Structural Requirements for Antimicrobial Activity of Phenolic Nor-Triterpenes from Celastraceae Species
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedures
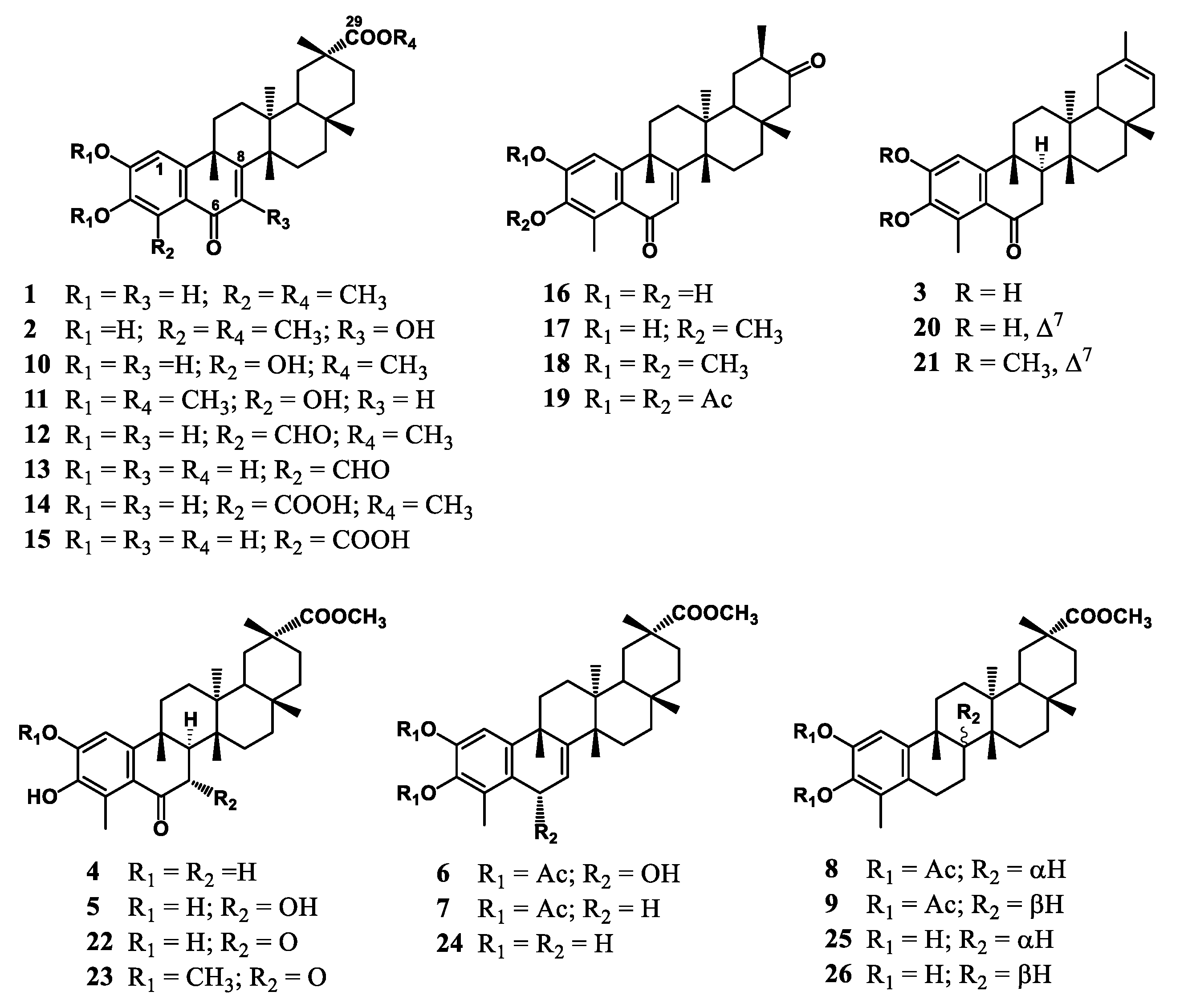
2.2. Phenolic Nor-Triterpenes
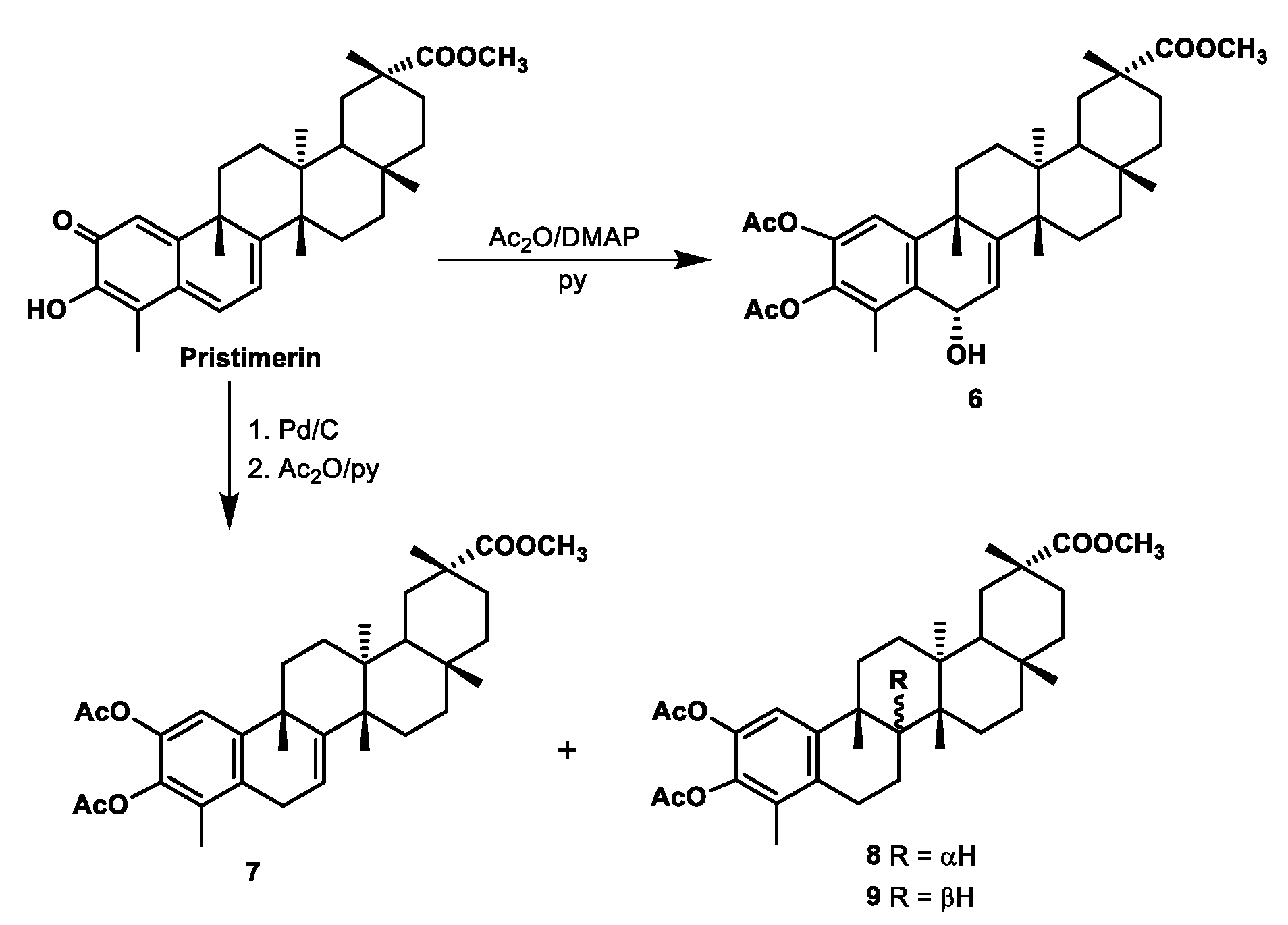
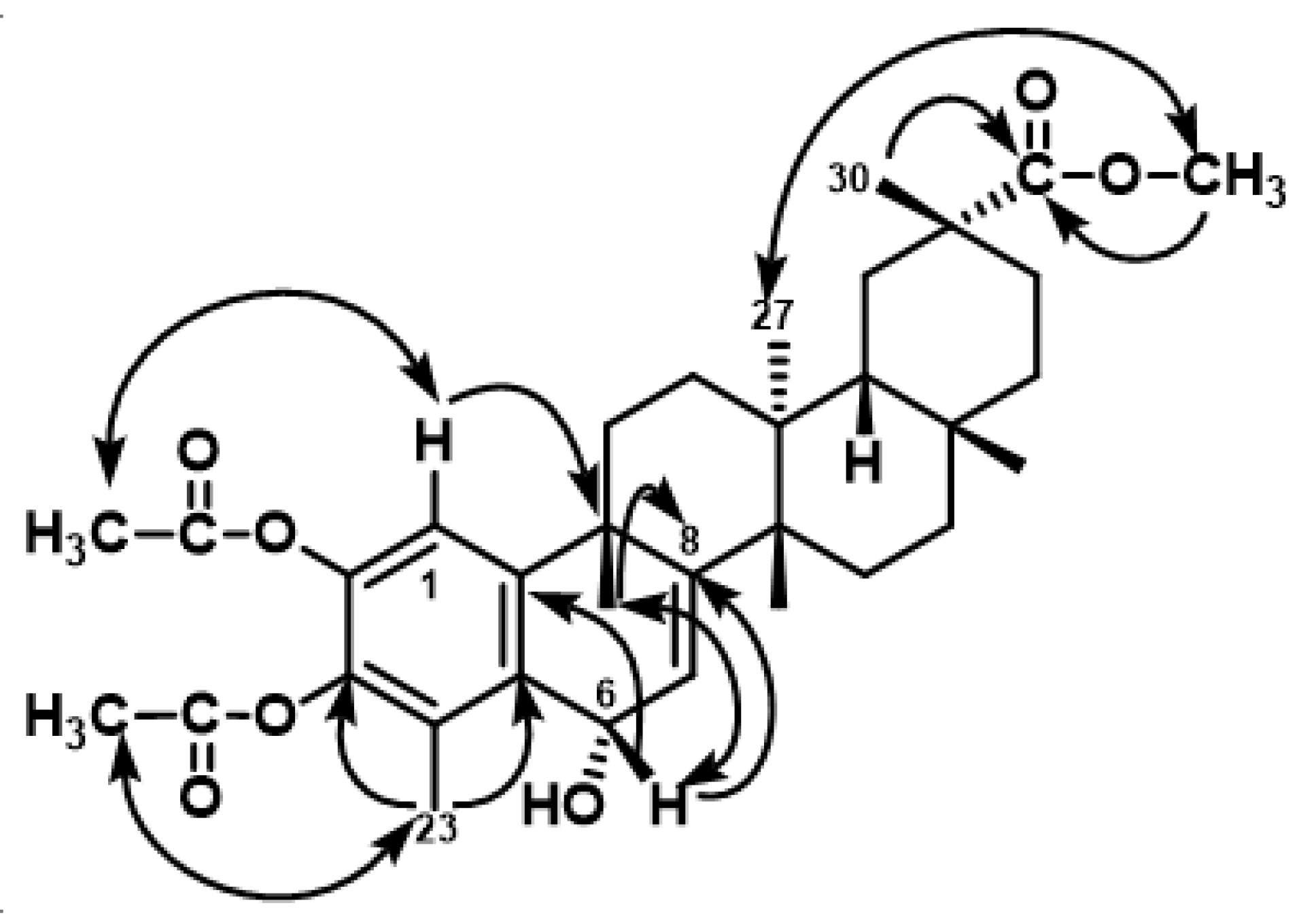
2.2.1. Preparation of Compound 6
2.2.2. Preparation of Compounds 7–9
2.3. Antimicrobial Activity
3. Results and Discussion
3.1. Chemistry
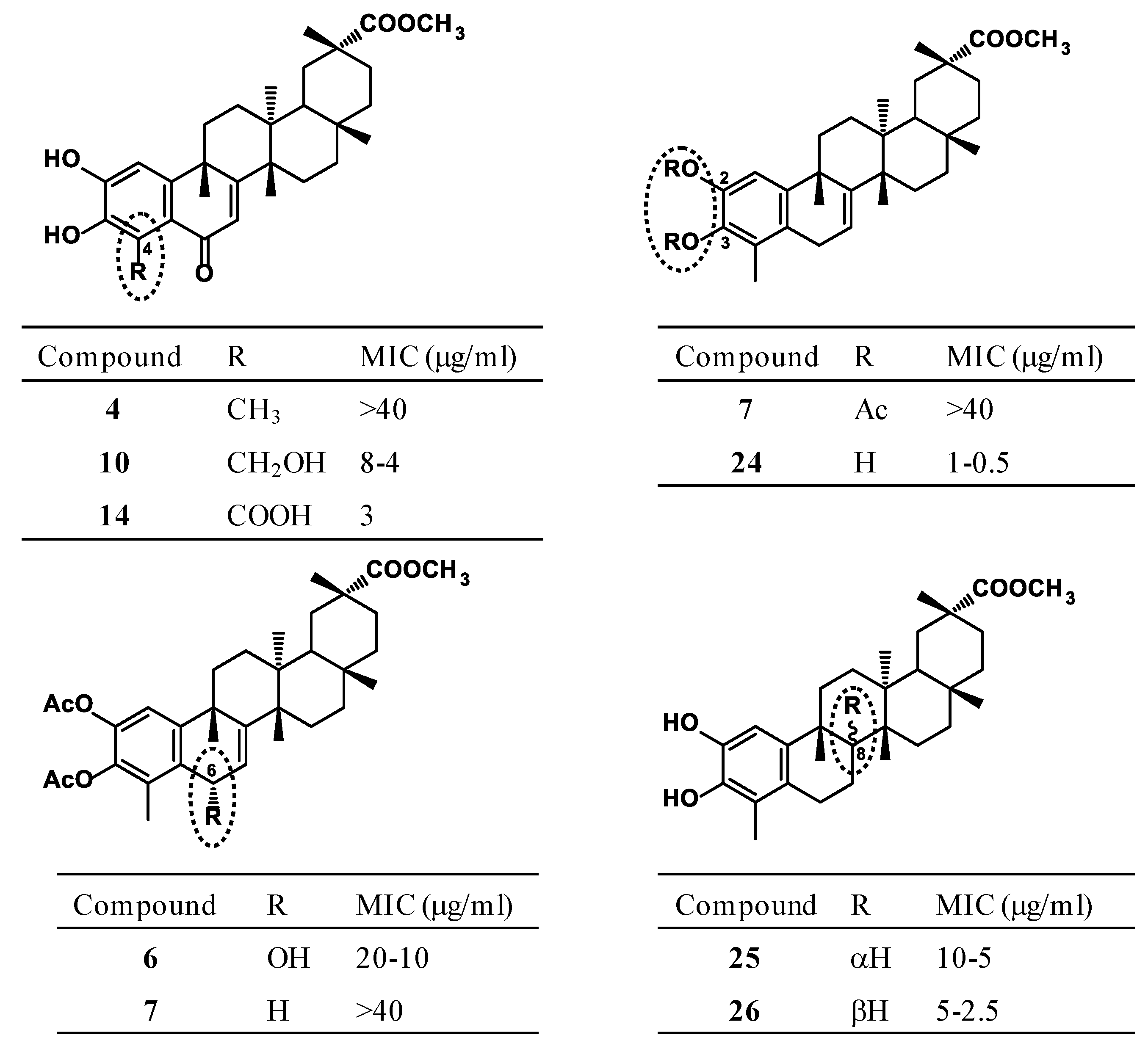
3.2. Antimicrobial Evaluation
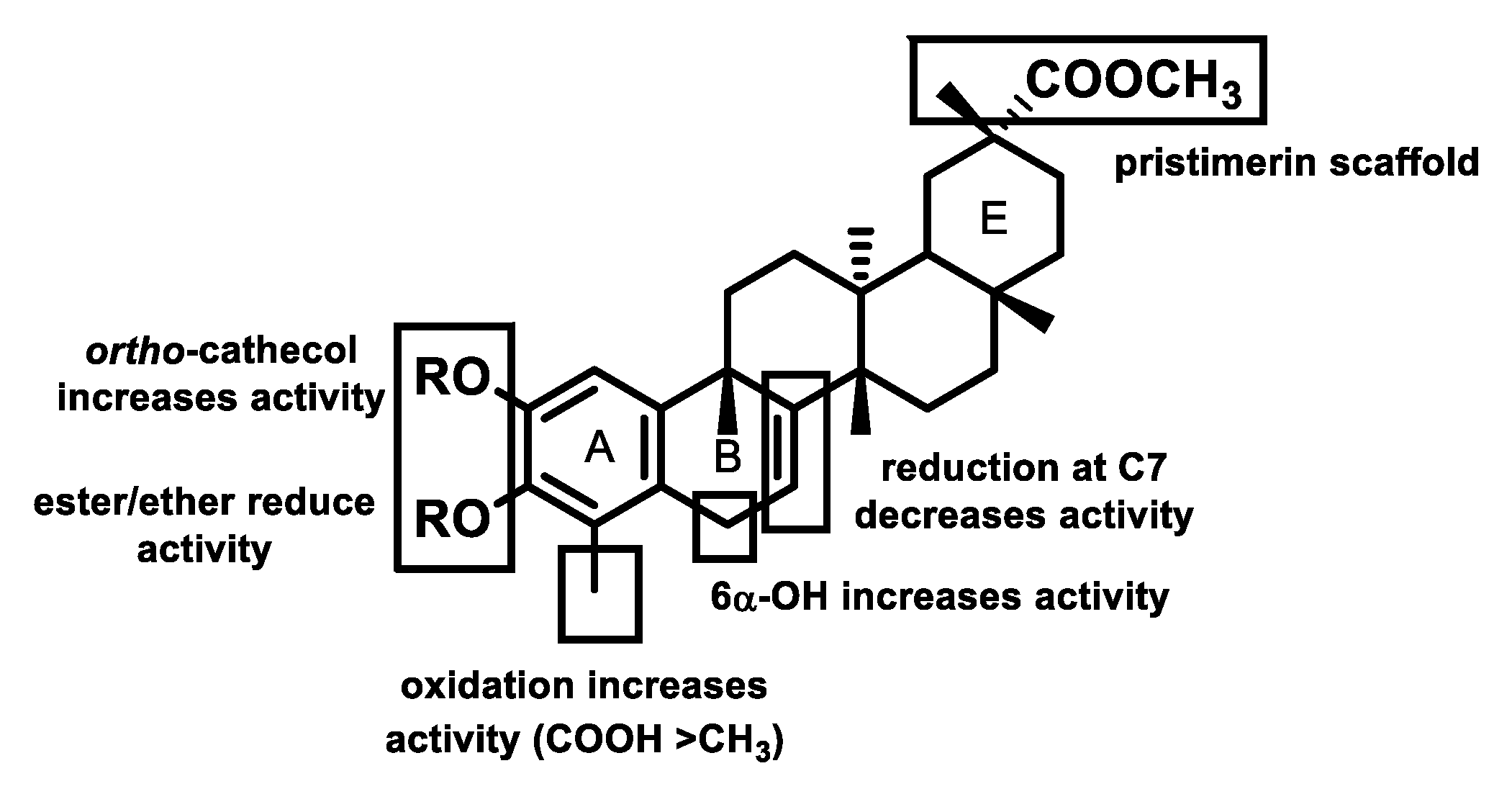
3.3. Structure–Activity Relationship Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Durand, G.A.; Raoult, D.; Dubourg, G. Antibiotic discovery: History, methods and perspectives. Int. J. Antimicrob. Agents 2019, 53, 371–382. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- González, A.G.; Bazzocchi, I.L.; Moujir, L.M.; Jiménez, I.A. Ethnobotanical uses of Celastraceae. Bioactive metabolites. In Studies in Natural Products Chemistry: Bioactive Natural Products; (Part D); Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2000; Volume 23, pp. 649–738. [Google Scholar]
- Niero, R.; de Andrade, S.F.; Cechinel, V.F. A review of the ethnopharmacology, phytochemistry and pharmacology of plants of the Maytenus genus. Curr. Pharm. Des. 2011, 17, 1851. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L. Triterpenoid quinonemethides and related compounds (celastroloids). In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: New York, NY, USA, 1996; Volume 67, pp. 1–123. [Google Scholar]
- Chen, S.-R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Yousef, B.A.; Hassan, H.M.; Zhang, L.-Y.; Jiang, Z.-Z. Anticancer potential and molecular targets of pristimerin: A mini review. Curr. Cancer Drug Targets 2017, 17, 100–108. [Google Scholar] [CrossRef]
- Alvarenga, N.; Ferro, E.A. Bioactive triterpenes and related compounds from Celastraceae. In Studies in Natural Products Chemistry: Bioactive Natural Products; (Part K); Bioactive triterpenes and related compounds from Celastraceae; Atta-ur-Rahman, Ed.; Elsevier Science Publisher: Amsterdam, The Netherlands, 2006; Volume 33, pp. 239–307. [Google Scholar]
- Bazzocchi, I.L.; Núñez, M.J.; Reyes, C.P. Diels-Alder adducts from Celastraceae species. Phytochem. Rev. 2018, 17, 669–690. [Google Scholar] [CrossRef]
- Li, P.P.; He, W.; Yuan, P.F.; Song, S.S.; Lu, J.T.; Wei, W. Celastrol induces mitochondria-mediated apoptosis in hepatocellular carcinoma Bel-7402 cells. Am. J. Chin. Med. 2015, 43, 137–148. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.C.; Oliveira, F.P.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Costa, E.V.; Silva, F.M.A.; Rocha, W.C.; Koolen, H.H.F.; et al. In vitro and in vivo anti-leukemia activity of the stem bark of Salacia impressifolia (Miers) A. C. Smith (Celastraceae). J. Ethnopharmacol. 2019, 231, 516–524. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Teng, H.; Li, C.; Wang, B.; Wang, J. Celastrol inhibits microglial pyroptosis and attenuates inflammatory reaction in acute spinal cord injury rats. Int. Immunopharmacol. 2019, 66, 215–223. [Google Scholar] [CrossRef]
- Santos, V.A.F.F.M.; Santos, D.P.; Castro-Gamboa, I.; Zanoni, M.V.B.; Furlan, M. Evaluation of antioxidant capacity and synergistic associations of quinonemethide triterpenes and phenolic substances from Maytenus ilicifolia (Celastraceae). Molecules 2010, 15, 6956–6973. [Google Scholar]
- Liao, L.M.; Silva, G.A.; Monteiro, M.R.; Albuquerque, S. Trypanocidal activity of quinonemethide triterpenoids from Cheiloclinium cognatum (Hippocrateaceae). Z. Naturforsch C 2008, 63, 207–210. [Google Scholar] [CrossRef]
- Avilla, J.; Teixidó, A.; Velázquez, C.; Alvarenga, N.; Ferro, E.; Canela, R. Insecticidal activity of Maytenus species (Celastraceae) nortriterpene quinone methides against Codling Moth, Cydia pomonella (L.) (Lepidoptera: Tortricidae). J. Agric. Food Chem. 2000, 48, 88–92. [Google Scholar] [CrossRef]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Luis, J.G. The relationship between structure and antimicrobial activity in quinones from the Celastraceae. Biochem. Syst. Ecol. 1990, 18, 25–28. [Google Scholar] [CrossRef]
- González, A.G.; Ravelo, A.G.; Bazzocchi, I.L.; Jiménes, J.; González, C.M.; Luis, J.G.; Ferro, E.A.; Gutiérrez, A.; Moujir, L. Biological study of triterpenoquinones from Celastraceae. Il Farmaco 1988, 43, 501–505. [Google Scholar]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Luis, J.G. Mode of action of netzahualcoyone. Antimicrob. Agents Chemother. 1991, 35, 211–213. [Google Scholar] [CrossRef] [Green Version]
- Moujir, L.; Gutiérrez-Navarro, A.M.; González, A.G.; Ravelo, A.G.; Jiménez, J. Biological properties of netzahualcoyone: Conditions for activity. Biomed. Lett. 1991, 46, 7–15. [Google Scholar]
- González, A.G.; Alvarenga, N.L.; Ravelo, A.G.; Jiménez, I.A.; Bazzocchi, I.L.; Canela, N.J.; Moujir, L. Antibiotic phenol nor-triterpenes from Maytenus canariensis. Phytochemistry 1996, 43, 129–132. [Google Scholar] [CrossRef]
- Rodríguez, F.M.; López, M.R.; Jiménez, I.A.; Moujir, L.; Ravelo, A.G.; Bazzocchi, I.L. New phenolic triterpenes from Maytenus blepharodes.Semisynthesis of 6-deoxoblepharodol from pristimerin. Tetrahedron 2005, 61, 2513–2519. [Google Scholar] [CrossRef]
- De León, L.; Beltrán, B.; Moujir, L. Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis. Planta Med. 2005, 71, 313–319. [Google Scholar] [CrossRef]
- De León, L.; Moujir, L. Activity and mechanism of the action of zeylasterone against Bacillus subtilis. J. Appl. Microbiol. 2008, 104, 1266–1274. [Google Scholar] [CrossRef]
- De León, L.; López, M.R.; Moujir, L. Antibacterial properties of zeylasterone, a triterpenoid isolated from Maytenus blepharodes, against Staphylococcus aureus. Microbiol. Res. 2010, 165, 617–626. [Google Scholar] [CrossRef]
- López, M.R.; de León, L.; Moujir, L. Antibacterial properties of phenolic triterpenoids against Staphylococcus epidermidis. Planta Med. 2011, 77, 726–729. [Google Scholar] [CrossRef]
- Shirota, O.; Morita, H.; Takeya, K.; Itokawa, H. Cytotoxic aromatic triterpenes from Maytenus ilicifolia and Maytenus chuchuhuasca. J. Nat. Prod. 1994, 57, 1675–1681. [Google Scholar] [CrossRef]
- González, A.G.; Alvarenga, N.L.; Rodríguez, F.; Ravelo, A.G.; Jiménez, I.A.; Bazzocchi, I.L.; Gupta, M.P. New phenolic and quinone-methide triterpenes from Maytenus species (Celastraceae). Nat. Prod. Lett. 1995, 7, 209–218. [Google Scholar] [CrossRef]
- Ankli, A.; Heilmann, J.; Heinrich, M.; Sticher, O. Cytotoxic cardenolides and antibacterial terpenoids from Crossopetalum gaumeri. Phytochemistry 2000, 54, 531–537. [Google Scholar] [CrossRef]
- Gamlath, C.; Gunaherath, K.B.; Gunatilaka, A.A.L. Studies on terpenoids and steroids. Part 10. Structures of four new natural phenolic D:A-friedo-24-noroleanane triterpenoids. J. Chem. Soc. Perkin Trans. I 1987, 2849–2853. [Google Scholar] [CrossRef]
- Kamal, G.M.; Gunaherath, K.B.; Gunatilaka, A.A.L. Studies on terpenoids and steroids. Part 10. Structures of four new natural phenolic D:A-friedo-24-noroleanane triterpenoids. J. Chem. Soc. Perkin Trans. I 1983, 2845–2850. [Google Scholar] [CrossRef]
- Gunatilaka, A.A.L.; Wimalasiri, W.R. Studies on terpenoids and steroids. Part 22. Structure and some reactions of pristimerin leucotriacetate. J. Chem. Res. S 1992, 1, 30–31. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility test for Bacteria that Grow Aerobically, 9th ed.; Approved standard M07-A9; Clinical and Laboratory Standards: Wayne, PA, USA, 2012. [Google Scholar]
- Barzic, A.I.; Ioan, S. Antibacterial drugs-From basic concepts to complex therapeutic mechanisms of polymer systems. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; Science, Technology and Medicine: London, UK, 2015. [Google Scholar]
- Software-Predicted Lipophilicity of the Compounds was Calculated with the ALOGPS 2.1 Program. Available online: www.vcclab.org/lab/ alogps/ accessible via internet on-line Lipophilicity/Aqueous Solubility Calculation Software. (accessed on 20 May 2019).

| Compound | S. a. | S. e. | S. s. | E. f. | B. s. | B. a. | B. c. | B. m. | B. p. | clog P | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | >40 | >40 | >40 | >40 | 40–20 | >40 | 40–20 | 40 | >40 | 5.43 | |
| 3 | >40 | >40 | >40 | >40 | 40–20 | >40 | 40 | >40 | >40 | 6. 41 | |
| 5 | >40 | >40 | >40 | >40 | 40–20 | >40 | >40 | >40 | >40 | 5.26 | |
| 6 | >40 | 5–2.5 | >40 | 20–10 | 1 | 20–10 | 2.5 | 10–5 | 10–5 | 5.95 | |
| 10 | >40 | >40 | >40 | >40 | 8–4 | - | >40 | - | - | 5.42 | [21] |
| 12 | 40 | 20 | >40 | >40 | 10 | 7 | 12 | 20 | 25 | 5.67 | [22] |
| 13 | 25 | 20 | 40 | >40 | 13 | >40 | 20 | >40 | 10 | 5.04 | [22] |
| 14 | 6 | >40 | >40 | >40 | 3 | 4 | 5 | 4 | 6 | 5.21 | [23] |
| 15 | >40 | >40 | >40 | >40 | 13 | >40 | 30 | >40 | >40 | 4.67 | [23] |
| 16 | >40 | >40 | >40 | >40 | 14–12 | - | >40 | >40 | >40 | 5.58 | [20] |
| 17 | >40 | >40 | >40 | >40 | 39–35 | - | >40 | >40 | >40 | 6.20 | [20] |
| 20 | >40 | >40 | >40 | >40 | 25 | - | 40 | >40 | >40 | 6.86 | [20] |
| 22 | 30 | >40 | >40 | - | 8–4 | - | 10 | - | - | 5.79 | [21] |
| 24 | >40 | 0.625 | 10–5 | >40 | 1–0.5 | 5–2.5 | 2.5 | 20–10 | 5–2.5 | 7.09 | [21] |
| 25 | >40 | 20–10 | >40 | >40 | 10–5 | 40–20 | 20–10 | >40 | 20–10 | 6.41 | [21] |
| 26 | >40 | 2 | 20–10 | >40 | 5–2.5 | 10–5 | 10–5 | 40–20 | >40 | 6.41 | [21] |
| Control 3 | 2.5 | 2.5 | 0.6 | >20 | 8 | - | 10 | - | >20 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moujir, L.; R. López, M.; P. Reyes, C.; Jiménez, I.A.; L. Bazzocchi, I. Structural Requirements for Antimicrobial Activity of Phenolic Nor-Triterpenes from Celastraceae Species. Appl. Sci. 2019, 9, 2957. https://doi.org/10.3390/app9152957
Moujir L, R. López M, P. Reyes C, Jiménez IA, L. Bazzocchi I. Structural Requirements for Antimicrobial Activity of Phenolic Nor-Triterpenes from Celastraceae Species. Applied Sciences. 2019; 9(15):2957. https://doi.org/10.3390/app9152957
Chicago/Turabian StyleMoujir, Laila, Manuel R. López, Carolina P. Reyes, Ignacio A. Jiménez, and Isabel L. Bazzocchi. 2019. "Structural Requirements for Antimicrobial Activity of Phenolic Nor-Triterpenes from Celastraceae Species" Applied Sciences 9, no. 15: 2957. https://doi.org/10.3390/app9152957
APA StyleMoujir, L., R. López, M., P. Reyes, C., Jiménez, I. A., & L. Bazzocchi, I. (2019). Structural Requirements for Antimicrobial Activity of Phenolic Nor-Triterpenes from Celastraceae Species. Applied Sciences, 9(15), 2957. https://doi.org/10.3390/app9152957







