lee, K.T.; Choi, N.S.; Cho, J. A Highly Cross-Linked Polymeric Binder for High-Performance Silicon Negative Electrodes in Lithium Ion Batteries. Angew. Chem. Int. Ed. 2012, 51, 8762–8767. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, Z.; Ye, H.; Mou, J.; Lei, D.; Liu, Y.; Lv, W.; Li, B.; Kang, F.; He, Y.-B. A Stable Cross-Linked Binder Network for SnO2 Anode with Enhanced Sodium-Ion Storage Performance. ChemistrySelect 2017, 2, 11365–11369. [Google Scholar] [CrossRef]
- Song, J.X.; Zhou, M.J.; Yi, R.; Xu, T.; Gordin, M.L.; Tang, D.H.; Yu, Z.X.; Regula, M.; Wang, D.H. Interpenetrated Gel Polymer Binder for High-Performance Silicon Anodes in Lithium-ion Batteries. Adv. Funct. Mater. 2014, 24, 5904–5910. [Google Scholar] [CrossRef]
- Rohan, R.; Kuo, T.C.; Chiou, C.Y.; Chang, Y.L.; Li, C.C.; Lee, J.T. Low-cost and sustainable corn starch as a high-performance aqueous binder in silicon anodes via in situ cross-linking. J. Power Sources 2018, 396, 459–466. [Google Scholar] [CrossRef]
- Chevrier, V.L.; Ceder, G. Challenges for Na-ion Negative Electrodes. J. Electrochem. Soc. 2011, 158, A1011–A1014. [Google Scholar] [CrossRef]
- Vukovic, M.; Brankovic, Z.; Poleti, D.; Recnik, A.; Brankovic, G. Novel simple methods for the synthesis of single-phase valentinite Sb2O3. J. Sol-Gel Sci. Technol. 2014, 72, 527–533. [Google Scholar] [CrossRef]
- Shi, Y.T.; Lv, W.; Niu, S.Z.; He, Y.B.; Zhou, G.M.; Chen, G.H.; Li, B.H.; Yang, Q.H.; Kang, F.Y. A Carbon-Sulfur Hybrid with Pomegranate-like Structure for Lithium-Sulfur Batteries. Chem. Asian J. 2016, 11, 1343–1347. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.H.; Cundy, C.S.; Garforth, A.A.; Zholobenko, V.L. Mesoporous ZSM-5 catalysts: Preparation, characterisation and catalytic properties. Part I: Comparison of different synthesis routes. Microporous Mesoporous Mater. 2006, 89, 78–87. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Zhang, F.X.; Jin, J. Interface Chemistry Guided Long-Cycle-Life Li-S Battery. Nano Lett. 2013, 13, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.Z.; Fu, Z.W. Electrochemical reaction of lithium with nanostructured thin film of antimony trioxide. Electrochem. Commun. 2006, 8, 1250–1256. [Google Scholar] [CrossRef]
- Hou, H.S.; Jing, M.J.; Yang, Y.C.; Zhang, Y.; Zhu, Y.R.; Song, W.X.; Yang, X.M.; Ji, X.B. Sb porous hollow microspheres as advanced anode materials for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 2971–2977. [Google Scholar] [CrossRef]
- Jin, J.; Huang, S.Z.; Shu, J.; Wang, H.E.; Li, Y.; Yu, Y.; Chen, L.H.; Wang, B.J.; Su, B.L. Highly porous TiO2 hollow microspheres constructed by radially oriented nanorods chains for high capacity, high rate and long cycle capability lithium battery. Nano Energy 2015, 16, 339–349. [Google Scholar] [CrossRef]
- Bao, W.Z.; Liu, L.; Wang, C.Y.; Choi, S.; Wang, D.; Wang, G.X. Facile Synthesis of Crumpled Nitrogen-Doped MXene Nanosheets as a New Sulfur Host for Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702485. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, C.F.; Wen, G.W. Equivalent circuit model analysis on electrochemical impedance spectroscopy of lithium metal batteries. J. Power Sources 2015, 294, 67–74. [Google Scholar] [CrossRef]
- Yang, X.; Ma, J.J.; Wang, H.J.; Chai, Y.Q.; Yuan, R. Partially reduced Sb/Sb2O3@C spheres with enhanced electrochemical performance for lithium ion storage. Mater. Chem. Phys. 2018, 213, 208–212. [Google Scholar] [CrossRef]
- Wang, Z.M.; Cheng, Y.; Li, Q.; Chang, L.M.; Wang, L.M. Facile synthesis of one-dimensional hollow Sb2O3@TiO2 composites as anode materials for lithium ion batteries. J. Power Sources 2018, 389, 214–221. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, C.; Wang, H.; Yang, J.; Hu, P.; Guo, L. 3D nest-shaped Sb2O3/RGO composite based high-performance lithium-ion batteries. Nanoscale 2016, 8, 17131–17135. [Google Scholar] [CrossRef] [PubMed]




| Materials | Current Density (mA/g) | Cycle Number | Specific Capacity (mAh/g) | Ref |
|---|---|---|---|---|
| octahedral Sb2O3 | 200 | 50 | 640.8 | [30] |
| carbon-coated Sb/Sb2O3 | 100 | 100 | 686 | [51] |
| hollow Sb2O3@TiO2 | 100 | 100 | 593 | [52] |
| bundle shaped Sb2O3 | 20 | 100 | 594.1 | [25] |
| 3D nest-shaped Sb2O3/RGO composites | 50 | 100 | 562 | [53] |
| CMC-FA@ Sb2O3 | 200 | 200 | 611.4 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, H.; Yang, K.; Yang, Y.; Ma, J.; Pan, K.; Wang, G.; Ren, F.; Pang, H. Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder. Appl. Sci. 2019, 9, 2677. https://doi.org/10.3390/app9132677
Liu Y, Wang H, Yang K, Yang Y, Ma J, Pan K, Wang G, Ren F, Pang H. Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder. Applied Sciences. 2019; 9(13):2677. https://doi.org/10.3390/app9132677
Chicago/Turabian StyleLiu, Yong, Haichao Wang, Keke Yang, Yingnan Yang, Junqing Ma, Kunming Pan, Guangxin Wang, Fengzhang Ren, and Huan Pang. 2019. "Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder" Applied Sciences 9, no. 13: 2677. https://doi.org/10.3390/app9132677
APA StyleLiu, Y., Wang, H., Yang, K., Yang, Y., Ma, J., Pan, K., Wang, G., Ren, F., & Pang, H. (2019). Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder. Applied Sciences, 9(13), 2677. https://doi.org/10.3390/app9132677






