Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine
Abstract
:1. Introduction
2. Nanotechnology and Nanomedicine
2.1. Nanoparticles
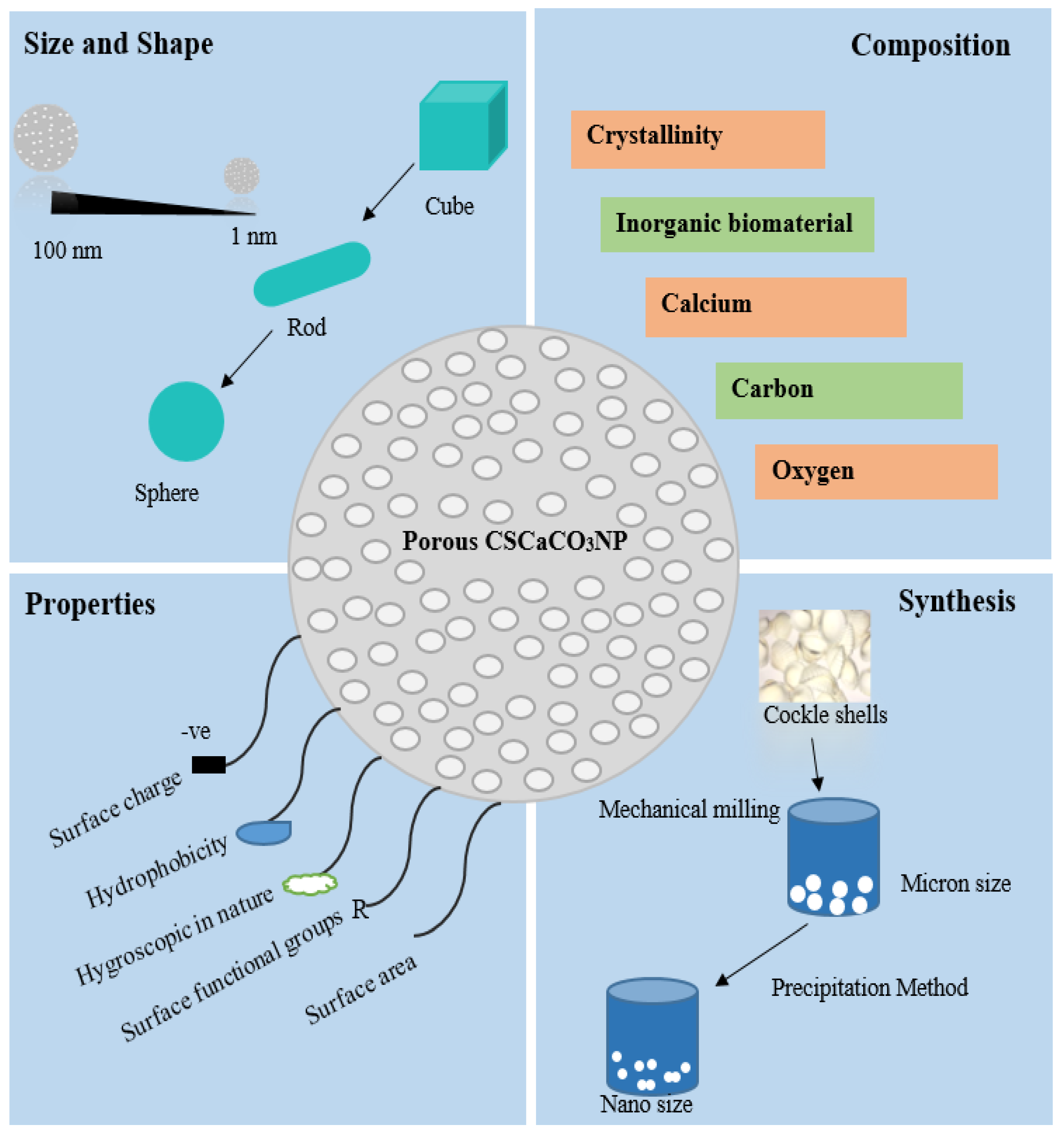
Types of Nanoparticles
2.2. Polymorphism of CaCO3NP
2.2.1. Aragonite
2.2.2. Advantages of Aragonite over Other Polymorphs
2.3. Cockle Shell (Anadara Granosa)
2.4. Synthesis of CaCO3 Aragonite from Cockle Shell
2.5. Characterization and Physicochemical Properties of CSCaCO3NP
2.6. Safety of CaCO3 Aragonite Nanoparticles
2.7. Drug Delivery System
2.7.1. Drug Loading Efficiency and Encapsulation Efficiency
2.7.2. Mechanism of Release Action of Drug from CSCaCO3NPs
2.8. Therapeutic Application of CSCaCO3 Aragonite Nanoparticles in Drug Delivery
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CSCaCO3NP | Cockle shell-derived calcium carbonate nanoparticles |
| CaCO3 | Calcium carbonate |
| BET | Brunauer–Emmet–Teller |
| TEM | Transmission electron microscope |
| MPNs | Metal phenolic nanoparticles |
| MOFs | Metal organic frameworks |
| FE-SEM | Field emission scanning electron microscope |
| BS-12 | Dodecyl dimethyl betaine |
| GIT | Gastrointestinal tracts |
| LUV | Large unilamellar vesicles |
| SUV | Small unilamellar vesicles |
| MLV | Multilamellar vesicles |
| ROS | Reactive oxygen species |
References
- Hasan, S.; Hasan, S. A Review on Nanoparticles: Their Synthesis and Types A Review on Nanoparticles: Their Synthesis and Types. Res. J. Recent Sci. 2015, 4, 7–10. [Google Scholar]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomedicine 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and promises. Mol. Pharmacol. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Mohd Abd Ghafar, S.L.; Hussein, M.Z.; Rukayadi, Y.; Abu Bakar Zakaria, M.Z. Surface-functionalized cockle shell – based calcium carbonate aragonite polymorph as a drug nanocarrier. Nanotechnol. Sci. Appl. 2017, 10, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Jafari, S.; Khosroushahi, A.Y. A sight on the current nanoparticle-based gene delivery vectors. Nanoscale Res. Lett. 2014, 9, 1–9. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium Carbonate Nanoparticles; Potential in Bone and Tooth Disorders. Pharm. Sci. 2015, 20, 175–182. [Google Scholar]
- Küther, J.; Seshadri, R.; Knoll, W.; Tremel, W. Templated growth of calcite, vaterite and aragonite crystals on self-assembled monolayers of substituted alkylthiols on gold. J. Mater. Chem. 1998, 8, 641–650. [Google Scholar] [CrossRef]
- Moghimi, S.M. Nanomedicine: current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef]
- Fu, W.; Mohd Noor, M.H.; Yusof, L.M.; Ibrahim, T.A.T.; Keong, Y.S.; Jaji, A.Z.; Zakaria, M.Z.A.B. In vitro evaluation of a novel pH sensitive drug delivery system based cockle shell-derived aragonite nanoparticles against osteosarcoma. J. Exp. Nanosci. 2017, 12, 166–187. [Google Scholar] [CrossRef]
- Jaji, A.Z.; Bakar, M.Z.; Mahmud, R.; Loqman, M.Y.; Hezmee, M.N.; Isa, T.; Wenliang, F.; Hammadi, N.I. Synthesis, characterization, and cytocompatibility of potential cockle shell aragonite nanocrystals for osteoporosis therapy and hormonal delivery. Nanotechnol. Sci. Appl. 2017, 10, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Awang-Hazmi, A.J.; Zuki, A.B.Z.; Noordin, M.M.; Jalila, A.; Norimah, Y. Mineral composition of the cockle (Anadara granosa) shells of West Coast of Peninsular Malaysia and it’s potential as biomaterial for use in bone repair. j. Amin Vet. Adv.. 2007, 6, 591–594. [Google Scholar]
- Wang, L.; Sondi, I.; Matijević, E. Preparation of uniform needle-like aragonite particles by homogeneous precipitation. J. Colloid Interface Sci. 1999, 218, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ruiz, I.; Delgado-López, J.M.; Durán-Olivencia, M.A.; Iafisco, M.; Tampieri, A.; Colangelo, D.; Prat, M.; Gómez-Morales, J. PH-responsive delivery of doxorubicin from citrate-apatite nanocrystals with tailored carbonate content. Langmuir 2013, 29, 8213–8221. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.N.; Bakar, M.Z.B.A.; Ali, M.E.; Hussein, M.Z.B.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. A novel method for the synthesis of calcium carbonate (aragonite) nanoparticles from cockle shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Jaji, A.Z.; Zakaria, Z.A.B.; Mahmud, R.; Loqman, M.Y.; Hezmee, M.N.M.; Abba, Y.; Isa, T.; Mahmood, S.K. Safety assessments of subcutaneous doses of aragonite calcium carbonate nanocrystals in rats. J. Nanoparticle Res. 2017, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Danmaigoro, A.; Selvarajah, G.T.; Noor, M.H.M.; Mahmud, R.; Zakaria, M.Z.A.B. Development of cockleshell (Anadara granosa) derived CaCO3 nanoparticle for doxorubicin delivery. J. Comput. Theor. Nanosci. 2017, 14, 5074–5086. [Google Scholar] [CrossRef]
- Hammadi, N.I.; Abba, Y.; Hezmee, M.N.M.; Razak, I.S.A.; Jaji, A.Z.; Isa, T.; Mahmood, S.K.; Zakaria, M.Z.A.B. Formulation of a Sustained Release Docetaxel Loaded Cockle Shell-Derived Calcium Carbonate Nanoparticles against Breast Cancer. Pharm. Res. 2017, 34, 1193–1203. [Google Scholar] [CrossRef]
- Kamba, A.S.; Ismail, M.; Azmi Tengku Ibrahim, T.; Zakaria, Z.A.B. Biocompatibility of bio based calcium carbonate nanocrystals aragonite polymorph on nih 3T3 fibroblast cell line. African J. Tradit. Complement. Altern. Med. 2014, 11, 31–38. [Google Scholar] [CrossRef]
- Hamidu, A.; Mokrish, A.; Mansor, R.; Shameha, I.; Razak, A.; Danmaigoro, A.; Jaji, A.Z.; Bakar, Z.A. Modi fi ed methods of nanoparticles synthesis in pH-sensitive nano-carriers production for doxorubicin delivery on MCF-7 breast cancer cell line. Int. J. Nanomed. 2019, 14, 3615–3627. [Google Scholar]
- Danmaigoro, A.; Selvarajah, G.T.; Mohd Noor, M.H.; Mahmud, R.; Abu Bakar, M.Z. Toxicity and Safety Evaluation of Doxorubicin-Loaded Cockleshell-Derived Calcium Carbonate Nanoparticle in Dogs. Adv. Pharmacol. Sci. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Kiranda, H.K.; Mahmud, R.; Abubakar, D.; Zakaria, Z.A. Fabrication, characterization and cytotoxicity of spherical-shaped conjugated gold-cockle shell derived calcium carbonate nanoparticles for biomedical applications. Nanoscale Res. Lett. 2018, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Popović, Z.; Liu, W.; Chauhan, V.P.; Lee, J.; Wong, C.; Greytak, A.B.; Insin, N.; Nocera, D.G.; Fukumura, D.; Jain, R.K.; et al. A nanoparticle size series for invivo fluorescence imaging. Angew. Chemie Int. Ed. 2010, 49, 8649–8652. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Mansour, H.M.; Zhang, Y.; Deng, X.; Chen, Y.; Wang, J.; Pan, Y.; Zhao, J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012, 426, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, L.M.G.; Brandao, D.A.; Rocha, F.R.G.; Marsiglio, R.P.; Longo, I.B.; Primo, F.L.; Tedesco, A.C.; Guimaraes-Stabili, M.R.; Rossa, C. Local administration of curcumin-loaded nanoparticles effectively inhibits inflammation and bone resorption associated with experimental periodontal disease. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Perret, P.; Bacot, S.; Gèze, A.; Gentil Dit Maurin, A.; Debiossat, M.; Soubies, A.; Blanc-Marquis, V.; Choisnard, L.; Boutonnat, J.; Ghezzi, C.; et al. Biodistribution and preliminary toxicity studies of nanoparticles made of Biotransesterified β–cyclodextrins and PEGylated phospholipids. Mater. Sci. Eng. C 2018, 85, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wu, S.; Cheng, Y.; You, J.; Chen, Y.; Li, M.; He, C.; Zhang, X.; Yang, T.; Lu, Y.; et al. MiR-375 delivered by lipid-coated doxorubicin-calcium carbonate nanoparticles overcomes chemoresistance in hepatocellular carcinoma. Nanomedicine Nanotechnology, Biol. Med. 2017, 13, 2507–2516. [Google Scholar] [CrossRef]
- Bhatia, S. Natural Polymer Drug Delivery Systems: Nanoparticles, Plants, and Algae; Spronger International Publishing: Basilea, Switzerland, 2016; pp. 1–225. ISBN 9783319411293. [Google Scholar]
- Christopher, A. lipinski Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar]
- Prashantha Kumar, B.R.; Soni, M.; Bharvi Bhikhalal, U.; Kakkot, I.R.; Jagadeesh, M.; Bommu, P.; Nanjan, M.J. Analysis of physicochemical properties for drugs from nature. Med. Chem. Res. 2010, 19, 984–992. [Google Scholar] [CrossRef]
- Lu, Y.; Park, K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013, 453, 198–214. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hörter, D.; Dressman, J. B Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2002, 46, 75–87. [Google Scholar] [CrossRef]
- Chen, Y.C.; Shie, M.Y.; Wu, Y.H.A.; Lee, K.X.A.; Wei, L.J.; Shen, Y.F. Anti-inflammation performance of curcumin-loaded mesoporous calcium silicate cement. J. Formos. Med. Assoc. 2017, 116, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Nanocarriers. Pharm. Res. 2007, 24, 2333–2334. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef]
- Ealias, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1–21. [Google Scholar]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2012, 64, 102–115. [Google Scholar] [CrossRef]
- Tiwari, D.K.; Behari, J.; Sen, P. Application of Nanoparticles in Waste Water Treatment. Carbon Nanotub. 2008, 3, 417–433. [Google Scholar]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Avasthi, P.; Marshall, W.F. NIH Public Access. Int. Soc. Differ. 2012, 83, 1–29. [Google Scholar]
- Gaucher, G.; Marchessault, R.H.; Leroux, J.C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release 2010, 143, 2–12. [Google Scholar] [CrossRef]
- Gaucher, G.; Satturwar, P.; Jones, M.C.; Furtos, A.; Leroux, J.C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. 2010, 76, 147–158. [Google Scholar] [CrossRef]
- Ji, S.; Lin, X.; Yu, E.; Dian, C.; Yan, X.; Li, L.; Zhang, M.; Zhao, W.; Dian, L. Curcumin-loaded mixed micelles: Preparation, characterization, and in vitro antitumor activity. J. Nanotechnol. 2018, 2018, 9103120. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent advances on liposomal nanoparticles: synthesis, characterization and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 788–799. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, F.; Gao, J.; Wang, L.; Huang, G. Liposomes for systematic delivery of vancomycin hydrochloride to decrease nephrotoxicity: Characterization and evaluation. Asian J. Pharm. Sci. 2015, 10, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.Z.H.; Jahan, R.; Bagul, U. Nanopharmaceuticals: A New Perspective of Drug Delivery System. asian J. Biomed. Pharm. Sci. 2012, 2, 11–20. [Google Scholar]
- Drulis-Kawa, Z.; Dorotkiewicz-Jach, A. Liposomes as delivery systems for antibiotics. Int. J. Pharm. 2010, 387, 187–198. [Google Scholar] [CrossRef]
- Mofazzal Jahromi, M.A.; Al-Musawi, S.; Pirestani, M.; Fasihi Ramandi, M.; Ahmadi, K.; Rajayi, H.; Mohammad Hassan, Z.; Kamali, M.; Mirnejad, R. Curcumin-loaded Chitosan Tripolyphosphate Nanoparticles as a safe, natural and effective antibiotic inhibits the infection of Staphylococcusaureus and Pseudomonas aeruginosa in vivo. Iran. J. Biotechnol. 2014, 12, 1–8. [Google Scholar] [CrossRef]
- Ghadi, A.; Mahjoub, S.; Tabandeh, F.; Talebnia, F. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Casp. J. Intern. Med. 2014, 5, 156–161. [Google Scholar]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliv. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Tianhong, D.; Masamitsu, T.; Ying-yang, H.; Micheal, R.H. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti. Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA . Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef]
- Ali, H.; Collnot, E.; Windbergs, M.; Lehr, C. Nanomedicines for the treatment of inflammatory bowel diseases. Eur. J. Nanomed. 2013, 5, 23–38. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Lou, Y.; Wu, J.Y.; Miao, J.; Lu, X.Y.; Gao, J.Q. Enhancement of oral bioavailability of salmon calcitonin through chitosan-modified, dual drug-loaded nanoparticles. Int. J. Pharm. 2019, 557, 170–177. [Google Scholar] [CrossRef]
- Xie, S.; Tao, Y.; Pan, Y.; Qu, W.; Cheng, G.; Huang, L.; Chen, D.; Wang, X.; Liu, Z.; Yuan, Z. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J. Control. Release 2014, 187, 101–117. [Google Scholar] [CrossRef]
- Tianhong, D.; Masamitsu, T.; Ying-Ying, H.; Michael, R.H. Chitosan Preparations for Wounds and Burns: Antimicrobial and Wound-Healing Effects. In Expert Rev Anti Infect Ther.; 2012; Volume 9, pp. 857–879. [Google Scholar]
- Hee, T.; Eun, J.; Jaie, Y.; Woon, J.; Su, C. Efficient gene delivery by urocanic acid-modified chitosan. J. Control. Release 2003, 93, 389–402. [Google Scholar]
- Segura, T.; Shea, L.D. Materials for non-viral gene delivery. Annu. Rev. Mater. Res. 2001, 25–46. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 1–22. [Google Scholar] [CrossRef]
- Reilly, R.M. Carbon nanotubes: Potential benefits and risks of nanotechnology in nuclear medicine. J. Nucl. Med. 2007, 48, 1039–1042. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intractracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar] [CrossRef]
- Liu, R.; Yu, T.; Shi, Z.; Wang, Z. The preparation of metal – organic frameworks and their biomedical application. Int. J. Nanomedicine 2016, 11, 1187–1200. [Google Scholar] [CrossRef]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Proc. Natl. Acad. Sci. 2008, 105, 11623–11627. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Al haydar, M.; Abid, H.R.; Sunderland, B.; Wang, S. Metal organic frameworks as a drug delivery system for flurbiprofen. Drug Des. Devel. Ther. 2017, 11, 2685–2695. [Google Scholar] [CrossRef]
- Sajid, M. Toxicity of nanoscale metal organic frameworks: A perspective. Environ. Sci. Pollut. Res. 2016, 14805–14807. [Google Scholar] [CrossRef]
- Hussain, K.; Hussain, T. Gold Nanoparticles:A Boon to Drug Delivery System. South Indian J. Biol. Sci. 2015, 1, 128. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D.K. Review on gold nanoparticles and their applications. Toxicol. Environ. Health Sci. 2011, 3, 193–205. [Google Scholar] [CrossRef]
- Khan, A.K.; Rashid, R.; Murtaza, G.; Zahra, A. Gold nanoparticles: Synthesis and applications in drug delivery. Trop. J. Pharm. Res. 2014, 13, 1169–1177. [Google Scholar] [CrossRef]
- Li, K.; Xiao, G.; Richardson, J.J.; Tardy, B.L.; Ejima, H.; Huang, W. Targeted Therapy against Metastatic Melanoma Based on Self-Assembled Metal-Phenolic Nanocomplexes Comprised of Green Tea Catechin. Adv. Sci. news 2019, 6, 1801688. [Google Scholar] [CrossRef]
- Zhang, X.; Parekh, G.; Guo, B.; Huang, X.; Dong, Y.; Han, W.; Chen, X.; Xiao, G. Polyphenol and self-assembly: Metal polyphenol nanonetwork for drug delivery and pharmaceutical applications. Futur. Drug Discov. 2019, 2631–3316. [Google Scholar] [CrossRef]
- Luo, W.; Xiao, G.; Tian, F.; Richardson, J.J.; Wang, Y.; Zhou, J.; Guo, J.; Liao, X.; Shi, B. Engineering robust metal-phenolic network membranes for uranium extraction from seawater. Energy Environ. Sci. 2019, 12, 607–614. [Google Scholar] [CrossRef]
- Caruso, F.; Best, J.P.; Kempe, K.; Cho, K.L.; Rahim, M.A.; Ejima, H.; Müllner, M. Coordination-Driven Multistep Assembly of Metal–Polyphenol Films and Capsules. Chem. Mater. 2014, 26, 1645–1653. [Google Scholar]
- Swain, S. Solid Lipid Nanoparticle: An Overview. Pharm. Regul. Aff. Open Access 2015, 04, 7689. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, A.S.; Grampurohit, N.D.; Gadhave, M.V.; Gaikwad, D.D.; Jadhav, S.L. Solid lipid nanoparticles: A promising drug delivery system. Int. Res. J. Pharm. 2012, 3, 100–107. [Google Scholar]
- Chirio, D.; Peira, E.; Dianzani, C.; Muntoni, E.; Gigliotti, C.; Ferrara, B.; Sapino, S.; Chindamo, G.; Gallarate, M. Development of solid lipid nanoparticles by cold dilution of microemulsions: Curcumin loading, preliminary in vitro Studies, and biodistribution. Nanomaterials 2019, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chaudhury, A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech 2010, 12, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Boonyuen, S.; Malaithong, M.; Prokaew, A. Decomposition study of calcium carbonate in shell. Thai J. Sci. Technol. 2015, 4, 115–122. [Google Scholar]
- Hariharan, M.; Varghese, N.; Cherian, A.B.; Sreenivasan, P.V.; Paul, J. Synthesis and characterisation of CaCO3 (Calcite) nano particles from cockle shells using chitosan as precursor. Int. j. Sci. Res, Publ. 2014, 4, 1–5. [Google Scholar]
- Kamba, S.A.; Ismail, M.; Hussein-Al-Ali, S.H.; Ibrahim, T.A.T.; Zakaria, Z.A.B. In vitro delivery and controlled release of doxorubicin for targeting osteosarcoma bone cancer. Molecules 2013, 18, 10580–10598. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, P.; Wang, Y.; Du, J.; Zhou, Q.; Zhu, Z.; Yang, X.; Yuan, J. Biocompatibility of porous spherical calcium carbonate microparticles on hela cells. World J. Nano Sci. Eng. 2012, 02, 25–31. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Kirboga, S.; Oner, M. Effect of the experimental parameters on calcium carbonate precipitation. Chem. Eng. Trans. 2013, 32, 2119–2124. [Google Scholar]
- Griesshaber, E.; Schmahl, W.W.; Neuser, R.; Pettke, T.; Blüm, M.; Mutterlose, J.; Brand, U. Crystallographic texture and microstructure of terebratulide brachiopod shell calcite: An optimized materials design with hierarchical architecture. Am. Mineral. 2007, 92, 722–734. [Google Scholar] [CrossRef]
- Chang, R.; Kim, S.; Lee, S.; Choi, S.; Kim, M.; Park, Y. Calcium carbonate precipitation for CO2 storage and utilization: A review of the carbonate crystallization and polymorphism. Front. Energy Res. 2017, 5, 1–12. [Google Scholar] [CrossRef]
- Isa, T.; Zakaria, Z.A.B.; Rukayadi, Y.; Hezmee, M.N.M.; Jaji, A.Z.; Imam, M.U.; Hammadi, N.I.; Mahmood, S.K. Antibacterial activity of ciprofloxacin-encapsulated cockle shells calcium carbonate (Aragonite) nanoparticles and its biocompatability in macrophage J774A.1. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B. A pH-sensitive, biobased calcium carbonate aragonite nanocrystal as a novel anticancer delivery system. Biomed Res. Int. 2013, 2013, 587451. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Z.; Ma, H. Synthesis of aragonite by carbonization from dolomite without any additives. Int. J. Miner. Process. 2013, 123, 25–31. [Google Scholar] [CrossRef]
- Bharatham, H.; Zakaria, M.Z.A.B.; Perimal, E.K.; Yusof, L.M.; Hamid, M. Mineral and physiochemical evaluation of Cockle shell (Anadara granosa) and other selected Molluscan shell as potential biomaterials. Sains Malaysiana 2014, 43, 1023–1029. [Google Scholar]
- Islam, K.N.; Bakar, M.Z.B.A.; Noordin, M.M.; Hussein, M.Z.B.; Rahman, N.S.B.A.; Ali, M.E. Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa). Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Hu, Z.; Shao, M.; Cai, Q.; Ding, S.; Zhong, C.; Wei, X.; Deng, Y. Synthesis of needle-like aragonite from limestone in the presence of magnesium chloride. J. Mater. Process. Technol. 2009, 209, 1607–1611. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Bala, H.; Pan, Y.; Zhao, J.; Zhao, X.; Wang, Z. Facile preparation of CaCO3 nanoparticles with self-dispersing properties in the presence of dodecyl dimethyl betaine. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 297, 179–182. [Google Scholar] [CrossRef]
- Saidykhan, L.; Bakar, M.Z.B.A.; Rukayadi, Y.; Kura, A.U.; Latifah, S.Y. Development of nanoantibiotic delivery system using cockle shell-derived aragonite nanoparticles for treatment of osteomyelitis. Int. J. Nanomedicine 2016, 11, 661–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayward, B.W.; Morley, M.; Riley, J.; Smith, N.; Stace, G. Addition to the mollusca from kawerua, north auckland. Auckl. Inst. Mus. 1995, 193, 183–193. [Google Scholar]
- Mohd Abd Ghafar, S.L.; Hussein, M.Z.; Abu Bakar Zakaria, Z. Synthesis and characterization of cockle shell-based calcium carbonate aragonite polymorph nanoparticles with surface functionalization. J. Nanoparticles 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Stupp, S.I.; Braun, P.V. Molecular manipulation of microstructures: Biomaterials, ceramics, and semiconductors. Science 1997, 277, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E. Processing and characterization of cockle shell calcium carbonate (CaCO3) bioceramic for potential application in bone tissue engineering. J. Mater. Sci. Eng. 2014, 02, 2–6. [Google Scholar] [CrossRef]
- Zhou, M.; Wei, Z.; Qiao, H.; Zhu, L.; Yang, H.; Xia, T. Particle size and pore structure characterization of silver nanoparticles prepared by confined arc plasma. J. Nanomater. 2009, 2009, 1–5. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Sechi, M. Targeted therapy using nanotechnology: Focus on cancer. Int. J. Nanomedicine 2014, 9, 467–483. [Google Scholar]
- Cho, E.J.; Holback, H.; Liu, K.C.; Abouelmagd, S.A.; Park, J.; Yeo, Y. Nanoparticle characterization: State of the art, challenges, and emerging technologies. Mol. Pharm. 2013, 10, 2093–2110. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Zhao, X.; Bala, H.; Wang, Z. Synthesis of nanosized calcium carbonate (aragonite) via a polyacrylamide inducing process. Powder Technol. 2006, 163, 134–138. [Google Scholar] [CrossRef]
- Guo, F.; Li, Y.; Xu, H.X.; Zhao, G.Q.; He, X.J. Size-controllable synthesis of calcium carbonate nanoparticles using aqueous foam films as templates. Mater. Lett. 2007, 61, 4937–4939. [Google Scholar] [CrossRef]
- Kitamura, M. Crystallization and transformation mechanism of calcium carbonate polymorphs and the effect of magnesium ion. J. Colloid Interface Sci. 2001, 327, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.I.; Han, S.W.; Choi, H.J. Nanoparticle-Directed Crystallization of Calcium Carbonate. Adv. Mater. 2001, 13, 1999–2002. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, G.; Fang, Y.; Dai, W.; Ma, N. Facile synthesis of mesoporous calcium carbonate particles with finger citron residue as template and their adsorption performances for Congo red. Adsorpt. Sci. Technol. 2018, 36, 872–887. [Google Scholar] [CrossRef]
- Mahmood, S.K.; Zakaria, M.Z.A.B.; Razak, I.S.B.A.; Yusof, L.M.; Jaji, A.Z.; Tijani, I.; Hammadi, N.I. Preparation and characterization of cockle shell aragonite nanocomposite porous 3D scaffolds for bone repair. Biochem. Biophys. Rep. 2017, 10, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Shafiu Kamba, A.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B. Synthesis and characterisation of calcium carbonate aragonite nanocrystals from cockle shell powder (Anadara granosa). J. Nanomater. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, Z.M.; Ye, L.; Zhang, A.Y.; Feng, Z.G. A pH-sensitive nano drug delivery system of doxorubicin-conjugated amphiphilic polyrotaxane-based block copolymers. Biomater. Sci. 2013, 1, 1282–1291. [Google Scholar] [CrossRef]
- Redhead, H.M.; Davis, S.S.; Illum, L. Drug delivery in poly (lactide-co-glycolide) nanoparticles surface modified with poloxamer 407 and poloxamine 908: In vitro characterisation and in vivo evaluation. J. Control. Release 70 2001, 70, 353–363. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Nagda, C.D.; Chotai, N.P.; Patel, S.B.; Soni, T.J.; Patel, U.L. Preparation and in vitro evaluation of bioadhesive microparticulate system. Int. J. Pharm. Sci. Nanotechnol. 2006, 1, 257–266. [Google Scholar]
- Vedantam, P.; Huang, G.; Tzeng, T.R.J. Size-dependent cellular toxicity and uptake of commercial colloidal gold nanoparticles in DU-145 cells. Cancer Nanotechnol. 2013, 4, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Ghaji, M.S.; Abu, Z.; Zakaria, B.; Shameha, A.R.I.; Noor, M.; Hezmee, M.; Hazilawati, H. Novelty to synthesis nanoparticles from cockle shall via mechanical method to delivery and controlled Release of cytarabine. J. Comput. Theor. Nanosci. 2017, 14, 1–9. [Google Scholar]
- Linga Raju, C.; Narasimhulu, K.V.; Gopal, N.O.; Rao, J.L.; Reddy, B.C.V. Electron paramagnetic resonance, optical and infrared spectral studies on the marine mussel Arca burnesi shells. J. Mol. Struct. 2002, 608, 201–211. [Google Scholar] [CrossRef]
- Sanoj Rejinold, N.; Muthunarayanan, M.; Chennazhi, K.P.; Nair, S.V.; Jayakumar, R. Curcumin loaded fibrinogen nanoparticles for cancer drug delivery. J. Biomed. Nanotechnol. 2011, 7, 521–534. [Google Scholar] [CrossRef]
- Lin, P.C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for physicochemical characterization of nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.S.; Skirtach, A.; Volodkin, D. Controlling the Vaterite CaCO3 Crystal Pores. Design of Tailor-Made Polymer Based Microcapsules by Hard Templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Dalgleish, T.; Williams, J.M.G.; Golden, A.-M.J.; Perkins, N.; Barrett, L.F.; Barnard, P.J.; Au Yeung, C.; Murphy, V.; Elward, R.; Tchanturia, K.; et al. Text Book of Modern Txicology, 4th ed.; Wiley: New Jersey, NJ, USA, 2007; Volume 136, pp. 23–42. ISBN 978-0-470-46206-5. [Google Scholar]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef] [PubMed]
- Shafiu Kamba, A.; Zakaria, Z.A.B. Osteoblasts growth behaviour on bio-based calcium carbonate aragonite nanocrystal. Biomed Res. Int. 2014, 2014, 215097. [Google Scholar] [CrossRef]
- Auriemma, G.; Mencherini, T.; Russo, P.; Stigliani, M.; Aquino, R.P.; Del Gaudio, P. Prilling for the development of multi-particulate colon drug delivery systems: Pectin vs. pectin-alginate beads. Carbohydr. Polym. 2013, 92, 367–373. [Google Scholar] [CrossRef]
- Paul, W.; Sharma, C.P. Ceramic Drug Delivery: A perspective. J. Biomater. Appl. 2007, 17, 0253–0264. [Google Scholar] [CrossRef] [PubMed]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines Based Drug Delivery Systems for Anti-Cancer Targeting and Treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.-J.; Cheng, F.-F.; Zheng, T.-T.; Wang, C.; Zhu, J.-J. Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery. J. Mater. Chem. 2011, 21, 12034. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.J.; Cao, S.W.; Chen, F. Hierachically nanostructured mesoporous spheres of calcium silicate hydrate: Surfactant-free sonochemical synthesis and drug-delivery system with ultrahigh drug-loading capacity. Adv. Mater. 2010, 22, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Farzan, M. Nanoparticles for targeted delivery of therapeutics and small interfering RNAs in hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 12022–12041. [Google Scholar] [CrossRef]
- Montalbán, M.; Coburn, J.; Lozano-Pérez, A.; Cenis, J.; Víllora, G.; Kaplan, D. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef]
- Seleci, M.; Ag Seleci, D.; Joncyzk, R.; Stahl, F.; Blume, C.; Scheper, T. Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials 2016, 17, 33–41. [Google Scholar] [CrossRef]
- Isa, T.; Zakaria, Z.A.B.; Rukayadi, Y.; Hezmee, M.N.M.; Jaji, A.Z.; Imam, M.U.; Hammadi, N.I.; Mahmood, S.K. Antibacterial activity of ciprofloxacin–encapsulated cockle shells calcium carbonate (Aragonite) nanoparticles and its biocompatability in macrophage J774A.1. Int. J. Mol. Sci. Artic. 2016, 17, 713. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- Jain, D.; Banerjee, R. Comparison of ciprofloxacin hydrochloride-loaded protein, lipid, and chitosan nanoparticles for drug delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 105–112. [Google Scholar] [CrossRef]
- Han, S.C.; Kim, D.G.; Han, E.H.; Kim, Y.B.; Hwang, I.C.; Kim, C.Y. Toxicity study of a new camptothecin anti-cancer agent CKD-602 in dogs: 4-Week continuous intravenous dose by infusion pump and 4-week repeated intravenous dose. Regul. Toxicol. Pharmacol. 2010, 58, 275–284. [Google Scholar] [CrossRef]
- Lanz-Landázuri, A.; Martínez De Ilarduya, A.; García-Alvarez, M.; Muñoz-Guerra, S. Poly(β,L-malic acid)/Doxorubicin ionic complex: A pH-dependent delivery system. React. Funct. Polym. 2014, 81, 45–53. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.Q.; Niu, J.; Liu, W.; Peng, S.F.; Liu, C.M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef]
- Kadota, K.; Furukawa, R.; Tozuka, Y.; Shimosaka, A.; Shirakawa, Y.; Hidaka, J. Formation mechanism of non-spherical calcium carbonate particles in the solution using cluster-moving Monte Carlo simulation. J. Mol. Liq. 2014, 194, 115–120. [Google Scholar] [CrossRef]
- Manocha, B.; Margaritis, A. Controlled Release of Doxorubicin from Doxorubicin/γ-Polyglutamic Acid Ionic Complex. J. Nanomater. 2010, 2010, 1–9. [Google Scholar] [CrossRef]
- Giodini, L.; Re, F.L.; Campagnol, D.; Marangon, E.; Posocco, B.; Dreussi, E.; Toffoli, G. Nanocarriers in cancer clinical practice: a pharmacokinetic issue. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 583–599. [Google Scholar] [CrossRef]
- Adhikari, P.; Pal, P.; Das, A.K.; Ray, S.; Bhattacharjee, A.; Mazumder, B. Nano lipid-drug conjugate: An integrated review. Int. J. Pharm. 2017, 529, 629–641. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Svenskaya, Y.; Parakhonskiy, B.; Haase, A.; Atkin, V.; Lukyanets, E.; Gorin, D.; Antolini, R. Anticancer drug delivery system based on calcium carbonate particles loaded with a photosensitizer. Biophys. Chem. 2013, 182, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Zhou, X.; Xing, D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials 2014, 35, 4185–4194. [Google Scholar] [CrossRef]
- Mukhopdhyay, S. Nanotoxicology: Assessment of toxicological properties of nanomaterial. J. Nanomed. Res. 2017, 6, 9–10. [Google Scholar] [CrossRef]
- Sahari, F.; Mijan, N.A. Cockle shell as an alternative construction material for artificial reef. In Proceedings of the International Conference on Creativity and Innovation for Sustainable Developmement, At International Islamic University Malaysia, Kuala Lumpur, Malaysia, 12–14 September 2011. [Google Scholar]

| Author’s Name | Drug Used | Zeta Potential (mV) before Drug Loading | Zeta Potential (mV) after Drug Loading | Size of Nanoparticles (nm) | Shape of Nanoparticles |
|---|---|---|---|---|---|
| Isa et al. [28] | Ciprofloxacin | −15.3 ± 2.0 | −13.0 ± 1.9 | 11.93–22.12 | Spherical |
| Fu et al. [15] | Doxorubicin | −46.17 ± 3.82 | −40.57 ± 3.80 | 20–60 | Spherical |
| Jaji et al. [16] | Teriparatide (PTH 1–34) | Nil | −27.6 ± 8.9 | 30 ± 50 | Spherical |
| Hammadi et al. [105] | Taxanes (Docetaxel) | −15.4 ± 0.9 | −21.7 ± 0.1 | 42.22 | Spherical |
| Danmaigoro et al. [32] | Doxorubicin | −12.1 | −34.7 | 24.90 | Spherical |
| Ghafar et al. [6] | Nil | −9.4 ± 0.8 | −28.9 ± 0.1 | <100 | Spherical |
| Kiranda et al. [82] | Nil | Nil | −16.4 ± 3.81 | 35 ± 16 | Spherical |
| Hamidu et al. [20] | Doxorubicin | −19.1 ± 3.9 | −17.8 ± 4.6 | 35.50 | Spherical |
| Ghaji et al. [124] | Cytarabine | −11 | −13.2 | 20–50 | Spherical |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad Mailafiya, M.; Abubakar, K.; Danmaigoro, A.; Musa Chiroma, S.; Bin Abdul Rahim, E.; Aris Mohd Moklas, M.; Abu Bakar Zakaria, Z. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Appl. Sci. 2019, 9, 2897. https://doi.org/10.3390/app9142897
Muhammad Mailafiya M, Abubakar K, Danmaigoro A, Musa Chiroma S, Bin Abdul Rahim E, Aris Mohd Moklas M, Abu Bakar Zakaria Z. Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Applied Sciences. 2019; 9(14):2897. https://doi.org/10.3390/app9142897
Chicago/Turabian StyleMuhammad Mailafiya, Maryam, Kabeer Abubakar, Abubakar Danmaigoro, Samaila Musa Chiroma, Ezamin Bin Abdul Rahim, Mohamad Aris Mohd Moklas, and Zuki Abu Bakar Zakaria. 2019. "Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine" Applied Sciences 9, no. 14: 2897. https://doi.org/10.3390/app9142897
APA StyleMuhammad Mailafiya, M., Abubakar, K., Danmaigoro, A., Musa Chiroma, S., Bin Abdul Rahim, E., Aris Mohd Moklas, M., & Abu Bakar Zakaria, Z. (2019). Cockle Shell-Derived Calcium Carbonate (Aragonite) Nanoparticles: A Dynamite to Nanomedicine. Applied Sciences, 9(14), 2897. https://doi.org/10.3390/app9142897






