Effect of Nanofluids on Boiling Heat Transfer Performance
Abstract
1. Introduction
- Application of nanofluids.
- Effect of nanoparticle type on boiling heat transfer.
- Effect of base fluid type on boiling heat transfer.
- Outline the effect of nanofluids on boiling critical heat flux.
- Outline the effect of nanofluids on boiling heat transfer coefficient.
2. Nanofluid Application in Engineering
2.1. Nanofluid Application in Heat Pipes
2.2. Nanofluid Application in Automobiles
2.3. Nanofluid in the Collector
2.4. Nanofluids in Other Applications
3. Effects of Nanofluid Physical Properties and Heated Surface on Boiling Heat Transfer
3.1. Effect of Nanoparticles on Boiling Heat Transfer
3.1.1. Effect of Nanoparticle Types on Boiling Heat Transfer
3.1.2. Effect of Nanoparticle Concentration on Boiling Heat Transfer
3.2. Effect of Base Fluid Type on Boiling Heat Transfer
3.3. Effect of Heated Surface on Boiling Heat Transfer of Nanofluids
4. Effect of Nanofluid on Critical Heat Transfer and Boiling Heat Transfer Coefficient
- (1)
- The addition of nanoparticles increases the thermal conductivity of the base fluid.
- (2)
- The deposition on the heated surface during boiling process reduces the contact angle and changes the wettability, so that the liquid is quickly replenished to the dry spots formed after the bubbles are detached.
- (1)
- The influence of the deposition morphology of the nanoparticles on the heated surface.
- (2)
- The size of the bubble diameter.
- (3)
- Liquid convection effect on the heated surface.
- (1)
- The thermal conductivity of nanoparticles compared to heated surfaces.
- (2)
- Bubble detachment.
- (3)
- Microscopic motion between multiple solutions.
5. The Direction of Nanofluid Research
- (1)
- Scanning electron microscopy is used to analyze the sedimentary morphology and atomic force microscopy is used to analyze the roughness of the deposited surface.
- (2)
- Analysis of the generation and detachment of boiling bubbles by high-speed cameras.
- (3)
- Analysis of the wettability of the heated surface.
- (1)
- By disposing the nanoparticles with different thermal conductivity on the heated surface and not escaping due to the severity boiling during the boiling process. The effects on the critical heat flux and boiling heat transfer coefficient in the pure solution are investigated and the bubbles around a single nanoparticle are observed.
- (2)
- The current boiling heat transfer devices are all boiling in a large container saturated pool boiling, so the influence of the multicomponent solution on the boiling heat transfer can be observed by externally condensing the device, and the relative movement between the multicomponent solutions can be clearly observed. The motion and the disturbance of the bubble were observed and analyzed, and the effects on the critical heat flux and boiling heat transfer coefficient were investigated.
- (3)
- By investigating the boiling heat transfer performance of mixed nanoparticles in multisolution, the relative motion between base liquids and the influence of micromotion of different nanoparticles due to their density difference and specific surface difference on the critical heat flux and boiling heat transfer coefficient were analyzed.
- (4)
- To classify the heat transfer between different nanoparticles and different base liquid nanofluids and to correlate the effects of nanofluids on boiling heat transfer to a general purpose test of boiling critical heat flux and boiling heat transfer coefficient. Subsequent further in-depth research laid the foundation.
6. Conclusions
- (1)
- The boiling heat transfer performance of nanofluids is affected by the type, concentration of nanoparticles, base type, and heating surface. Nanoparticle deposition causes the wettability and the number of gasification core points on the heated surface to change, which has an effect on the boiling heat transfer performance. In addition, the nanoparticle deposition surface changes the generation state of the boiling bubble and the escape frequency per unit time.
- (2)
- The nanofluid has a strong strengthening effect on the critical heat flux. It is mainly manifested that when the concentration of the nanoparticles is too large and the heating surface roughness begins to decrease, the critical heat flux no longer increases and begins to deteriorate. In addition, the deposition morphology of different nanoparticles increases the capillary force, thereby enhancing the liquid replenishing ability after the bubble is detached. This in turn increases the critical heat flux.
- (3)
- The influence of nanofluids on the boiling heat transfer coefficient is still different. The main explanation and analysis is attributed to the formation of a porous deposit on the heated surface of the nanoparticles, which increases the heat transfer resistance of the heated surface and reduces the boiling heat transfer coefficient. In addition, the deposition of nanoparticles on the heated surface causes the critical heat flux to increase faster than superheat, resulting in an increase in the maximum boiling heat transfer coefficient. There is also a large increase in the thermal conductivity of the base liquid to the base liquid, so that the boiling heat transfer coefficient is enhanced. The perturbation of the boiling surface by the nanoparticles increases the thinning of the liquid microlayer on the heated surface, the bubble disturbance is enhanced, and the boiling heat transfer coefficient is enhanced.
- (4)
- In view of the divergence of the nanofluids on the boiling heat transfer coefficient, it is found that the dispersion of nanoparticles in the base liquid with higher viscosity has a positive effect on the boiling heat transfer performance because the nanoparticles have a great effect on the thermal conductivity of the base liquid, and the stability is good. Therefore, the deposition of the nanoparticles on the heated surface is small, and thus the enhancement of the thermal conductivity of the base liquid is stronger than the microscopic movement of nanoparticles on the heated surface and the boiling heat transfer performance is enhanced. When the base liquid is a multicomponent solution, the relative movement between the solutions enhances the microscopic motion of the nanoparticles due to the different evaporation order during the boiling process. Therefore, the disturbance of the bubble on the heating surface is enhanced, and the boiling heat transfer performance is enhanced.
- (5)
- Compared with the thermal conductivity of the heated surface, the deposition of the low thermal conductivity nanoparticle on the heated surface reduces the heat dissipation rate and improves the wall superheat of the heated surface. Then the enhancement of the boiling heat transfer coefficient of the nanofluid should be attributed to the thermal conductivity of the nanoparticle to the base fluid and the influence of the microscopic motion of the nanoparticle on the disturbance of the heated surface bubble. The movement path in the boiling process can be observed by using a component analyzer by appropriately marking the nanoparticles. In-depth research and analysis on the effects of microscopic motion of nanoparticles on boiling heat transfer. In addition, because the surface of the nanoparticle is deposited on the heating surface to change the bubble generation state, it is necessary to use a high-speed camera to observe and analyze the bubble generation state around the deposition of a single nanoparticle.
Author Contributions
Acknowledgments
Conflicts of Interest
References and Note
- Leidenfrost, J.G. De Aquae Communis Nonnullis Qualitatibus Tractatus. (Ovenius, Duisburg, 1756); transl. Wares, C. On the fixation of water in diverse fire. Int. J. Heat Mass Trans. 1966, 9, 1153–1166. [Google Scholar] [CrossRef]
- Jakob, M.; Fritz, W. Versuche ueber den verdampfungsvorgang. Forsch. Geb. Ingenieurwes 1931, 2, 437–447. [Google Scholar]
- Jakob, M.; Linke, W. Heat Transfer from a Horizontal Plate. Forsch. Geb. Ingenieurwes 1933, 4, 434. [Google Scholar]
- Jacob, M. Heat transfer in evaporation and condensation. Mech. Eng. 1936, 58, 643–660. [Google Scholar]
- Kutateladze, S.S. AHydrodynamic Theory of Changes in Boiling Process under Free Convection. Isv Akad Nauk, USSR, Otd Tekh Nauk 1951, 4, 529–935. [Google Scholar]
- Kutateladze, S.S. Heat Transfer in Condensation and Boiling. Report No. AEC-tr-3770, 1952. [Google Scholar]
- Akhgar, A.; Toghraie, D. An experimental study on the stability and thermal conductivity of water-ethylene glycol/TiO2-MWCNTs hybrid nanofluid: Developing a new correlation. Powder Technol. 2018, 338, 806–818. [Google Scholar] [CrossRef]
- Kakavandi, A.; Akbari, M. Experimental investigation of thermal conductivity of nanofluids containing of hybrid nanoparticles suspended in binary base fluids and propose a new correlation. Int. J. Heat Mass Transf. 2018, 124, 742–751. [Google Scholar] [CrossRef]
- Aparna, Z.; Michael, M.M.; Pabi, S.K.; Ghosh, S. Diversity in thermal conductivity of aqueous Al2O3- and Ag-nanofluids measured by transient hot-wire and laser flash methods. Exp. Therm. Fluid Sci. 2018, 94, 231–245. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R.; Sharma, K.V. Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf. 2018, 116, 1143–1152. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, M.H. The boiling phenomenon of alumina nano-fluid near critical heat flux. Int. J. Heat Mass Transf. 2013, 54, 718–728. [Google Scholar] [CrossRef]
- Liu, J.; Lu, S.; Chen, Q.; Zhang, J.H.; Yao, S.G. Stability of Nanofluids for Heat Pipe. Mater. Sci. Forum 2014, 789, 6–11. [Google Scholar] [CrossRef]
- Ozsoy, A.; Corumlu, V. Thermal performance of a thermosyphon heat pipe evacuated tube solar collector using silver-water nanofluid for commercial applications. Renew. Energy 2018, 122, 26–34. [Google Scholar] [CrossRef]
- Ding, Y.L.; Alias, H.; Wen, D.S.; Williams, R.A. Heat transfer of aqueous suspensions of carbon nanotubes (CNT nanofluids). Int. J. Heat Mass Transf. 2006, 49, 240–250. [Google Scholar] [CrossRef]
- Subramani, J.; Nagarajan, P.K.; Mahian, O.; Sathyamurthy, R. Efficiency and heat transfer improvements in a parabolic trough solar collector using TiO2 nanofluids under turbulent flow regime. Renew. Energy 2017. [Google Scholar] [CrossRef]
- Park, K.J.; Jung, D.; Shim, S.E. Nucleate boiling heat transfer in aqueous solutions with carbon nanotubes up to critical heat fluxes. Int. J. Multiph. Flow 2009, 35, 525–532. [Google Scholar] [CrossRef]
- Jaikumar, A.; Gupta, A.; Kandlikar, S.G.; Yang, C.Y.; Su, C.Y. Scale effects of graphene and graphene oxide coatings on pool boiling enhancement mechanisms. Int. J. Heat Mass Transf. 2017, 109, 357–366. [Google Scholar] [CrossRef]
- Kim, K.M.; Bang, I.C. Effects of graphene oxide nanofluids on heat pipe performance and capillary limits. Int. J. Therm. Sci. 2016, 100, 346–356. [Google Scholar] [CrossRef]
- Mahian, O.; Kianifar, A.; Kalogirou, S.A.; Pop, I.; Wongwises, S. A review of the applications of nanofluids in solar energy. Int. J. Heat Mass Transf. 2013, 57, 582–594. [Google Scholar] [CrossRef]
- Kim, D.E.; Yu, D.I.; Jerng, D.W.; Kim, M.H.; Ahn, H.S. Review of boiling heat transfer enhancement on micro/nanostructured surfaces. Exp. Therm. Fluid Sci. 2015, 66, 173–196. [Google Scholar] [CrossRef]
- Yao, S.G.; Teng, Z.C.; Huang, X.G.; Song, Y.D.; Zhang, S.L. Study on Boiling Heat Transfer of Ethylene Glycol/Deionized Water Based Al2O3 Nanofluids Under Different Pressures. Nanosci. Nanotechnol. Lett. 2019, 11, 222–228. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F.; Peyghambarzadeh, S.M.; Vaeli, N. Upward Flow Boiling to DI-Water and Cuo Nanofluids Inside the Concentric Annuli. J. Appl. Fluid Mech. 2015, 8, 651–659. [Google Scholar]
- Nikkhah, V.; Sarafraz, M.M.; Hormozi, F. Application of Spherical Copper Oxide (II) Water Nano-fluid as a Potential Coolant in a Boiling Annular Heat Exchanger. Chem. Biochem. Eng. Q. 2015, 29, 405–415. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Arya, H.; Saeedi, M.; Ahmadi, D. Flow boiling heat transfer to MgO-therminol 66 heat transfer fluid: Experimental assessment and correlation development. Appl. Therm. Eng. 2018, 138, 552–562. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Peyghambarzadeh, S.M. Nucleate Pool BoilingHeat Transfer to Al2O3-Water and TiO2-Water Nanofluids on Horizontal Smooth Tubes with DissimilarHomogeneous Materials. Chem. Biochem. Eng. Q. 2012, 26, 199–206. [Google Scholar]
- Salari, E.; Peyghambarzadeh, S.M.; Sarafraz, M.M.; Hormozi, F.; Nikkhah, V. Thermal behavior of aqueous iron oxide nano-fluid as a coolant on a flat disc heater under the pool boiling condition. Heat Mass Transf. 2017, 53, 265–275. [Google Scholar] [CrossRef]
- Ciloglu, D.; Bolukbasi, A. A comprehensive review on pool boiling of nanofluids. Appl. Therm. Eng. 2015, 84, 45–63. [Google Scholar] [CrossRef]
- Liang, G.T.; Mudawar, I. Review of pool boiling enhancement with additives and nanofluid. Int. J. Heat Mass Transf. 2018, 124, 423–453. [Google Scholar] [CrossRef]
- Kundan, A.; Plawsky, J.L.; Wayner, P.C., Jr.; Chao, D.F.; Sicker, R.J.; Motil, B.J.; Lorik, T.; Chestney, L.; Eustace, J.; Zoldak, J. Thermocapillary Phenomena and Performance Limitations of a Wickless Heat Pipe in Microgravity. Phys. Rev. Lett. 2015, 114, 146105. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.; Alekseik, Y.; Alekseik, O.; Khairnasov, S.; Baturkin, V.; Ho, T.; Celotti, L. Heat pipes with variable thermal conductance property developed for space applications. In Proceedings of the Joint 18th IHPC and 12th IHPS, Jeju, Korea, 12–16 June 2016. [Google Scholar]
- Guptaa, N.K.; Tiwaria, A.K.; Ghosh, S.K. Heat transfer mechanisms in heat pipes using—A review. Exp. Therm. Fluid Sci. 2018, 90, 84–100. [Google Scholar] [CrossRef]
- Mashaei, P.R.; Shahryari, M.H.; Fazeli, S.M. Hosseinalipour, Numerical simulation of nanofluid application in a horizontal mesh heat pipe with multiple heat sources: A smart fluid for high efficiency thermal system. Appl. Therm. Eng. 2016, 100, 1016–1030. [Google Scholar] [CrossRef]
- Sözen, A.; Menlik, T.; Gürü, M.; Boran, K.; Kılıç, F. A comparative investigation on the effect of fly-ash and alumina nanofluids on the thermal performance of two-phase closed thermo-syphon heat pipes. Appl. Therm. Eng. 2016, 96, 330–337. [Google Scholar] [CrossRef]
- Kumaresan, G.; Venkatachalapathy, S.; Godson, L.; Wongwises, S. Comparative study on heat transfer characteristics of sintered and mesh wick heat pipes using CuO nanofluids. Int. Commun. Heat Mass Transf. 2014, 57, 208–215. [Google Scholar] [CrossRef]
- Venkatachalapathy, S.; Kumaresan, G.; Suresh, S. Performance analysis of cylindrical heat pipe using nanofluids – An experimental study. Int. J. Multiph. Flow 2015, 72, 188–197. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, P.; Liu, Z. Application of water-based SiO2 functionalized nanofluid in a loop thermosyphon. Int. J. Heat Mass Transf. 2013, 56, 59–68. [Google Scholar] [CrossRef]
- Gunnasegaran, P.; Zulkifly, M.; Zamri, M. Optimization of SiO2 nanoparticle mass concentration and heat input on a loop heat pipe, Case Stud. Therm. Eng. 2015, 6, 238–250. [Google Scholar]
- Goshayeshi, H.R.; Izadi, F.; Bashirnezhad, K. Comparison of heat transfer performance on closed pulsating heat pipe for Fe3O4 and Fe2O3 for achieving an empirical correlation. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 43–49. [Google Scholar] [CrossRef]
- Goshayeshi, H.R.; Safaei, M.R.; Goodarzi, M.; Dahari, M. Particle size and type effects on heat transfer enhancement of Ferro-nanofluids in a pulsating heat pipe. Powder Technol. 2016, 301, 1218–1226. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Saidur, R.; Mehrali, M.; Latibari, S.T.; Akhiani, A.R.; Metselaar, H.S.C. A comprehensive review on grapheme nanofluids: Recent research, development and applications. Energy Convers. Manage. 2016, 111, 466–487. [Google Scholar] [CrossRef]
- Tharayil, T.; Asirvatham, L.G.; Ravindran, V.; Wongwises, S. Thermal performance of miniature loop heat pipe with graphene–water nanofluid. Int. J. Heat Mass Transf. 2016, 93, 957–968. [Google Scholar] [CrossRef]
- Buffone, C.; Coulloux, J.; Alonso, B.; Schlechtendahl, M.; Palermo, V.; Zurutuza, A.; Albertin, T.; Martin, S.; Molina, M.; Chikov, S.; et al. Capillary pressure in graphene oxide nanoporous membranes for enhanced heat transport in Loop Heat Pipes for aeronautics. Exp. Therm. Fluid Sci. 2016, 78, 147–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, X.Y.; Weng, J.H.; Shi, S.Y.; Han, H.; Chen, C.M. Experimental investigation of the heat transfer performance of an oscillating heat pipe with graphene nanofluids. Powder Technol. 2018, 332, 371–380. [Google Scholar] [CrossRef]
- Li, S.F.; Bao, Y.Y.; Wang, P.Y.; Liu, Z.H. Effect of nano-structure coating on thermal performance of thermosyphon boiling in micro-channels. Int. J. Heat Mass Transf. 2018, 124, 463–474. [Google Scholar] [CrossRef]
- Nazaria, M.A.; Ghasempoura, R.; Ahmadib, M.H.; Heydarian, G.; Shafiic, M.B. Experimental investigation of graphene oxide nanofluid on heat transfer enhancement of pulsating heat pipe. Int. Commun. Heat Mass Transf. 2018, 91, 90–94. [Google Scholar] [CrossRef]
- Hosseinian, A.; Isfahani, A.H.M.; Shirania, E. Experimental investigation of surface vibration effects on increasing the stability and heat transfer coeffcient of MWCNTs-water nanofluid in a flexible double pipe heat exchanger. Exp. Therm. Fluid Sci. 2018, 90, 275–285. [Google Scholar] [CrossRef]
- Subhedar, D.G.; Ramani, B.M.; Gupta, A. Experimental investigation of heat transfer potential of Al2O3/Water-Mono Ethylene Glycol nanofluids as a car radiator coolant. Case Stud. Therm. Eng. 2018, 11, 26–34. [Google Scholar] [CrossRef]
- Contreras, E.M.C.; Oliveira, G.A.; Filho, E.P.B. Experimental analysis of the thermohydraulic performance of graphene and silver nanofluids in automotive cooling systems. Int. J. Heat Mass Transf. 2019, 132, 375–387. [Google Scholar] [CrossRef]
- Tijani, A.S.; Sudirman, A.S.B. Thermos-physical properties and heat transfer characteristics of water/ anti-freezing and Al2O3/CuO based nanofluid as a coolant for car radiator. Int. J. Heat Mass Transf. 2018, 118, 48–57. [Google Scholar] [CrossRef]
- Chaurasia, P.; Kumar, A.; Yadav, A.; Rai, P.K.; Kumar, V.; Prasad, L. Heat transfer augmentation in automobile radiator using Al2O3– water based nanofluid. SN Appl. Sci. 2019, 257. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ozkaymak, M.; Sözen, A.; Menlik, T.; Fahed, A. Improving car radiator performance by using TiO2-water nanofluid. Eng. Sci. Technol. Int. J. 2018, 21, 996–1005. [Google Scholar] [CrossRef]
- Farhana, K.; Kadirgama, K.; Rahman, M.M.; Ramasamy, D.; Noor, M.M.; Najafi, G.; Samykano, M.; Mahamude, A.S.F. Improvement in the performance of solar collectors with nanofluids—A state-of-the-art review. Nano-Struct. Nano-Objects 2019, 18, 100276. [Google Scholar] [CrossRef]
- Bellos, E.; Said, Z.; Tzivanidis, C. The use of nanofluids in solar concentrating technologies: A comprehensive review. J. Clean. Prod. 2018, 196, 84–99. [Google Scholar] [CrossRef]
- Campos, C.; Vasco, D.; Angulo, C.; Burdiles, P.A.; Cardemil, J.; Palza, H. About the relevance of particle shape and graphene oxide on the behavior of direct absorption solar collectors using metal based nanofluids under different radiation intensities. Energy Convers. Manag. 2019, 181, 247–257. [Google Scholar] [CrossRef]
- Mahbubul, I.M.; Khan, M.M.A.; Ibrahim, N.I.; Ali, H.M.; Al-Sulaiman, F.A.; Saidur, R. Carbon nanotube nanofluid in enhancing the efficiency of evacuated tube solar collector. Renew. Energy 2018, 121, 36–44. [Google Scholar] [CrossRef]
- Allouhia, A.; Amine, M.B.; Saidur, R.; Kousksou, T.; Jamil, A. Energy and exergy analyses of a parabolic trough collector operated with nanofluids for medium and high temperature applications. Energy Convers. Manag. 2018, 155, 201–217. [Google Scholar] [CrossRef]
- Verma, S.K.; Tiwari, A.K.; Tiwari, S.; Chauhan, D.S. Performance analysis of hybrid nanofluids in flat plate solar collector as an advanced working fluid. Sol. Energy 2018, 167, 231–241. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B. Prediction of electrical conducivity of TiO2 water and ethylene glycol-based nanofluids for cooling application in low temperature PEM fuel cells. Energy Procedia 2019, 160, 550–557. [Google Scholar] [CrossRef]
- Ahmad, L.; Khan, M. Importance of activation energy in development of chemical covalent bonding in flow of Sisko magneto-nanofluids over a porous moving curved surface. Int. J. Hydrog. Energy 2019, 44, 10197–10206. [Google Scholar] [CrossRef]
- Sui, D.; Langåker, V.H.; Yu, Z.X. Investigation of thermophysical properties of Nanofluids for application in geothermal energy. Energy Procedia 2017, 105, 5055–5060. [Google Scholar] [CrossRef]
- Hayat, T.; Kiyani, M.Z.; Alsaedi, A.; Khan, M.I.; Ahmad, I. Mixed convective three-dimensional flow of Williamson nanofluid subject to chemical reaction. Int. J. Heat Mass Transf. 2018, 127, 422–429. [Google Scholar] [CrossRef]
- Khan, M.I.; Qayyum, S.; Hayat, T.; Khan, M.I.; Alsaedi, A. Entropy optimization in flow of Williamson nanofluid in the presence of chemical reaction and Joule heating. Int. J. Heat Mass Transf. 2019, 133, 959–967. [Google Scholar] [CrossRef]
- Kathiravan, R.; Kumar, R.; Gupta, A.; Chandra, R. Characterization and Pool Boiling Heat Transfer Studies of Nanofluids. J. Heat Transf. 2009, 131, 1–8. [Google Scholar] [CrossRef]
- Yang, Y.M.; Maa, J.R. Pool boiling of dilute surfactant solutions. J. Heat Transf. 1983, 105, 190–192. [Google Scholar] [CrossRef]
- Feng, Z.F.; Luo, X.P.; Zhang, J.X.; Xiao, J.; Yuan, W. Effects of electric field on flow boiling heat transfer in a vertical minichannel heat sink. Int. J. Heat Mass Transf. 2018, 124, 726–741. [Google Scholar] [CrossRef]
- Özdemir, M.R.; Sadaghiani, A.K.; Motezakker, A.R.; Paraparib, S.S.; Park, H.S.; Acar, H.Y.; Koşar, A. Experimental studies on ferrofluid pool boiling in the presence of external magnetic force. Appl. Therm. Eng. 2018, 139, 598–608. [Google Scholar] [CrossRef]
- Abdollahi, A.; Salimpour, M.R.; Etesami, N. Experimental analysis of magnetic field effect on the pool boiling heat transfer of a ferrofluid. Appl. Therm. Eng. 2017, 111, 1101–1110. [Google Scholar] [CrossRef]
- Ulset, E.T.; Kosinski, P.; Zabednova, Y.; Zhdaneev, O.V.; Struchalin, P.G.; Balakin, B.V. Photothermal boiling in aqueous nanofluids. Nano Energy 2018, 50, 339–346. [Google Scholar] [CrossRef]
- Yao, S.G.; Huang, X.G.; Song, Y.D.; Shen, Y.; Zhang, S.L. Effects of nanoparticle types and size on boiling feat transfer performance under different pressures. AIP Adv. 2018, 8, 025005. [Google Scholar] [CrossRef]
- Bang, I.C.; Chang, S.H. Boiling heat transfer performance and phenomena of Al2O3–water nano-fluids from a plain surface in a pool. Int. J. Heat Mass Transf. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F. Comparatively experimental study on the boiling thermal performance of metal oxide and multi-walled carbon nanotube nanofluids. Powder Technol. 2016, 287, 412–430. [Google Scholar] [CrossRef]
- Manetti, L.L.; Stephen, M.T.; Beck, P.A.; Cardoso, E.M. Evaluation of the heat transfer enhancement during pool boiling using low concentrations of Al2O3-water based nanofluid. Exp. Therm. Fluid Sci. 2017, 87, 191–200. [Google Scholar] [CrossRef]
- Pan, B.G.; Wang, W.H.; Chu, D.L.; Mei, L.Q.; Zhang, Q.H. Experimental investigation of hypervapotron heat transfer enhancement with alumina–water nanofluids. Int. J. Heat Mass Transf. 2016, 98, 738–745. [Google Scholar] [CrossRef]
- Ham, J.; Kim, H.; Shin, Y.; Cho, H. Experimental investigation of pool boiling characteristics in Al2O3 nanofluid according to surface roughness and concentration. Int. J. Therm. Sci. 2017, 114, 86–97. [Google Scholar] [CrossRef]
- Ahmed, O.; Hamed, M.S. Experimental investigation of the effect of particle deposition on pool boiling of nanofluids. Int. J. Heat Mass Transf. 2012, 55, 3423–3436. [Google Scholar] [CrossRef]
- Neto, A.R.; Oliveira, J.L.G.; Passos, J.C. Heat transfer coefficient and critical heat flux during nucleate pool boiling of water in the presence of nanoparticles of alumina, maghemite and CNTs. Appl. Therm. Eng. 2017, 111, 1493–1506. [Google Scholar] [CrossRef]
- Vafaei, S. Nanofluid pool boiling heat transfer phenomenon. Powder Technol. 2015, 277, 181–192. [Google Scholar] [CrossRef]
- Shahmoradi, Z.; Etesami, N.; Esfahany, M.N. Pool boiling characteristics of nanofluid on flat plate based on heater surface analysis. Int. Commun. Heat Mass Transf. 2013, 47, 113–120. [Google Scholar] [CrossRef]
- Sulaiman, M.Z.; Matsuo, D.; Enoki, K.; Okawa, T. Systematic measurements of heat transfer characteristics in saturated pool boiling of water-based nanofluids. Int. J. Heat Mass Transf. 2016, 102, 264–276. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Bahrami, M.; Jamialahmadi, M. The boiling performance of ZnO, a-Al2O3 and MWCNTs/water nanofluids: An experimental study. Exp. Therm. Fluid Sci. 2017, 80, 27–39. [Google Scholar] [CrossRef]
- Minakov, A.V.; Pryazhnikov, M.I.; Guzei, D.V.; Zeer, G.M.; Rudyak, V.Y. The experimental study of nanofluids boiling crisis on cylindrical heaters. Int. J. Therm. Sci. 2017, 116, 214–223. [Google Scholar] [CrossRef]
- Ham, J.; Cho, H. Theoretical analysis of pool boiling characteristics of Al2O3 nanofluid according to volume concentration and nanoparticle size. Appl. Therm. Eng. 2016, 108, 158–171. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F. Nucleate pool boiling heat transfer characteristics of dilute Al2O3–ethyleneglycol nanofluids. Int. Commun. Heat Mass Transf. 2014, 58, 96–104. [Google Scholar] [CrossRef]
- Raveshi, M.R.; Keshavarz, A.; Mojarrad, M.S.; Amiri, S. Experimental investigation of pool boiling heat transfer enhancement of alumina–water–ethylene glycol nanofluids. Exp. Therm. Fluid Sci. 2013, 44, 805–814. [Google Scholar] [CrossRef]
- Diao, Y.H.; Liu, Y.; Wang, R.; Zhao, Y.H.; Guo, L.; Tang, X. Effects of nanofluids and nanocoatings on the thermal performance of an evaporator with rectangular microchannels. Int. J. Heat Mass Transf. 2013, 67, 183–193. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Y.H.; Diao, Y.H. Experimental investigation of the nucleate pool boiling heat transfer characteristics of d-Al2O3-R141b nanofluids on a horizontal plate. Exp. Therm. Fluid Sci. 2014, 52, 88–96. [Google Scholar] [CrossRef]
- Shoghl, S.N.; Bahrami, M. Experimental investigation on pool boiling heat transfer of ZnO, and CuO water-based nanofluids and effect of surfactant on heat transfer coefficient. Int. Commun. Heat Mass Transf. 2013, 45, 122–129. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Hormozi, F. Pool boiling heat transfer to dilute copper oxide aqueous nanofluids. Int. J. Therm. Sci. 2015, 90, 224–237. [Google Scholar] [CrossRef]
- Karimzadehkhouei, M.; Shojaeian, M.; Sendur, K.; Mengüç, M.P.; Kosar, A. The effect of nanoparticle type and nanoparticle mass fraction on heat transfer enhancement in pool boiling. Int. J. Heat Mass Transf. 2017, 109, 157–166. [Google Scholar] [CrossRef]
- Umesh, V.; Raja, B. A study on nucleate boiling heat transfer characteristics of pentane and CuO-pentane nanofluid on smooth and milled surfaces. Exp. Therm. Fluid Sci. 2015, 64, 23–29. [Google Scholar] [CrossRef]
- Heris, S.Z. Experimental investigation of pool boiling characteristics of low-concentrated CuO/ ethylene glycol–water nanofluids. Int. Commun. Heat Mass Transf. 2011, 38, 1470–1473. [Google Scholar] [CrossRef]
- Sheikhbahai, M.; Esfahany, M.N.; Etesami, N. Experimental investigation of pool boiling of Fe3O4/ethylene glycolewater nanofluid in electric field. Int. J. Therm. Sci. 2012, 62, 149–153. [Google Scholar] [CrossRef]
- Hu, Y.W.; Li, H.R.; He, Y.R.; Wang, L.D. Role of nanoparticles on boiling heat transfer performance of ethylene glycol aqueous solution based graphene nanosheets nanofluid. Int. J. Heat Mass Transf. 2016, 96, 565–572. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, L.W.; Yu, Z.T.; Cen, K.F. An experimental investigation of transient pool boiling of aqueous nanofluids with graphene oxide nanosheets as characterized by the quenching method. Int. J. Heat Mass Transf. 2014, 73, 410–414. [Google Scholar] [CrossRef]
- Xing, M.B.; Yu, J.L.; Wang, R.X. Effects of surface modification on the pool boiling heat transfer of MWNTs/water nanofluids. Int. J. Heat Mass Transf. 2016, 103, 914–919. [Google Scholar] [CrossRef]
- Fan, L.W.; Li, J.Q.; Wu, Y.Z.; Zhang, L.; Yu, Z.T. Pool boiling heat transfer during quenching in carbon nanotube (CNT)- based aqueous nanofluids: Effects of length and diameter of the CNTs. Appl. Therm. Eng. 2017, 122, 555–565. [Google Scholar] [CrossRef]
- Amiri, A.; Shanbedi, M.; Amiri, H.; Heris, S.Z.; Kazi, S.N.; Chew, B.T.; Eshghi, H. Pool boiling heat transfer of CNT/water nanofluids. Appl. Therm. Eng. 2014, 71, 450–459. [Google Scholar] [CrossRef]
- Bolukbasi, A.; Ciloglu, D. Pool boiling heat transfer characteristics of vertical cylinder quenched by SiO2-water nanofluids. Int. J. Therm. Sci. 2011, 50, 1013–1021. [Google Scholar] [CrossRef]
- Hu, Y.W.; Li, H.R.; He, Y.R.; Liu, Z.Y.; Zhao, Y.H. Effect of nanoparticle size and concentration on boiling performance of SiO2 nanofluid. Int. J. Heat Mass Transf. 2017, 107, 820–828. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Investigations on the pool boiling heat transfer and critical heat flux of ZnO-ethylene glycol nanofluids. Appl. Therm. Eng. 2012, 37, 112–119. [Google Scholar] [CrossRef]
- He, Y.R.; Li, H.R.; Hu, Y.W.; Wang, X.Z.; Zhu, J.Q. Boiling heat transfer characteristics of ethylene glycol and water mixture based ZnO nanofluids in a cylindrical vessel. Int. J. Heat Mass Transf. 2016, 98, 611–615. [Google Scholar] [CrossRef]
- Pioro, I. Boiling heat transfer characteristics of thin liquid layers in a horizontally flat two-phase thermosyphon. In Proceedings of the Preprints of the 10th International Heat Pipe Conference, Stuttgart, Germany, 21–25 September 1997; p. 1. [Google Scholar]
- Ali, H.M.; Generous, M.M.; Ahmad, F.; Irfan, M. Experimental investigation of nucleate pool boiling heat transfer enhancement of TiO2-water based nanofluids. Appl. Therm. Eng. 2017, 113, 1146–1151. [Google Scholar] [CrossRef]
- Suriyawong, A.; Wongwises, S. Nucleate pool boiling heat transfer characteristics of TiO2–water nanofluids at very low concentrations. Exp. Therm. Fluid Sci. 2010, 34, 992–999. [Google Scholar] [CrossRef]
- Naphon, P.; Thongjing, C. Pool boiling heat transfer characteristics of refrigerant-nanoparticle mixtures. Int. Commun. Heat Mass Transf. 2014, 52, 84–89. [Google Scholar] [CrossRef]
- Chun, S.Y.; Bang, I.C.; Choo, Y.J.; Song, C.H. Heat transfer characteristics of Si and SiC nanofluids during a rapid quenching and nanoparticles deposition effects. Int. J. Heat Mass Transf. 2011, 54, 1217–1223. [Google Scholar] [CrossRef]
- Sadeghinezhad, E.; Mehrali, M.; Rosen, M.A.; Akhiani, A.R.; Latibari, S.T.; Mehrali, M.; Metselaar, H.S.C. Experimental investigation of the effect of graphene nanofluids on heat pipe thermal performance. Appl. Therm. Eng. 2016, 100, 775–787. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.M.; Jerng, D.W.; Kim, E.Y.; Ahn, H.S. Effect of aluminum oxide and reduced graphene oxide mixtures on critical heat flux enhancement. Int. J. Heat Mass Transf. 2018, 116, 858–870. [Google Scholar] [CrossRef]
- Zuber, N. Hydrodynamic Aspects of Boiling Heat Transfer. Ph.D. Thesis, Research Laboratory, Los Angeles and Ramo-Wooldridge Corporation, University of California, Los Angeles, CA, USA, 1959. [Google Scholar]
- Kandlikar, S.G. A theoretical model to predict pool boiling CHF incorporating effects of contact angle and orientation. J. Heat Transf. 2001, 123, 1071–1079. [Google Scholar] [CrossRef]
- Park, S.D.; Lee, S.W.; Kang, S.; Bang, I.C.; Kim, J.H.; Shin, H.S.; Lee, D.W.; Lee, D.W. Effects of nanofluids containing graphene/graphene-oxide nanosheets on critical heat flux. Appl. Phys. Lett. 2010, 97, 023103. [Google Scholar] [CrossRef]
- Liter, S.G.; Kaviany, M. Pool-boiling CHF enhancement by modulated porous-layer coating: Theory and experiment. Int. J. Heat Mass Transf. 2001, 44, 4287–4311. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmia, W.H.; Hamida, K.A.; Mamata, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO2-SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Chiam, H.W.; Azmi, W.H.; Usri, N.A.; Mamat, R.; Adam, N.M. Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci. 2017, 81, 420–429. [Google Scholar] [CrossRef]
- Kole, M.; Dey, T.K. Investigation of thermal conductivity, viscosity, and electrical conductivity of graphene based nanofluids. J. Appl. Phys. 2013, 113, 084307. [Google Scholar] [CrossRef]
- Li, X.K.; Zou, C.J. Thermo-physical properties of water and ethylene glycol mixture based SiC nanofluids: An experimental investigation. Int. J. Heat Mass Transf. 2016, 101, 412–417. [Google Scholar] [CrossRef]
- Seo, H.; Chu, J.H.; Kwon, S.Y.; Bang, I.C. Pool boiling CHF of reduced graphene oxide, graphene, and SiC-coated surfaces under highly wettable FC-72. Int. J. Heat Mass Transf. 2015, 82, 490–502. [Google Scholar] [CrossRef]
- Rahmati, A.R.; Reiszadeh, M. An experimental study on the effects of the use of multi-walled carbon nanotubes in ethylene glycol/water-based fluid with indirect heaters in gas pressure reducing stations. Appl. Therm. Eng. 2018, 134, 107–117. [Google Scholar] [CrossRef]
- Xu, J.H.; Bandyopadhyay, K.; Jung, D. Experimental investigation on the correlation between nano-fluid characteristics and thermal properties of Al2O3 nano-particles dispersed in ethylene glycol–water mixture. Int. J. Heat Mass Transf. 2016, 94, 262–268. [Google Scholar] [CrossRef]
- Guo, Y.F.; Zhang, T.T.; Zhang, D.R.; Wang, Q. Experimental investigation of thermal and electrical conductivity of silicon oxide nanofluids in ethylene glycol/water mixture. Int. J. Heat Mass Transf. 2018, 117, 280–286. [Google Scholar] [CrossRef]
- Ahmadi, M.A.; Ahmadi, M.H.; Alavi, M.F.; Nazemzadegan, M.R.; Ghasempour, R.; Shamshirband, S. Determination of thermal conductivity ratio of CuO/ethylene glycol nanofluid by connectionist approach. J. Taiwan Inst. Chem. Eng. 2018, 91, 383–395. [Google Scholar] [CrossRef]
- Sandhya, D.; Reddy, M.C.S.; Rao, V.V. Improving the cooling performance of automobile radiatorwith ethylene glycol water based TiO2 nanofluids. Int. Commun. Heat Mass Transf. 2016, 78, 121–126. [Google Scholar]
- Nikulin, A.; Khliyeva, O.; Lukianov, N.; Zhelezny, V.; Semenyuk, Y. Study of pool boiling process for the refrigerant R11, isopropanol and isopropanol/Al2O3 nanofluid. Int. J. Heat Mass Transf. 2018, 118, 746–757. [Google Scholar] [CrossRef]
- Trisaksri, V.; Wongwises, S. Nucleate pool boiling heat transfer of TiO2–R141b nanofluids. Int. J. Heat Mass Transf. 2009, 52, 1582–1588. [Google Scholar] [CrossRef]
- Watanabe, Y.; Enoki, K.; Okawa, T. Nanoparticle layer detachment and its influence on the heat transfer characteristics in saturated pool boiling of nanofluids. Int. J. Heat Mass Transf. 2018, 125, 171–178. [Google Scholar] [CrossRef]
- Xu, Z.G.; Zhao, C.Y. Influences of nanoparticles on pool boiling heat transfer in porous metals. Appl. Therm. Eng. 2014, 65, 34–41. [Google Scholar] [CrossRef]
- Xu, Z.G.; Zhao, C.Y. Enhanced boiling heat transfer by gradient porous metals in saturated pure water and surfactant solutions. Appl. Therm. Eng. 2016, 100, 68–77. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Coulombe, S.; Kietzig, A.M. Boiling heat transfer enhancement with stable nanofluids and laser textured copper surfaces. Int. J. Heat Mass Transf. 2018, 126, 287–296. [Google Scholar] [CrossRef]
- Dadjoo, M.; Etesami, N.; Esfahany, M.N. Influence of orientation and roughness of heater surface on critical heat flux and pool boiling heat transfer coefficient of nanofluid. Appl. Therm. Eng. 2017, 124, 353–361. [Google Scholar] [CrossRef]
- Sun, Y.L.; Chen, G.; Zhang, S.W.; Tang, Y.; Zeng, J.; Yuan, W. Pool boiling performance and bubble dynamics on microgrooved surfaces with reentrant cavities. Appl. Therm. Eng. 2017, 125, 432–442. [Google Scholar] [CrossRef]
- Gheitaghy, A.M.; Samimi, A.; Saffari, H. Surface structuring with inclined minichannels for pool boiling improvement. Appl. Therm. Eng. 2017, 126, 892–902. [Google Scholar] [CrossRef]
- Xu, Z.G.; Qu, Z.G.; Zhao, C.Y.; Tao, W.Q. Experimental correlation for pool boiling heat transfer on metallic foam surface and bubble cluster growth behavior on grooved array foam surface. Int. J. Heat Mass Transf. 2014, 77, 1169–1182. [Google Scholar] [CrossRef]
- Oyarzua, E.; Waltherbc, J.H.; Zambrano, H.A. Water thermophoresis in carbon nanotubes: The interplay between thermophoretic and friction forces. Phys. Chem. Chem. Phys. 2018, 20, 3672–3677. [Google Scholar] [CrossRef]
- Raza, M.Q.; Kumar, N.; Raj, R. Wettability-independent critical heat flux during boiling crisis in foaming solutions. Int. J. Heat Mass Transf. 2018, 126, 567–579. [Google Scholar] [CrossRef]
- Kumar, N.; Raza, M.Q.; Seth, D.; Raj, R. Aqueous ionic liquid solutions for boiling heat transfer enhancement in the absence of buoyancy induced bubble departure. Int. J. Heat Mass Transf. 2018, 122, 354–363. [Google Scholar] [CrossRef]
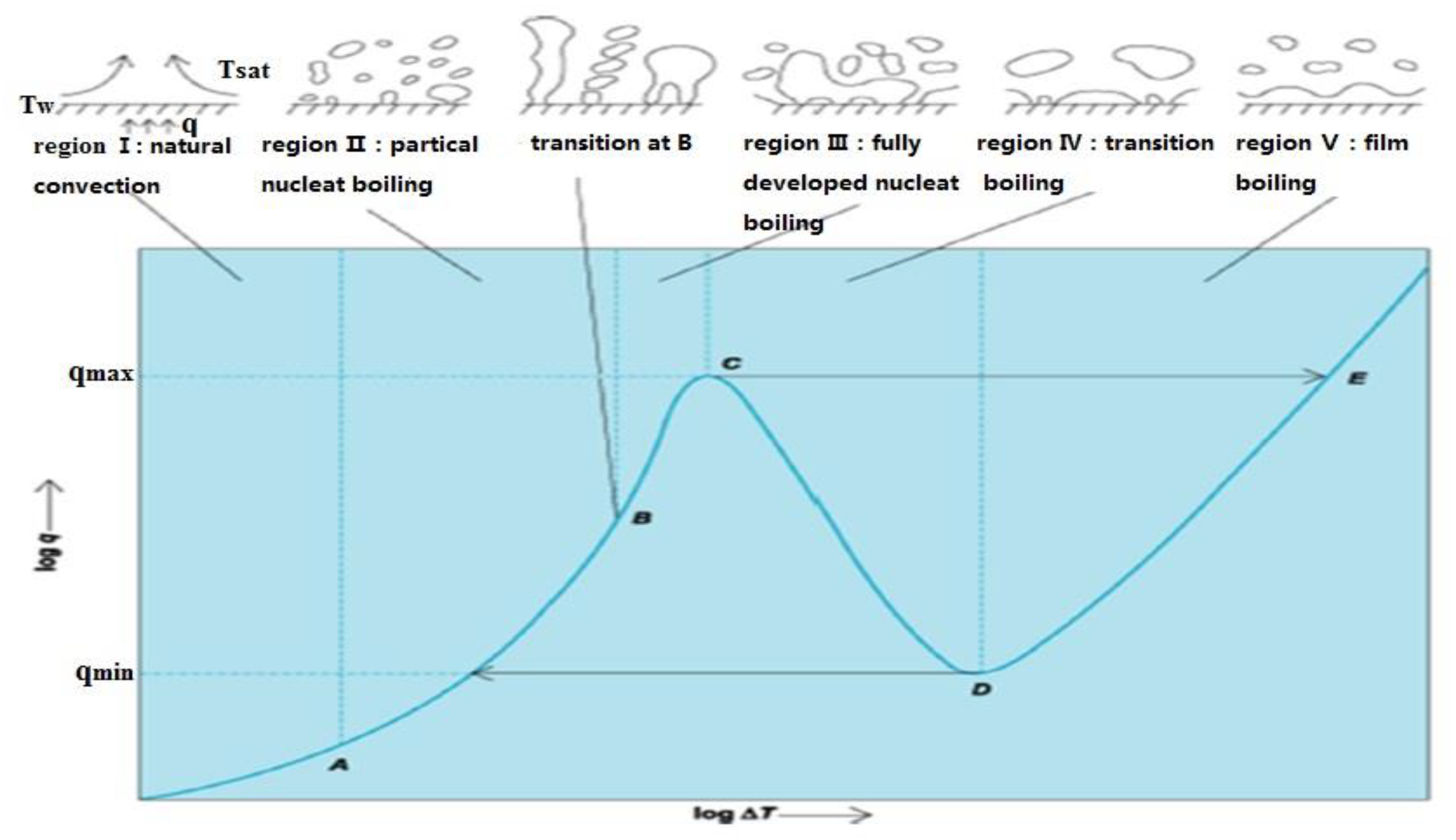
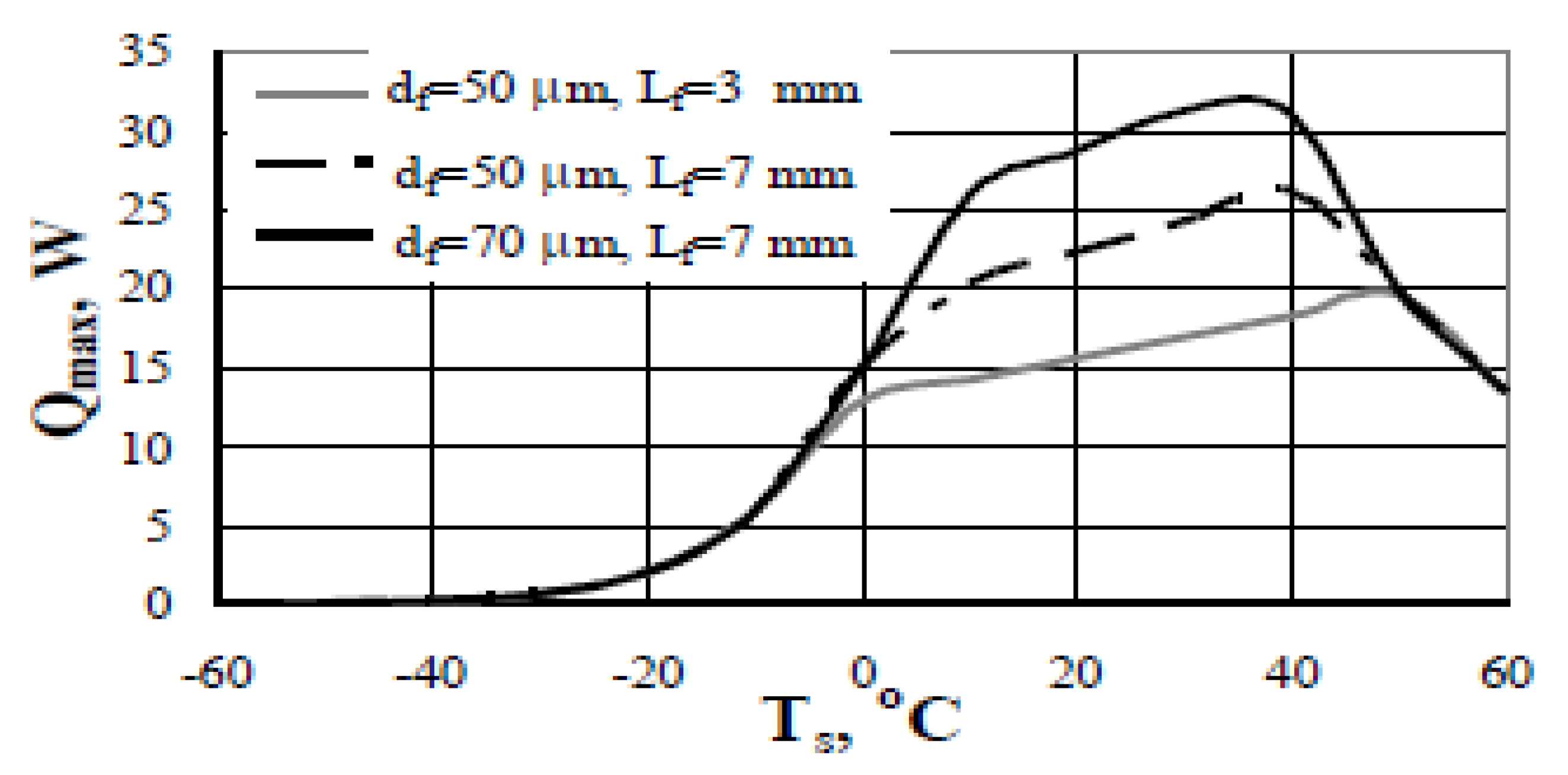
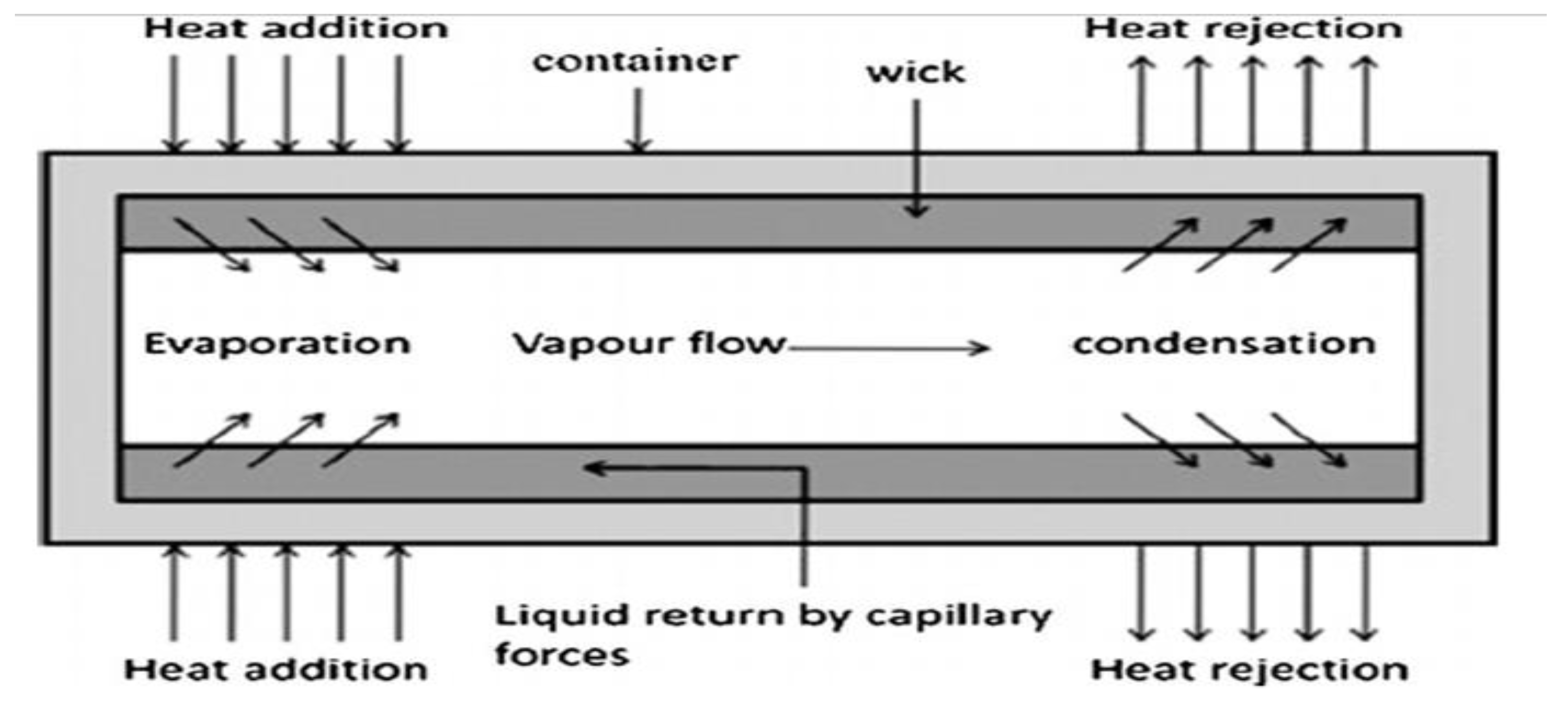

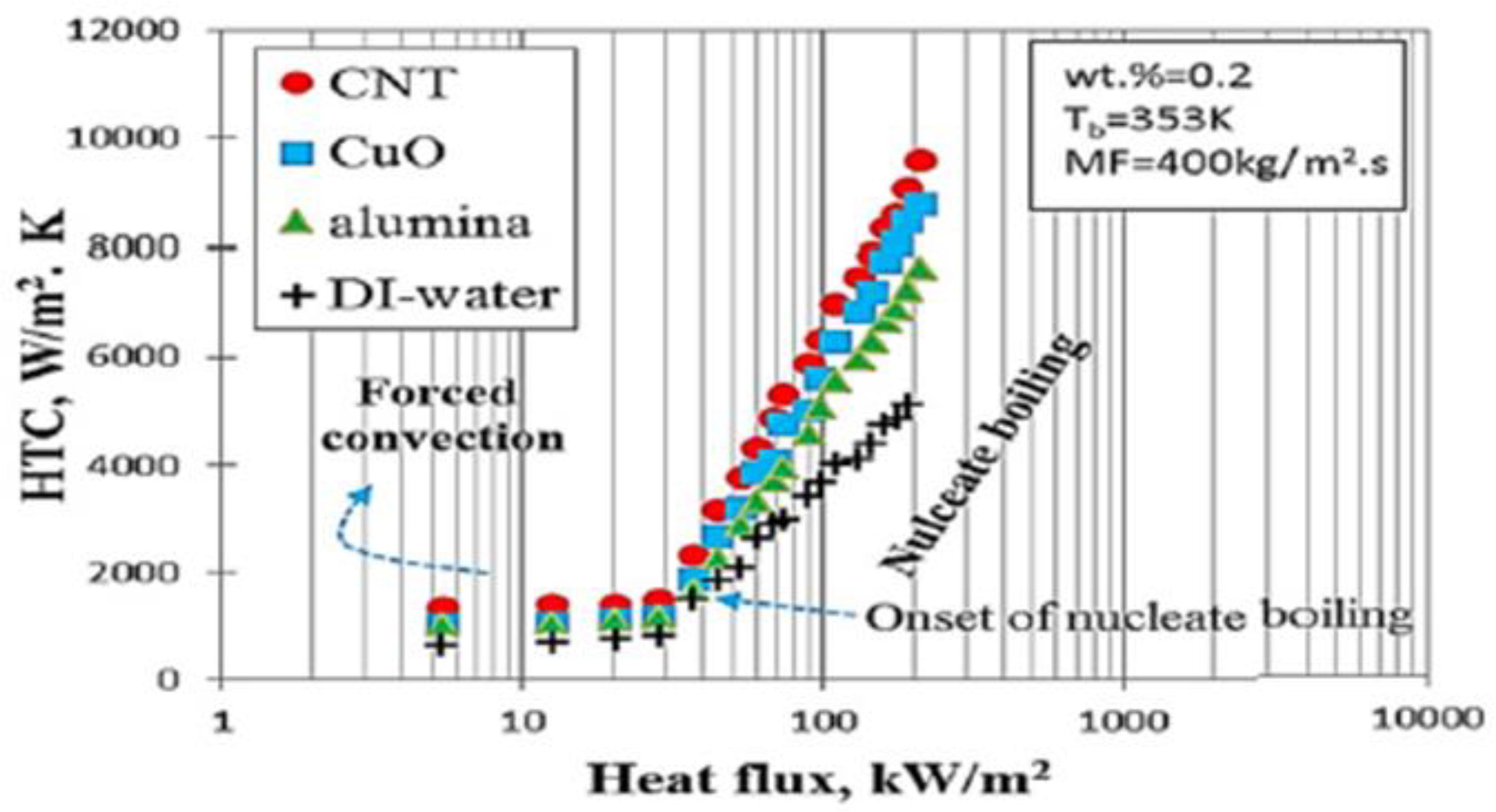

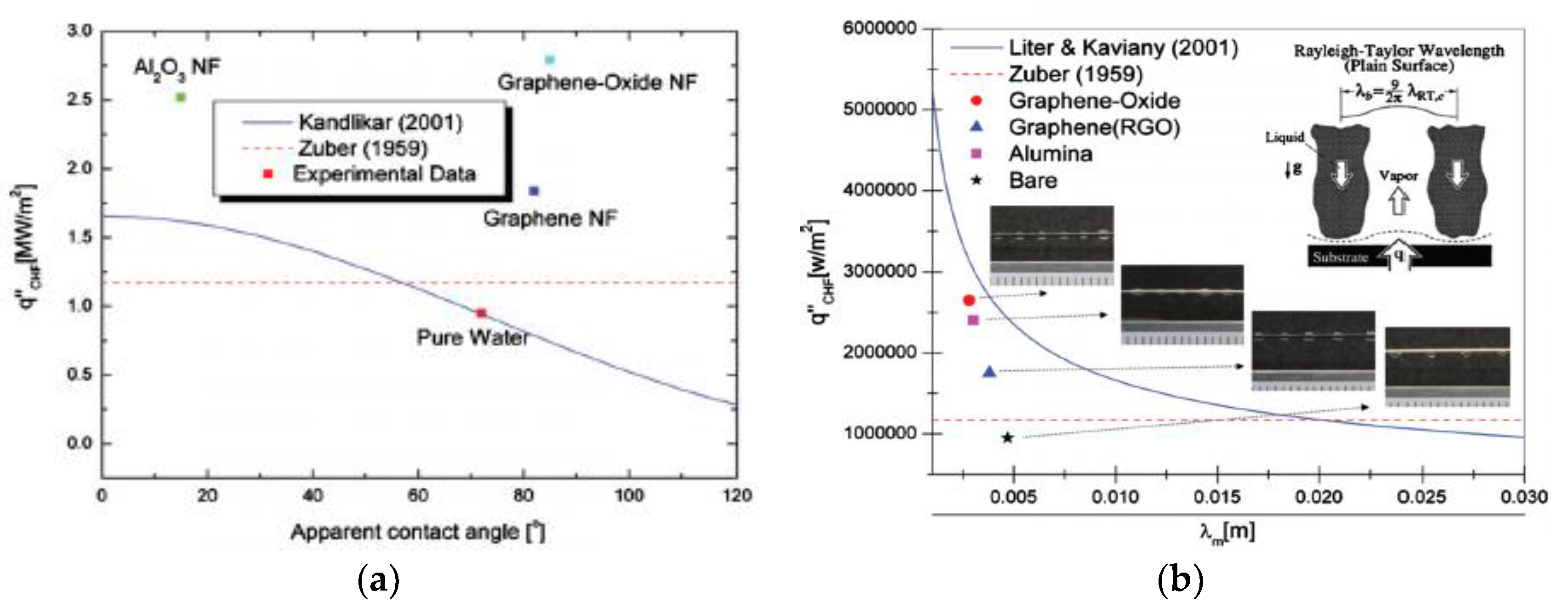
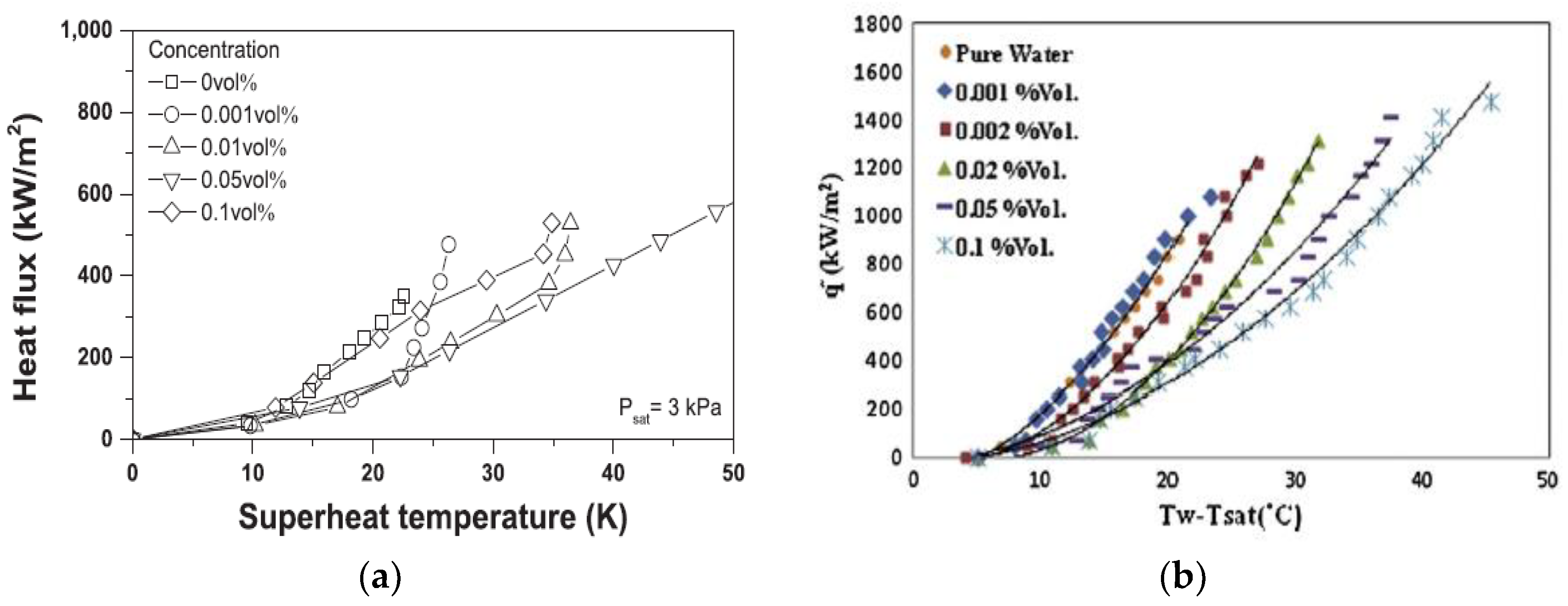
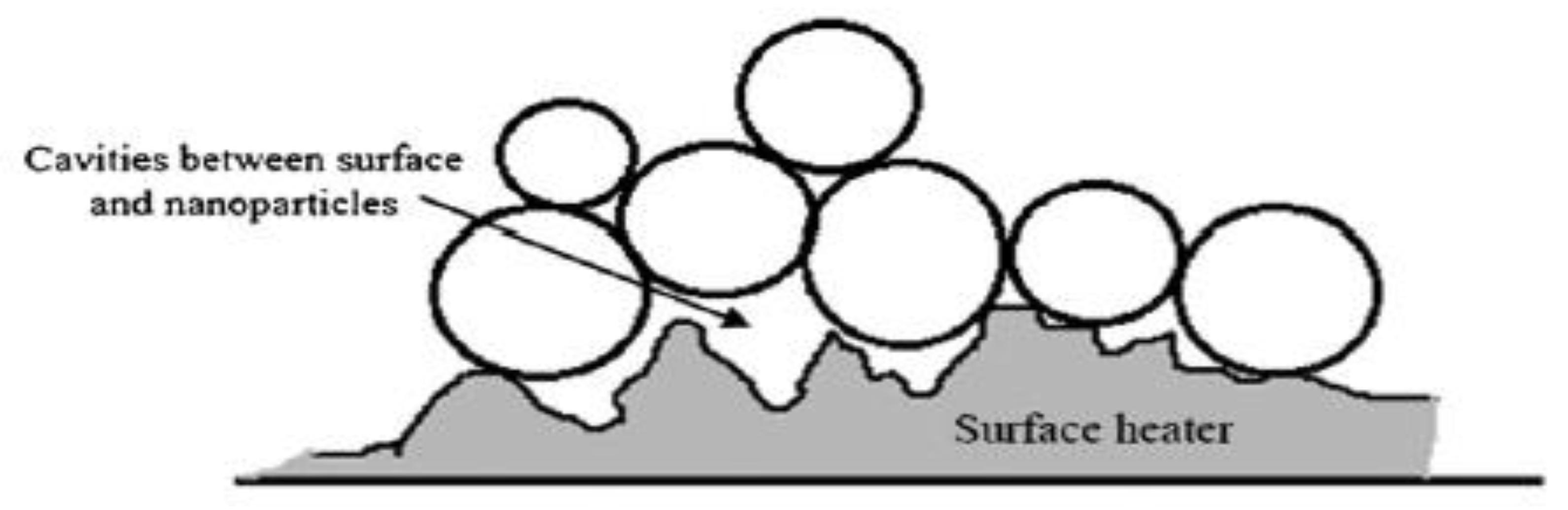
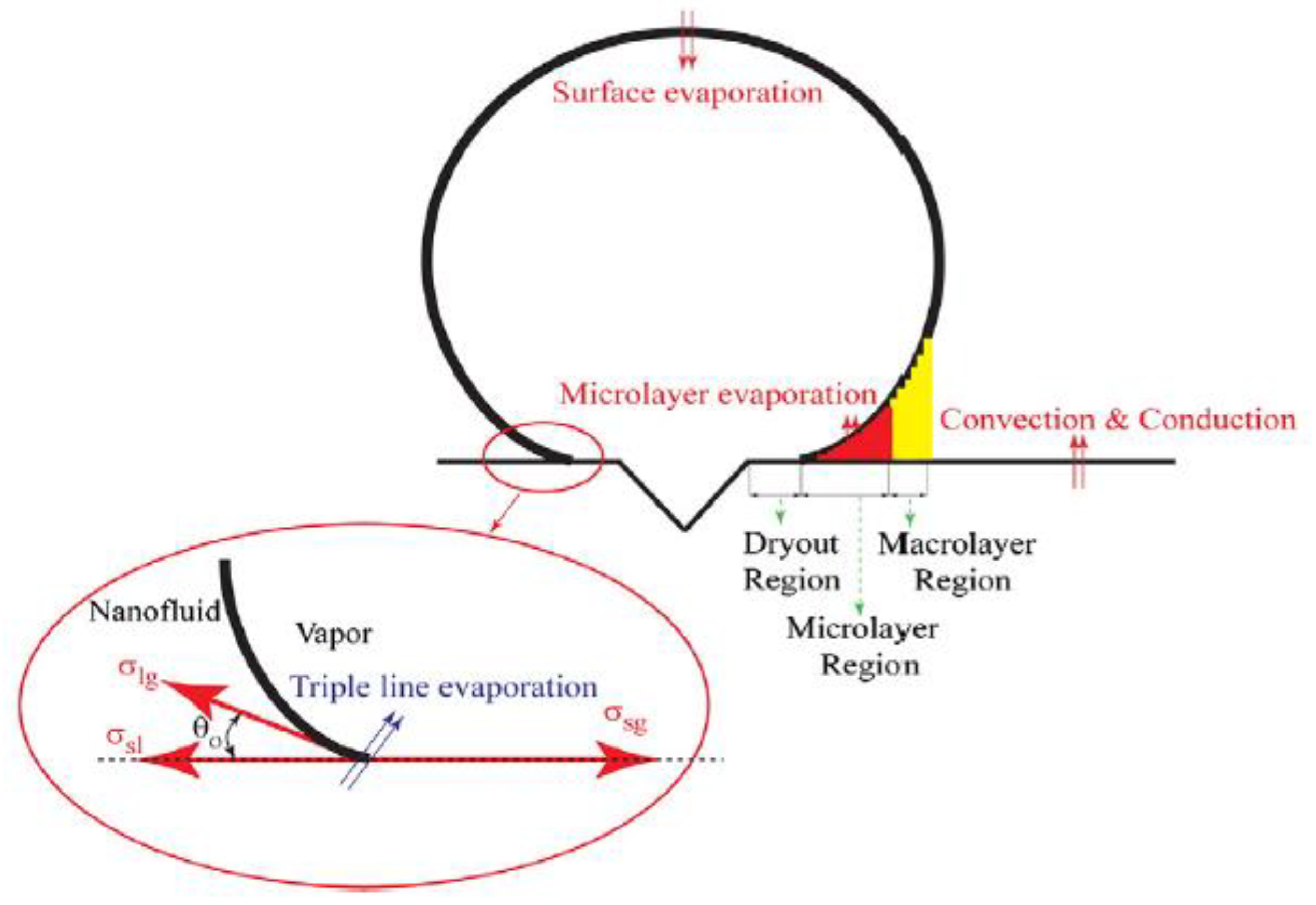
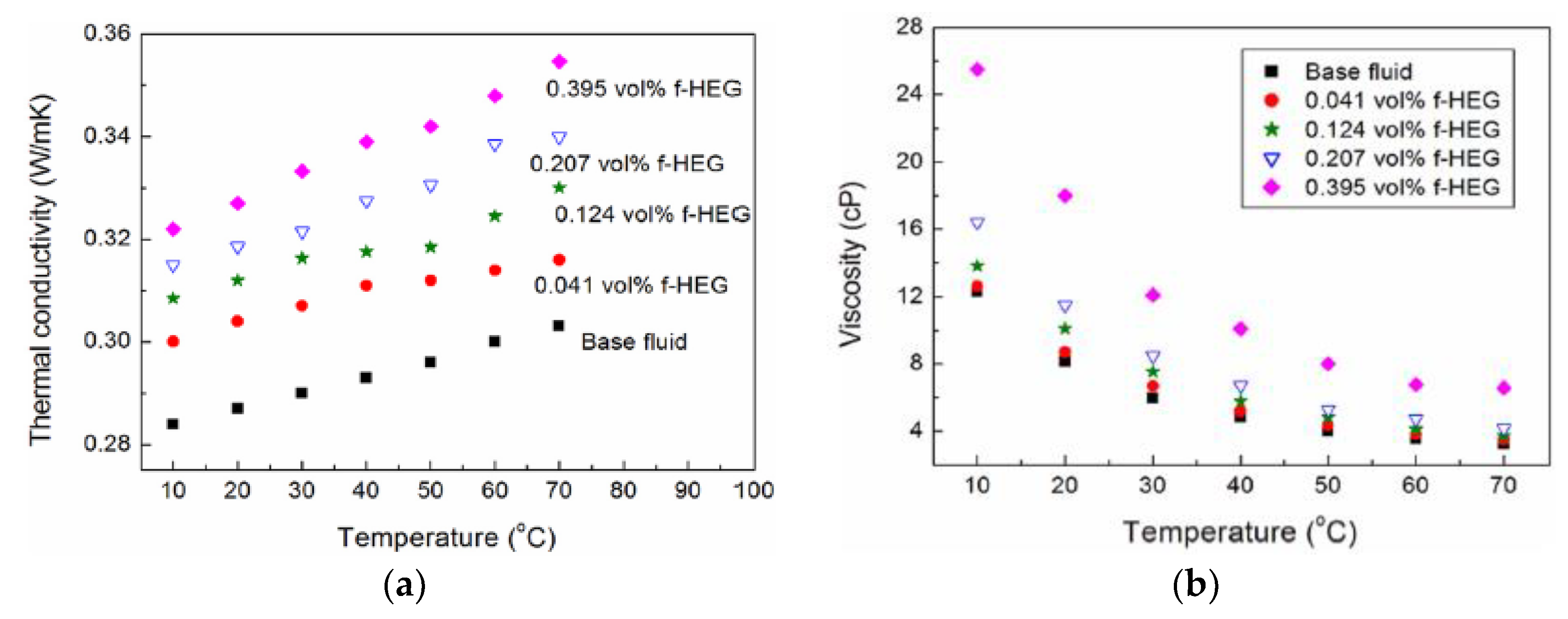
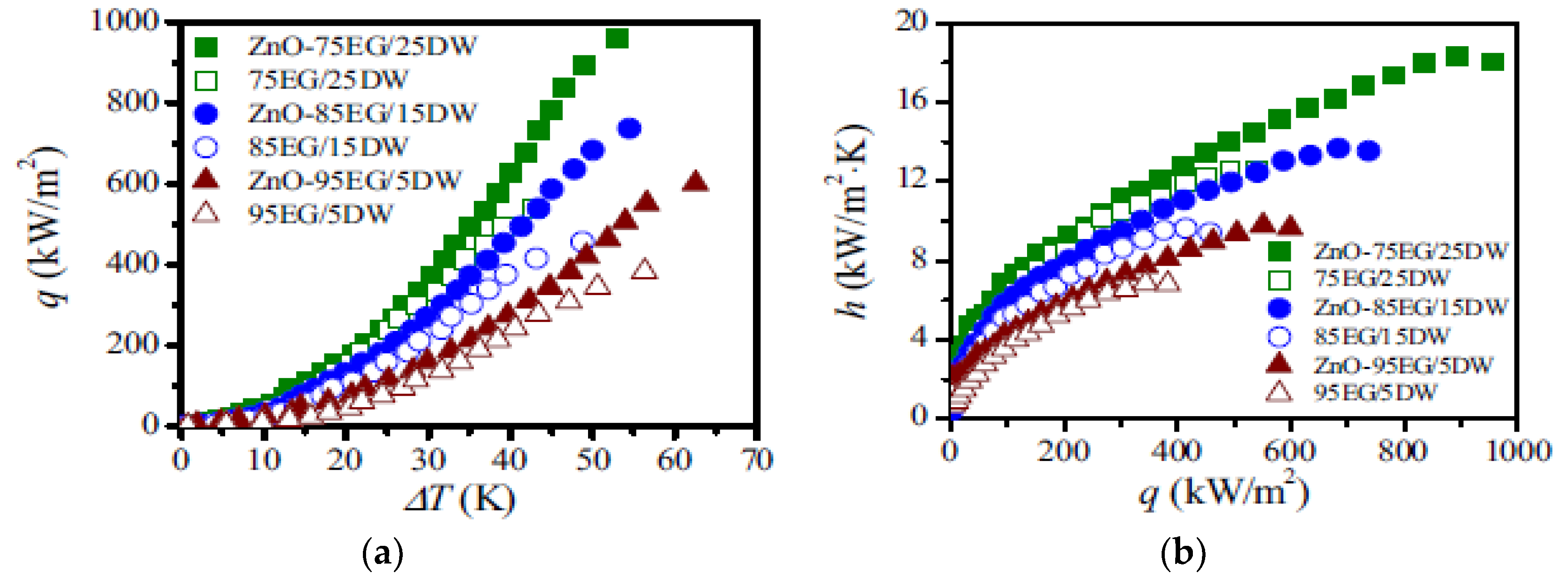

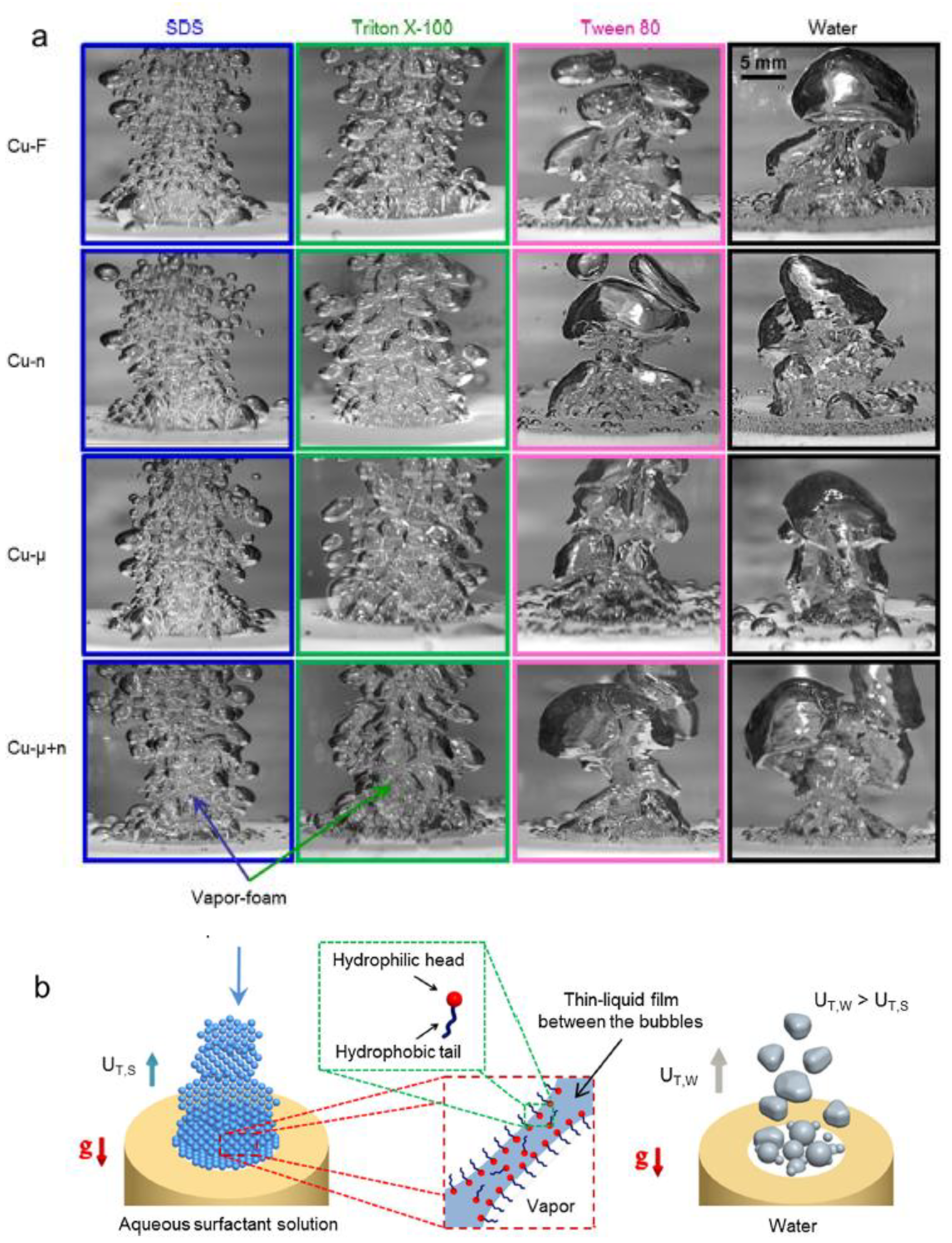
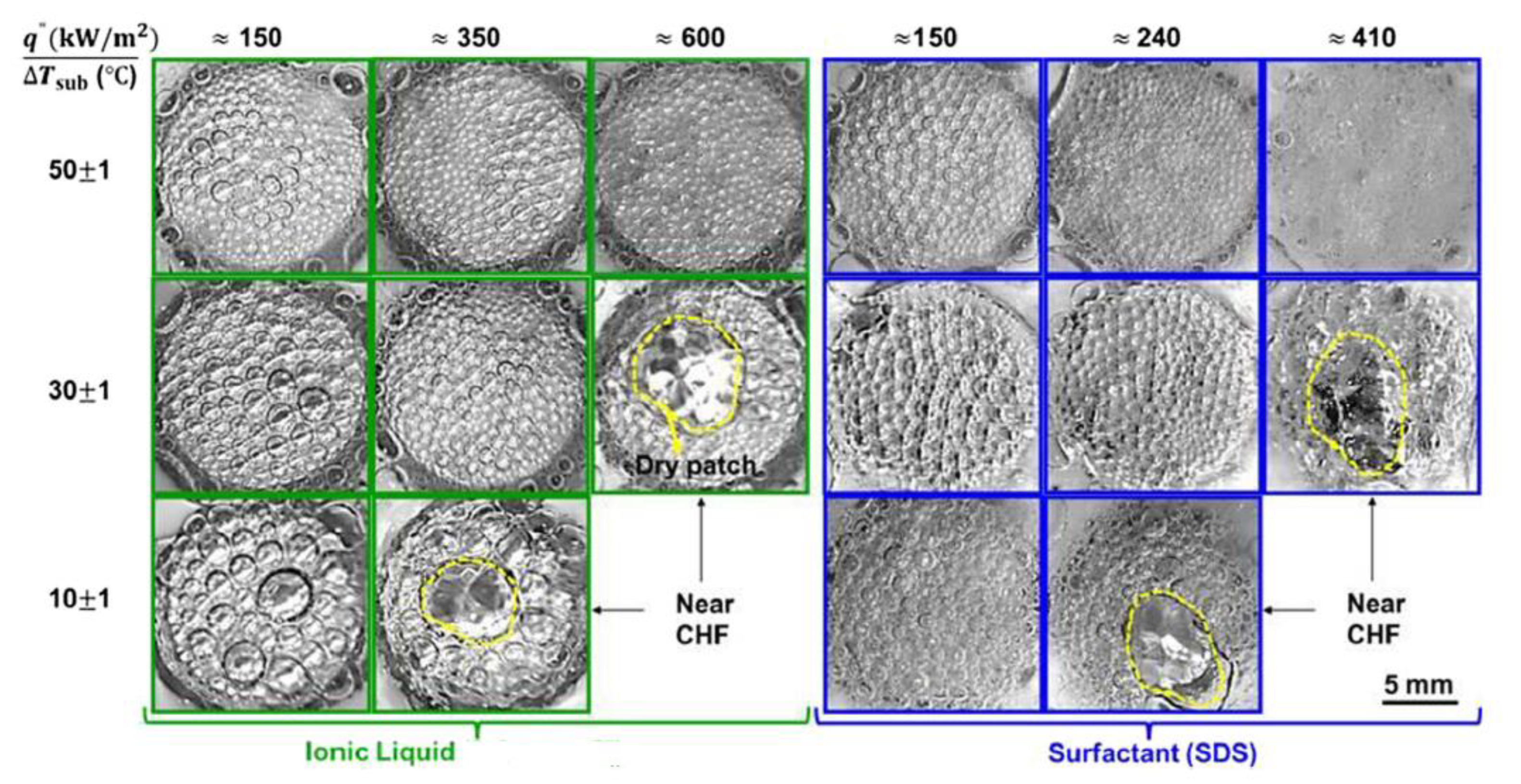
| Nanoparticles | Base Liquid | Concentration | Heated Surface | CHF/HTC | Reasons | |
|---|---|---|---|---|---|---|
| Al2O3 | DW | 0.5, 1, 2, 4 wt.% | copper surface | CHF enhancement HTC deterioration | CHF enhancement is due to surface characteristics of nanoparticle deposition | [70] |
| 0.1, 0.2, 0.3 wt.% | stainless steel rod | CHF enhancement HTC enhancement | Nanoparticle deposition causes changes in surface roughness and vaporized core points. | [71] | ||
| 0.0007, 0.007 Vol.% | copper block | HTC enhanced 15–75% (0.0007%) | HTC is affected by surface roughness. | [72] | ||
| 0.005, 0.05, 0.01, 0.1 wt.% | steel plate | CHF enhancement | The nanoparticles accelerate the liquid perturbation, increasing the frequency of bubble detachment. | [73] | ||
| 0.001, 0.01, 0.05, 0.1 wt.% | copper surface | CHF enhancement HTC deterioration | It subject to surface roughness and concentration. | [74] | ||
| 0.01, 0.1, 0.5 vol.% | copper surface | HTC enhancement (0.01%) | The effect of the thermal conductivity of the nanofluid is more pronounced than the effect of nanoparticle deposition on the surface. | [75] | ||
| 0.02, 0.1 vol.% | copper surface | CHF enhanced 26–37% HTC deterioration | CHF enhancement is due to the complete wetting behavior exhibited by the nanoparticles. HTC deterioration is due to the increase in wettability and thickness of the porous layer structure. | [76] | ||
| 0.001, 0.01, 0.1 Vol.% | copper surface | HTC depends not only on cavity size and surface wettability, but also on the heat flux range. | [77] | |||
| 0.001, 0.002, 0.02, 0.05, 0.1 Vol.% | flat plate heater | CHF enhancement HTC deterioration | The deposition of nanofluids on the surface causes changes in wettability and thermal resistance. | [78] | ||
| 0.04, 0.4, 1 kg/m3 | copper surface | CHF enhanced 2.5–3 times HTC enhancement | Wall superheat is reduced quickly. | [79] | ||
| 0.01, 0.05 wt.% | horizontal rod heater | HTC deterioration | Nanoparticle deposition causes a reduction in surface roughness. | [80] | ||
| 0.05–1 vol.% | cylindrical heater | CHF enhancement | Nanoparticle deposition causes an increase in surface roughness. | [81] | ||
| 0.025, 0.05, 0.075, 0.1 vol.% | Theoretical analysis | CHF enhancement | The bubble detachment frequency is lowered, the detachment diameter is increased, and the wettability is enhanced. | [82] | ||
| EG | 0.1, 0.2, 0.3 wt.% | stainless steel cylinder | HTC deterioration | The surface average roughness is reduced and the number of bubble nucleation is reduced. | [83] | |
| EG/DW | 0.05, 0.1, 0.25, 0.5, 0.75, 1 vol.% | copper cylinder | HTC enhancement, when concentration is 0.75%, HTC enhance 64% | In addition to the increased properties of the base fluid, changes in the state of the heated surface are the main reasons. | [84] | |
| R141b | 0.001, 0.01 vol.% | Microchannel surfaces | HTC enhancement | The low pressure causes an increase in the heat transfer coefficient. | [85] | |
| 0.001, 0.01, 0.1 vol.% | copper surface | HTC enhancement | The addition of SDBS resulted in less deposition of nanoparticles. | [86] | ||
| CuO | Water | 0.1, 0.2, 0.3 wt.% | stainless steel rod | CHF enhancement HTC enhancement | Nanoparticle deposition causes changes in surface roughness and vaporization core points. | [71] |
| 0.01, 0.02 wt.% | surface of rod heater | HTC enhancement | SDS significantly reduces surface tension. | [87] | ||
| 0.1, 0.2, 0.3, 0.4 wt.% | stainless steel cylinder | HTC enhancement | Surfactants increase surface wettability and the contact angle of the bubbles with the surface is reduced. | [88] | ||
| 0.001, 0.01, 0.05, 0.075, 0.2 wt.% | flat heater plate | HTC enhancement | The deposited nanoparticles have a positive effect on heat transfer. | [89] | ||
| pentane | 0.005, 0.01 vol.% | circular brass surfaces | HTC enhancement | Surface trenches are the dominant condition for increasing nuclear boiling. | [90] | |
| EG/DW | 0.1, 0.2, 0.3 wt.% | cylindrical cartridge heater | HTC enhancement. When concentration is 0.5%, HTC enhanced 55% | Nanoparticle deposits alter surface roughness and wettability, which changes bubble shape and behavior. | [91] | |
| Fe2O3 | Water | 0.02, 0.1 vol.% copper surface | copper surface | CHF enhance 26–37% HTC deterioration | CHF enhancement is due to the complete wetting behavior exhibited by the nanoparticles. HTC deterioration is due to the increase in wettability and thickness of the porous layer structure. | [76] |
| 0.05–1 vol.% | cylindrical heater | CHF enhancement | Nanoparticle deposition causes an increase in surface roughness. | [81] | ||
| Fe3O4 | Water | 0.01, 0.05, 0.075, 0.1, 0.2, 0.4 vol.% | cylindrical copper surface | When concentration is 0.1%, the HTC enhanced 43% | Surface characteristics have a significant effect on boiling heat transfer. | [67] |
| EG/DW | 0.01, 0.05, 0.1 vol.% | horizontal Ni–Cr wire | CHF enhancement HTC deterioration | The deposited layer increases surface wettability, resulting in significant CHF enhancement. | [92] | |
| GNs | EG/DW | 0.005, 0.01, 0.02, 0.05, 0.1 wt.% | heater wire | CHF enhancement HTC enhancement | The surface wettability caused by the deposition of nanoparticles is enhanced. | [93] |
| GONs | Water | 0.0001, 0.0002, 0.0005, 0.001 wt.% | Copper spheres | CHF non-monotonic change | Non-monotonic changes consistent with changes in surface wettability caused by GON | [94] |
| CNT | Water | 0.1, 0.2, 0.3 wt.% | stainless steel rod | CHF enhancement HTC enhancement | Nanoparticle deposition causes changes in surface roughness and vaporization core points. | [71] |
| 0.02, 0.1 vol.% | copper surface | CHF enhanced 26–37% HTC deterioration | CHF enhancement is due to the complete wetting behavior exhibited by the nanoparticles. HTC deterioration is due to the increase in wettability and thickness of the porous layer structure. | [76] | ||
| 0.01, 0.05 wt.% | horizontal rod heater | HTC enhancement | The larger size of the nanoparticles results in an increase in surface roughness. | [80] | ||
| 0.1, 0.3, 0.5, 0.7, 1 wt.% | cooper surface | HTC enhancement | The covalent modification of the nanofluid can avoid the deposition of nanoparticles and is beneficial to HTC enhancement. | [95] | ||
| 0.5 wt.% | stainless steel spheres | CHF enhancement | Longer and thicker CNTs tend to form microporous layers, increasing surface roughness. | [96] | ||
| 0.01, 0.05, 0.1 wt.% | heating element | CHF enhancement HTC enhancement | Using the covalent functionalization of cysteine and silver nanoparticles; the smaller the size, the increased surface area. | [97] | ||
| SiO2 | Water | 0.04, 0.4, 1 kg/m3 | copper surface | CHF enhanced 2.5–3 times HTC deterioration | The separation of the nanoparticle layer may significantly deteriorate the heat transfer coefficient of boiling. | [79] |
| 0.05–1 vol.% | cylindrical heater | CHF enhancement | Nanoparticle deposition causes an increase in surface roughness. | [81] | ||
| 0.001, 0.01, 0.05, 0.1 wt.% | vertical cylinder | HTC deterioration | The change in surface properties caused by the deposition of nanoparticles on the surface has a major influence on the quenching process. | [98] | ||
| EG/DW | 0.1, 0.25, 0.5, 0.75 vol.% | hot-wire | HTC enhancement with concentration of 0.25% | Boiling heat transfer changes caused by nanoparticle coatings. | [99] | |
| ZnO | Water | 0.01, 0.05 wt.% | horizontal rod heater | HTC deterioration | Nanoparticle deposition causes a reduction in surface roughness. | [80] |
| 0.01, 0.02 wt.% | surface of rod heater | HTC enhancement | SDS significantly reduces surface tension. | [87] | ||
| EG | 0.35, 0.62, 0.93, 1.6, 2.6 vol.% | cylindrical copper block | HTC firstly increase and then decrease with the concentration | It is affected by the surface roughness of the nanoparticle deposition. | [100] | |
| EG/DW | 5.25, 7.25, 8.25 wt.% | Ni–Cr wire | CHF enhancement HTC enhancement | Heating the coating reduces surface wettability. | [101] | |
| TiO2 | Water | 0.04, 0.4, 1 kg/m3 | copper surface | CHF enhanced 2.5–3 times | It depends on the concentration of the nanoparticles. | [79] |
| 0.001, 0.01, 0.05, 0.075, 0.2 wt.% | flat heater plate | HTC deterioration | The bubbles have a more spherical shape and their size is reduced. | [89] | ||
| 12, 15 wt.% | copper surface | HTC enhanced 124–138% | Verified by Pioro correlation [102] | [103] | ||
| 0.00005, 0.0001, 0.0005, 0.005, 0.01 wt.% | Horizontal circular plates | HTC firstly increase and then decrease with concentration, which 0.0001% is best. | Subject to surface roughness. | [104] | ||
| EG | 0.01, 0.025, 0.05, 0.075 vol.% | cylindrical brass surface | HTC deterioration | The bubble size decreases with increasing concentration. | [105] | |
| R141b | 0.01, 0.025, 0.05, 0.075 vol.% | cylindrical brass surface | HTC deterioration | The bubble size decreases with increasing concentration. | [105] |
| Experimental Number | Working Fluid | CHF (kW/m2) | Zuber (Kw/m2) | Kandlikar (kW/m2) |
|---|---|---|---|---|
| 1 | Distilled water | 1200 | 1108(−8%) | 1205(+0.4%) |
| 2 | Fe2O3–Water | 1514 | 1108(−36%) | 1507(−0.4%) |
| 3 | Al2O3–Water | 1542 | 1108(−39%) | 1507(−2%) |
| 4 | CNTs–Water (0.02%) | 1552 | 1108(−40%) | 1507(−3%) |
| 5 | CNTs–Water (0.1%) | 1496 | 1108(−35%) | 1507(+0.7%) |
| 6 | Distilled water/layers of Fe2O3 | 1526 | 1108(−37%) | 1507(−1%) |
| 7 | Distilled water/layers of Al2O3 | 1652 | 1108(−49%) | 1507(−9%) |
| 8 | Distilled water/layers of CNTs | 1612 | 1108(−45%) | 1507(−7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, S.; Teng, Z. Effect of Nanofluids on Boiling Heat Transfer Performance. Appl. Sci. 2019, 9, 2818. https://doi.org/10.3390/app9142818
Yao S, Teng Z. Effect of Nanofluids on Boiling Heat Transfer Performance. Applied Sciences. 2019; 9(14):2818. https://doi.org/10.3390/app9142818
Chicago/Turabian StyleYao, Shouguang, and Zecheng Teng. 2019. "Effect of Nanofluids on Boiling Heat Transfer Performance" Applied Sciences 9, no. 14: 2818. https://doi.org/10.3390/app9142818
APA StyleYao, S., & Teng, Z. (2019). Effect of Nanofluids on Boiling Heat Transfer Performance. Applied Sciences, 9(14), 2818. https://doi.org/10.3390/app9142818





