Chromium(VI) Removal by Polyvinyl Chloride (PVC)/Aliquat-336 Polymeric Inclusion Membranes in a Multiframe Flat Sheet Membrane Module
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Reagents
2.3. Membrane Preparation and Testing
2.4. Application of Design of Experiment (DOE)
3. Results and Discussion
3.1. Membrane Characterization
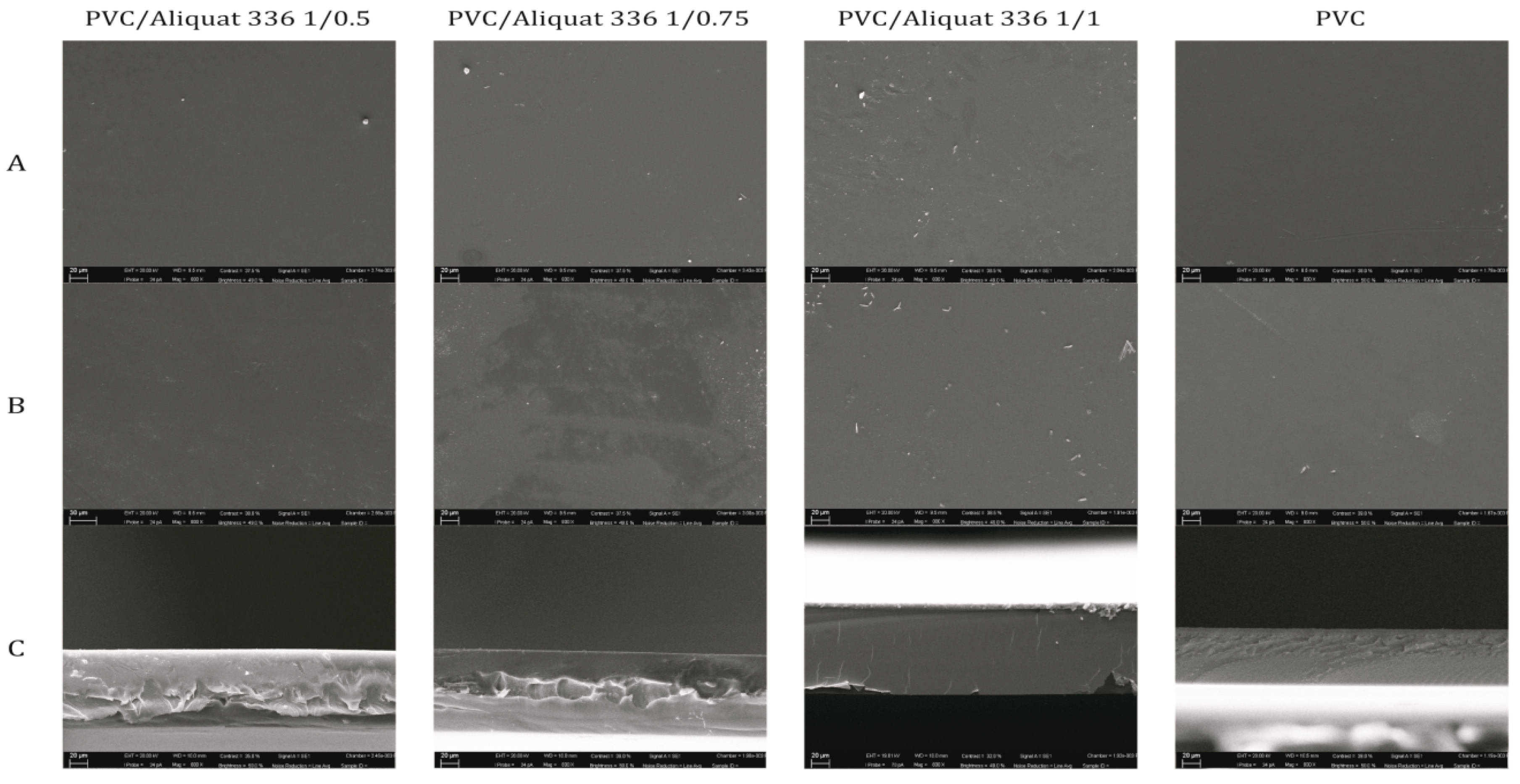
3.1.1. Scanning Electron Microscopy (SEM)
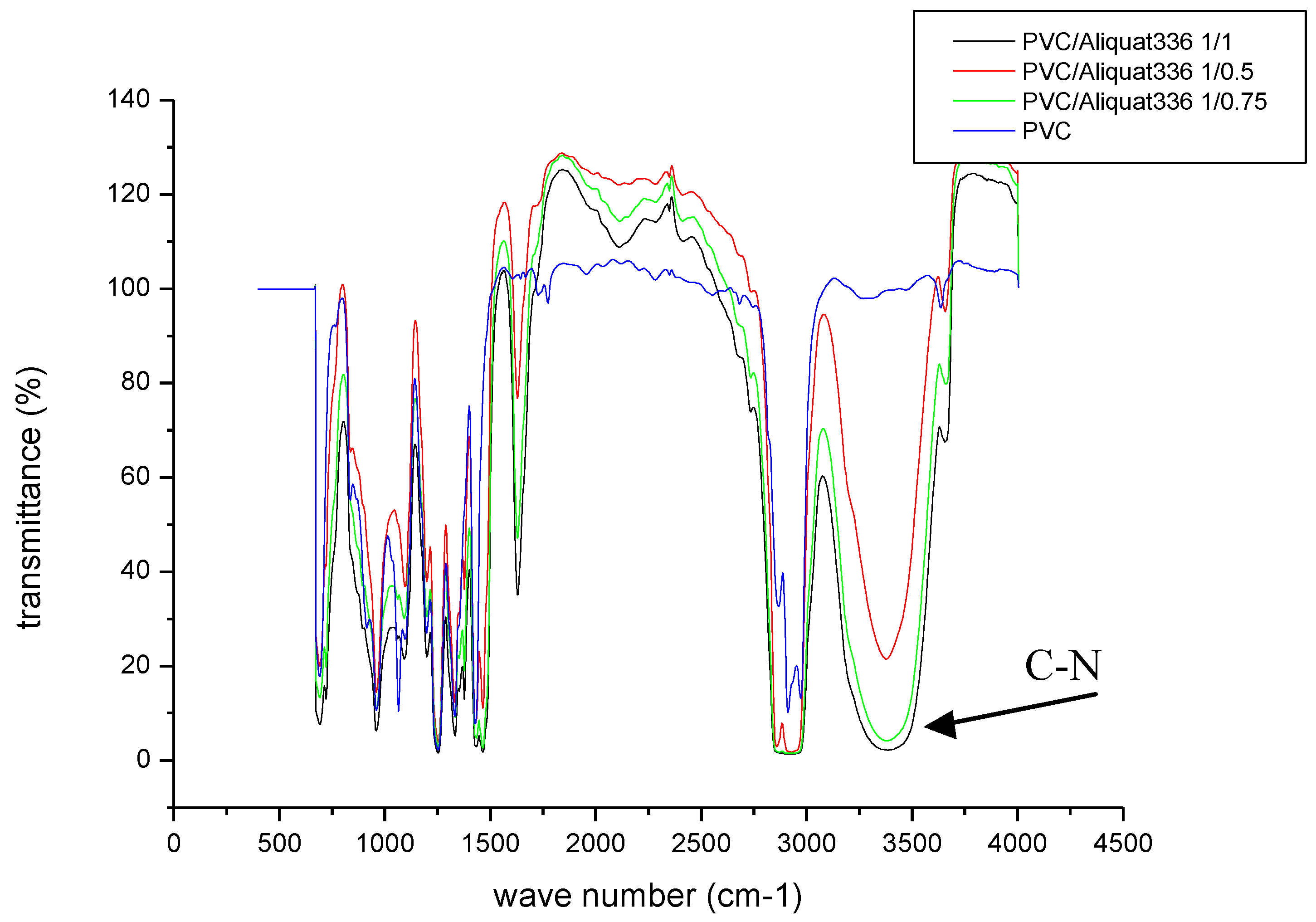
3.1.2. Infrared (IR) Spectrophotometry
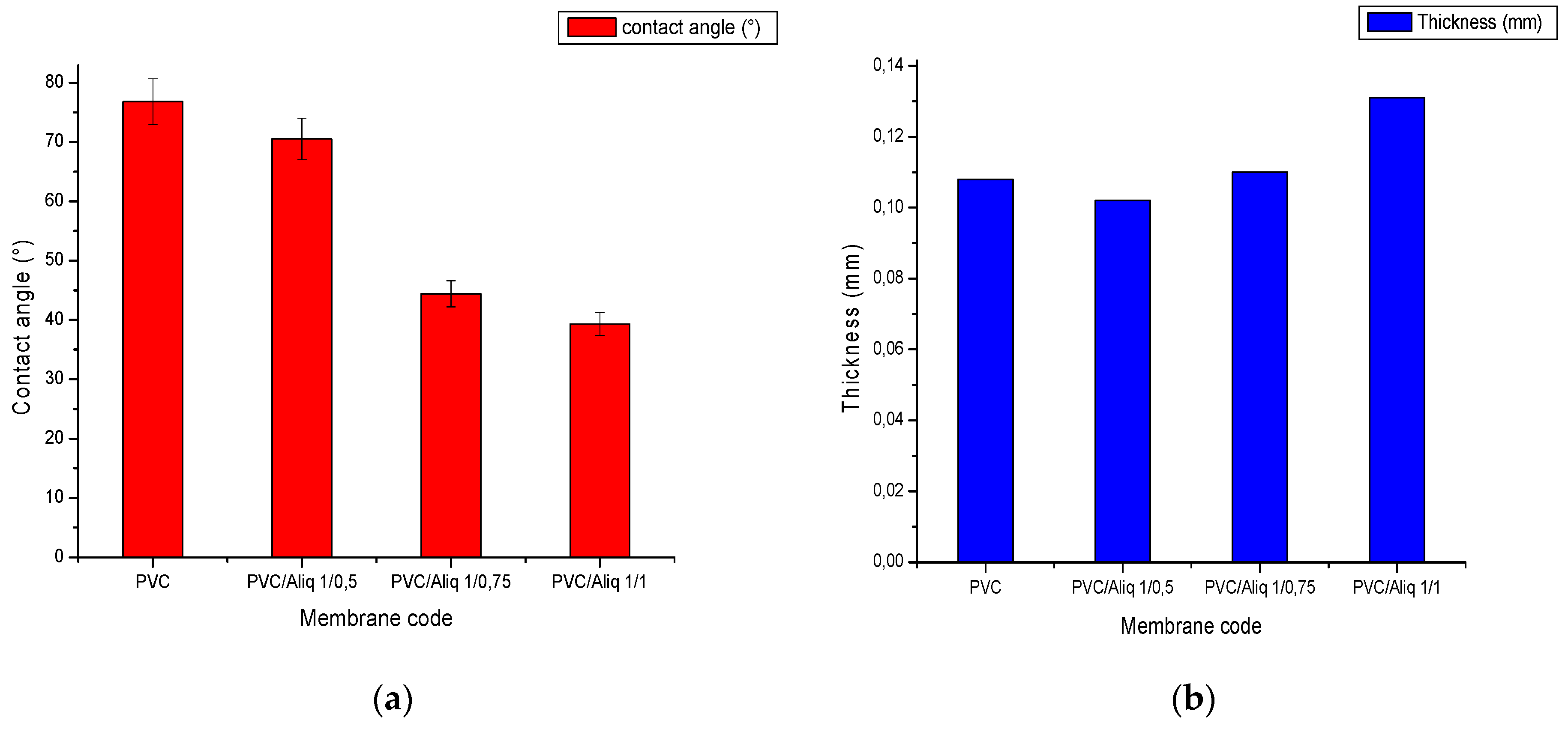
3.1.3. Membrane Thickness and Contact Angle
3.2. Design of Experiment (DOE)
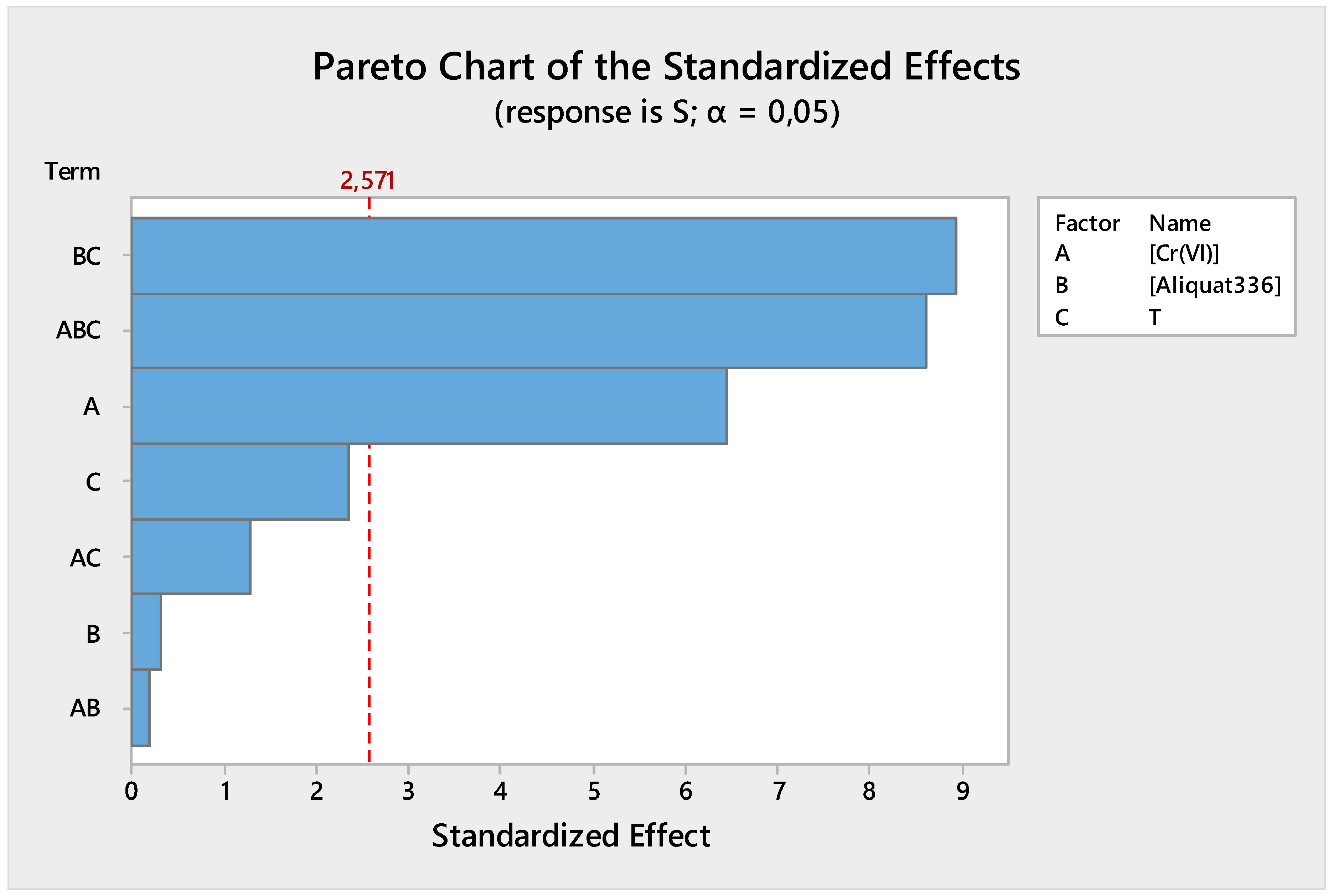
3.2.1. Pareto Chart
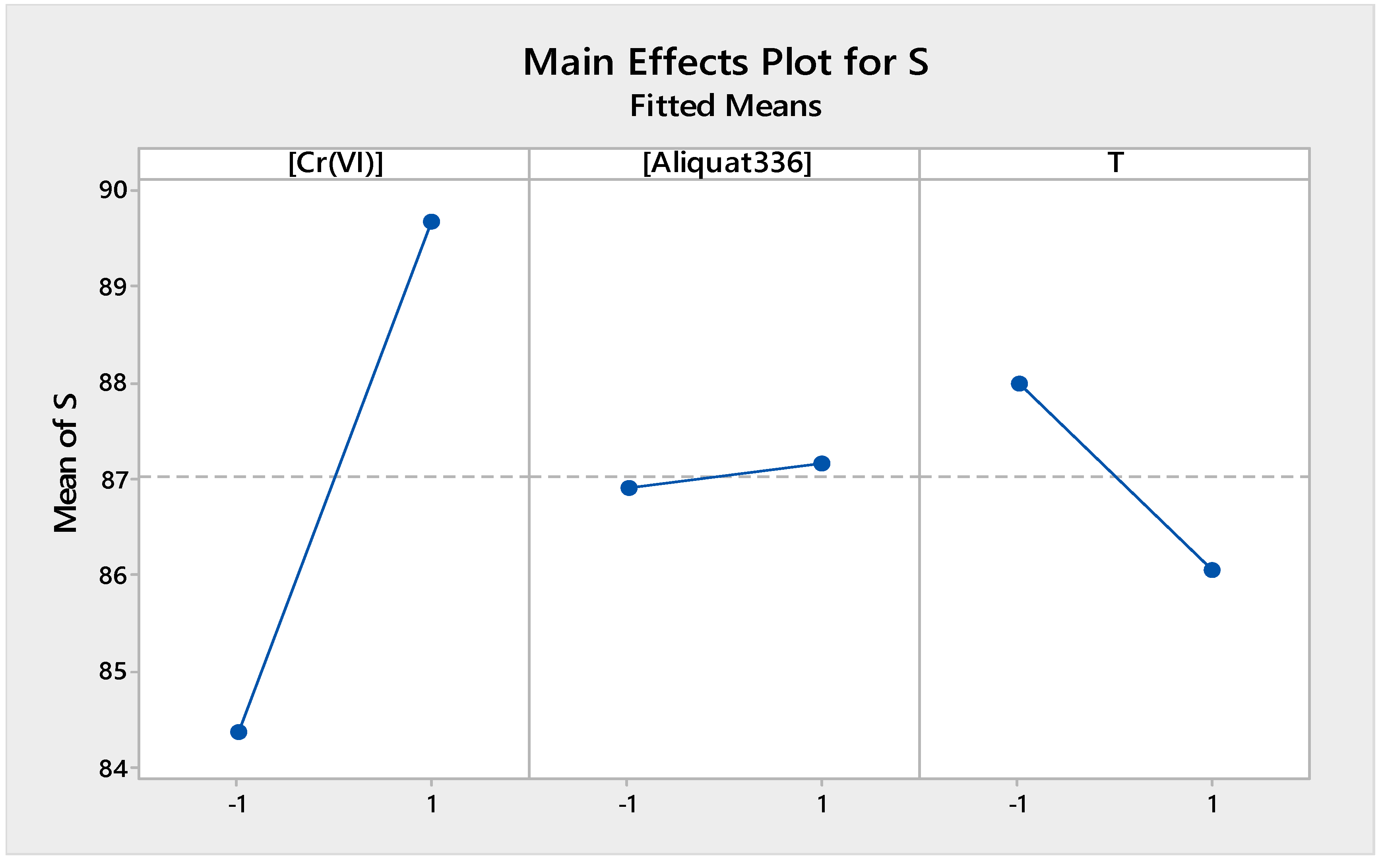
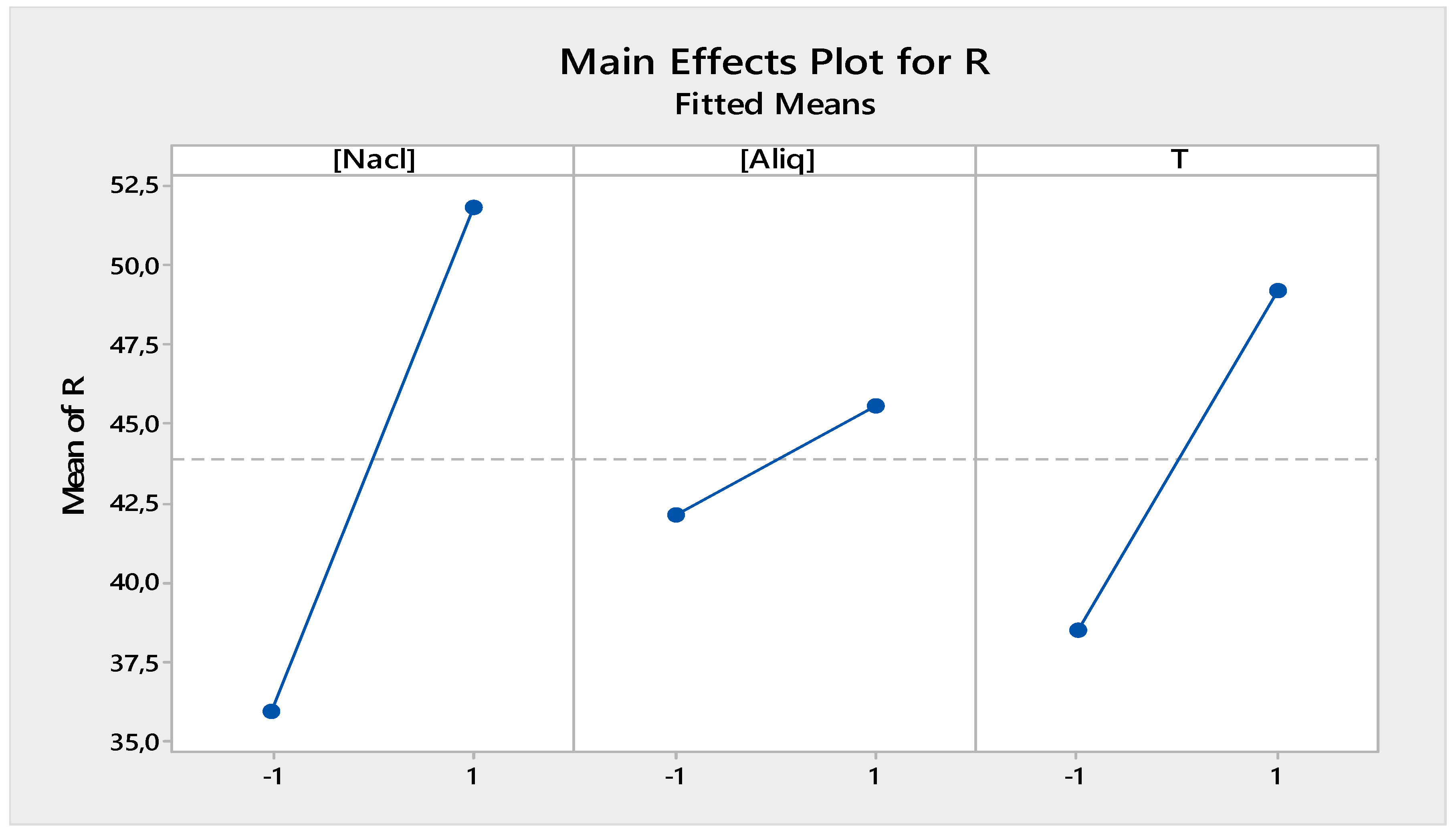
3.2.2. Main Effect Factors
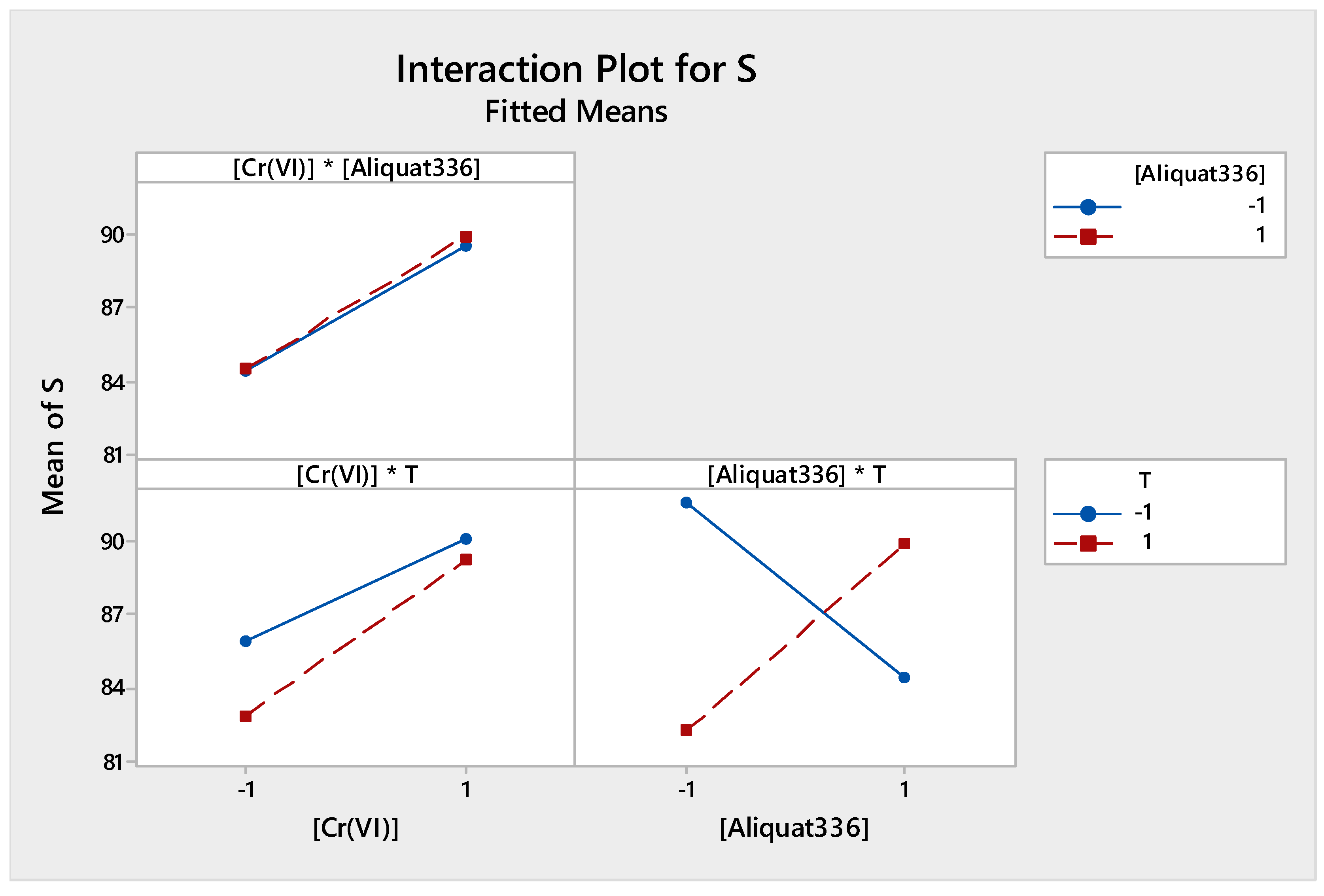
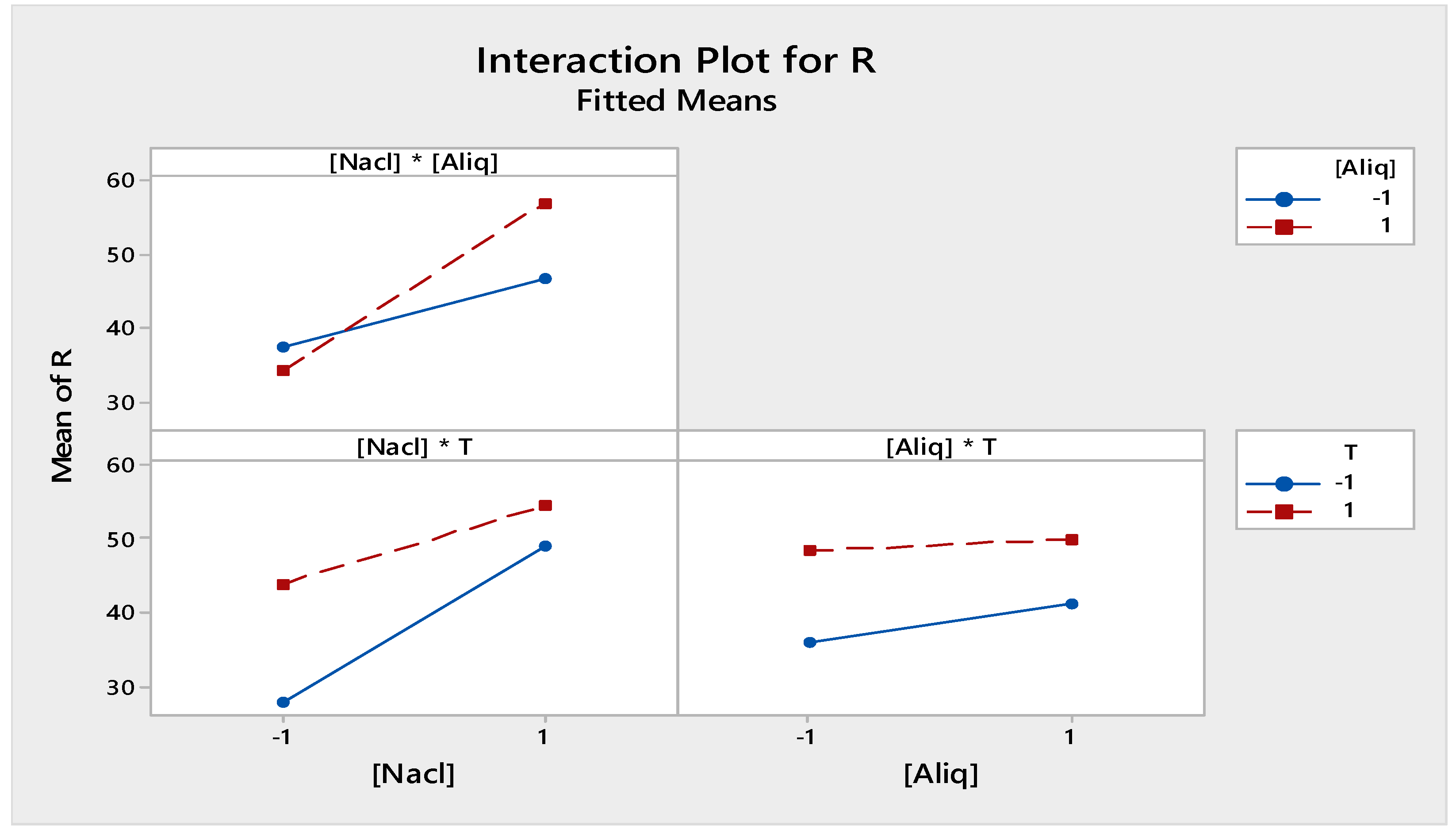
3.2.3. Interaction Effect Factors
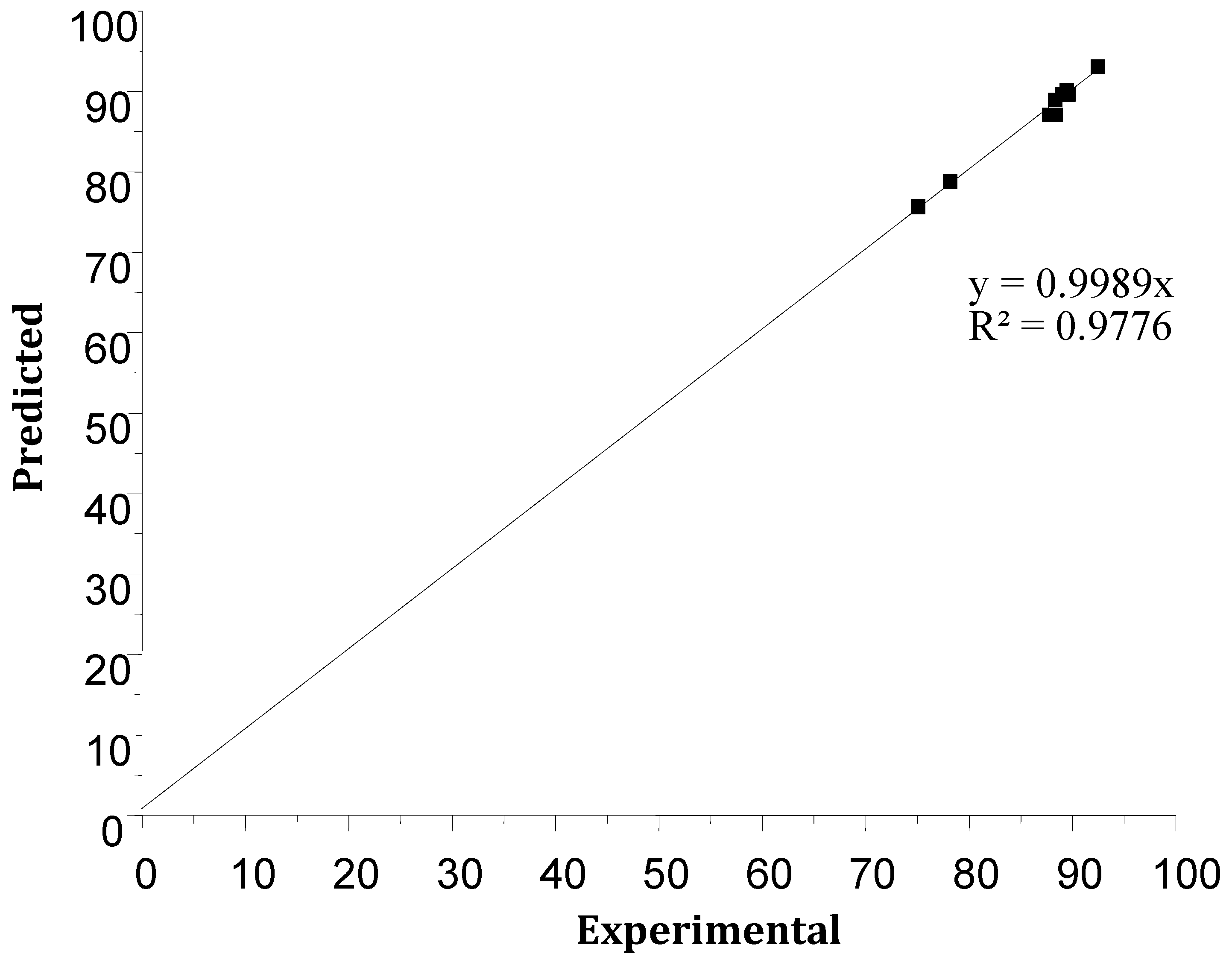
3.2.4. Mathematical Model and Analysis of Variance
Source Phase
Receiving Phase
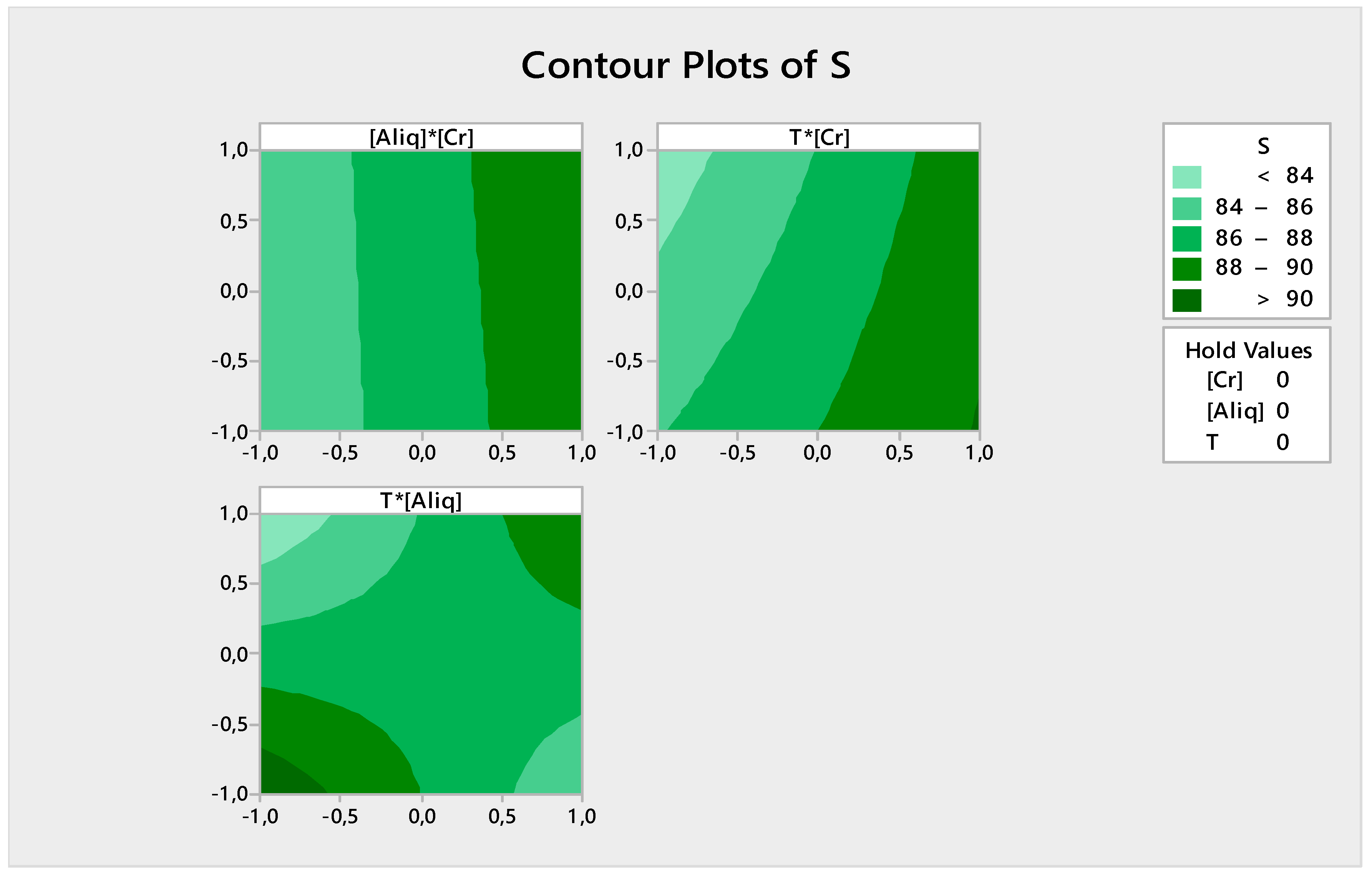
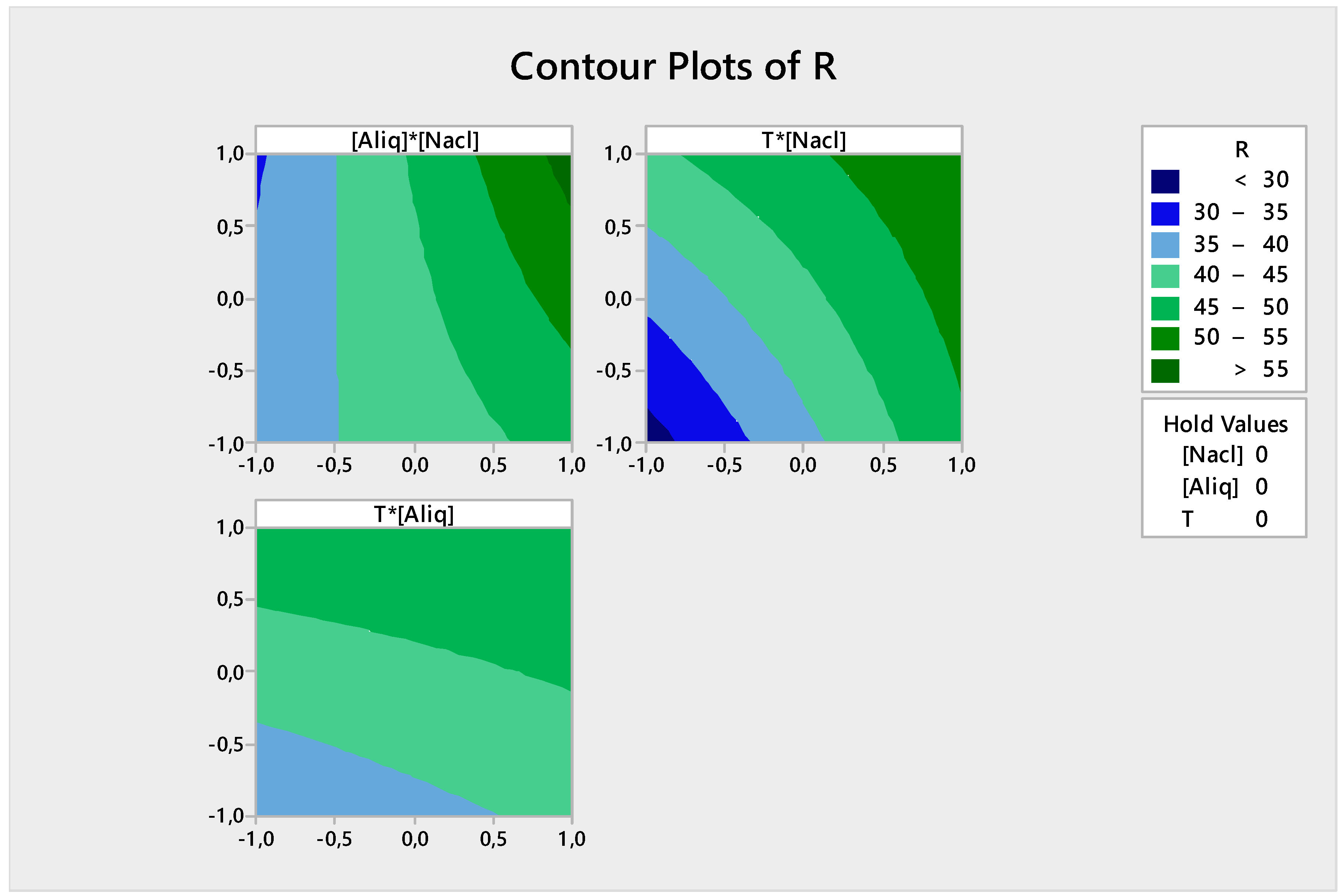
3.2.5. Response Contour
- Extraction is better at a high Cr(VI) concentration at whatever Aliquat-336 concentration;
- Extraction is high (>90%) at a high Cr(VI) concentration regardless of the temperature value; and
- The extraction efficiency is high (>90%) when the Aliquat-336 concentration and temperature are at their maximum or minimum values.
- The back-extraction efficiency of Cr(VI) is high (>55%) when both NaCl and Aliquat-336 concentrations are high;
- The back-extraction efficiency of Cr(VI) is >50% when the NaCl concentration and temperature reach their maximum values; and
- The back-extraction efficiency of Cr(VI) is >45% when the Aliquat-336 concentration and temperature are high.
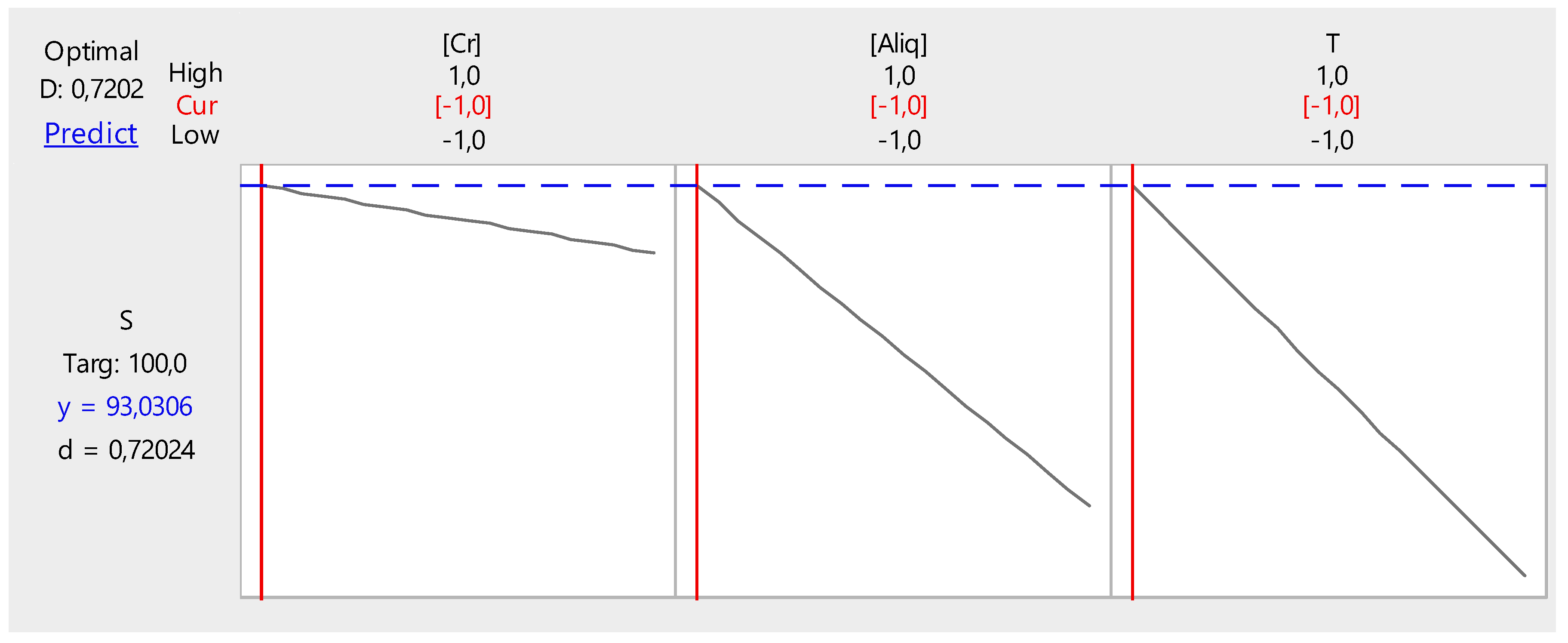
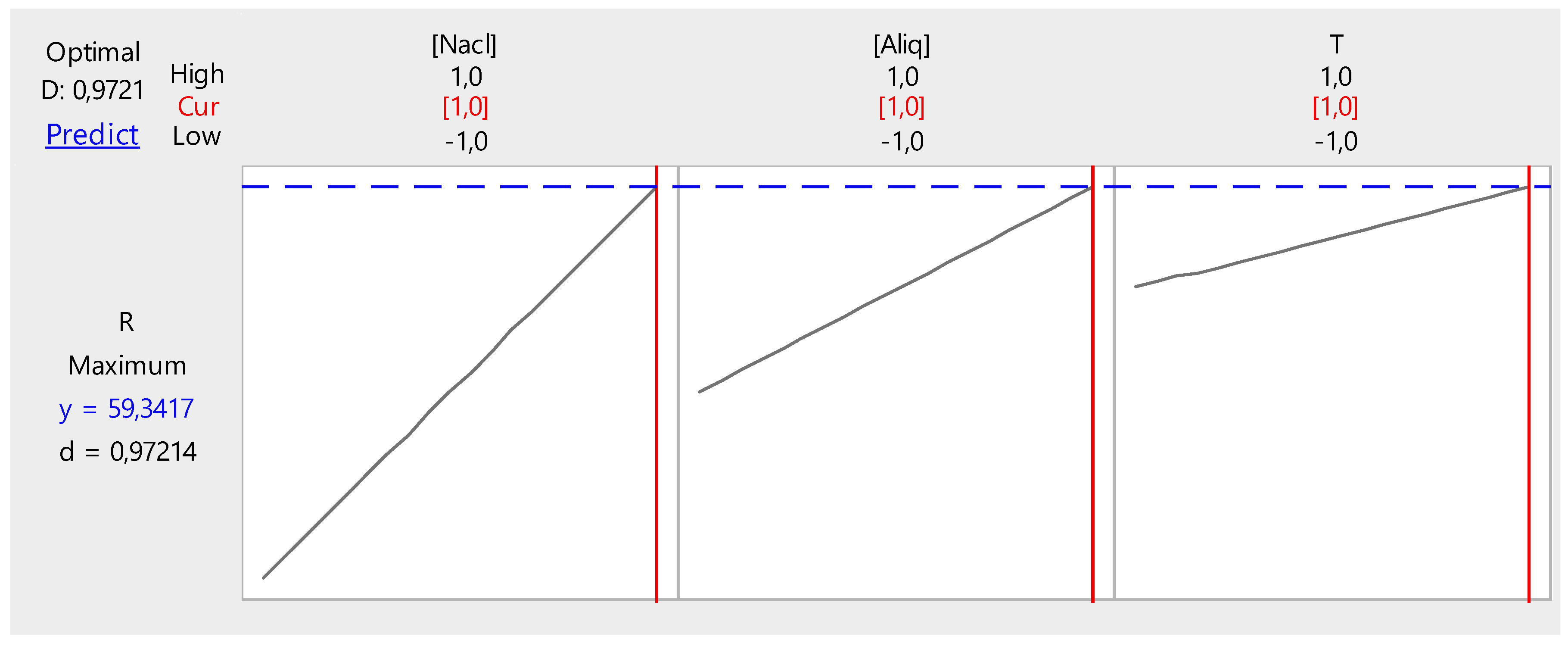
3.2.6. Optimization
- Cr(VI) concentration, 1.0 ppm;
- Temperature, 20 °C; and
- PVC/Aliquat-336 ratio, 1/0.5.
- NaCl concentration, 2 M;
- Temperature, 50 °C; and
- PVC/Aliquat-336 ratio, 1/1.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drioli, E.; Criscuoli, A.; Curcio, E. Membrane Contactors: Fundamentals, Applications and Potentialities, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–516. [Google Scholar]
- Bey, S.; Criscuoli, A.; Simone, S.; Figoli, A.; Benamor, M.; Drioli, E. Hydrophilic PEEK-WC hollow fibre membrane contactors for chromium (VI) removal. Desalination 2011, 283, 16–24. [Google Scholar] [CrossRef]
- Bey, S.; Criscuoli, A.; Figoli, A.; Leopold, A.; Simone, S.; Benamor, M.; Drioli, E. Removal of As(V) by PVDF hollow fibers membrane contactors using Aliquat-336 as extractant. Desalination 2010, 264, 193–200. [Google Scholar] [CrossRef]
- Aitali, S.; Kebiche-Senhadji, O.; Mansouri, L.; Benamor, M. Cationic dye (MB) removal using polymer inclusion membrane (PIMs). Procedia Eng. 2012, 33, 38–46. [Google Scholar] [Green Version]
- Yoshida, W.; Baba, Y.; Kubota, F.; Kolev, S.D.; Goto, M. Selective transport of scandium(III) across polymer inclusion membranes with improved stability which contain an amic acid carrier. J. Membr. Sci. 2019, 572, 291–299. [Google Scholar] [CrossRef]
- O’Rourke, M.; Cattrall, R.W.; Kolev, S.D.; Potter, I.D. The extraction and transport of organic molecules using polymer inclusion membrane. Solvent Extr. Res. Dev. 2009, 16, 1–12. [Google Scholar]
- Sellami, F.; Kebiche-Senhadji, O.; Marais, S.; Couvrat, N.; Fatyeyeva, K. Polymer inclusion membranes based on CTA/PBAT blend containing Aliquat-336 as extractant for removal of Cr(VI): Efficiency, stability and selectivity. J. React. Funct. Pol. 2019, 139, 120–132. [Google Scholar] [CrossRef]
- Bukhari, F.; Suah, M.; Ahmad, M. Preparation and characterization of polymer inclusion membrane based optode for determination of Al3+ ion. Anal. Chim. Acta 2017, 951, 133–139. [Google Scholar]
- Specht, C.; Cattrall, R.W.; Spassov, T.G.; Spassova, M.I.; Kolev, S.D. Polymer inclusion membranes as substrates for controlled in-situ gold nanoparticle synthesis. J. React. Funct. Pol. 2018, 130, 81–89. [Google Scholar] [CrossRef]
- Faye, M.C.; Denna, J.; Camitan, R.B.; Yabut, D.A.; Rivera, B.A.; Coo, L. Determination of Cu(II) in environmental water samples using polymer inclusion membrane-TAC optode in a continuous flow system. Sens. Act. B Chem. 2018, 260, 445–451. [Google Scholar]
- Vera, R.; Fontàs, C.; Galceran, J.; Serra, O.; Anticó, E. Polymer inclusion membrane to access Zn speciation: Comparison with root uptake. Sci. Total Environ. 2018, 622–623, 316–324. [Google Scholar] [CrossRef]
- Annane, K.; Sahmoune, A.; Montels, P.; Tingry, S. Polymer inclusion membrane extraction of cadmium(II) with Aliquat 336 in micro-channel cell. Chem. Eng. Res. Des. 2015, 94, 605–610. [Google Scholar] [CrossRef]
- Bukhari, F.; Suah, M. Preparation and characterization of a novel Co(II) optode based on polymer inclusion membrane. Anal. Chem. Res. 2017, 12, 40–46. [Google Scholar]
- Oberta, A.; Wasilewski, J.; Wódzki, R. Structure and transport properties of polymer inclusion membranes for Pb(II) separation. Desalination 2011, 271, 132–138. [Google Scholar] [CrossRef]
- Vera, R.; Insa, S.; Fontàs, C. Enriqueta Anticó. A new extraction phase based on a polymer inclusion membrane for the detection of chlorpyrifos, diazinon and cyprodinil in natural water samples. Talanta 2018, 185, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Elias, G.; Díez, S.; Fontàs, C. System for mercury preconcentration in natural waters based on a polymer inclusion membrane incorporating an ionic liquid. J. Hazard. Mater. 2019, 371, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Turgut, H.I.; Eyupoglu, V.; Kumbasar, R.A.; Sisman, I. Alkyl chain length dependent Cr(VI) transport by polymer inclusion membrane using room temperature ionic liquids as carrier and PVDF-co-HFP as polymer matrix. Sep. Purif. Technol. 2017, 175, 406–417. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Yang, F.; Zhao, Z.; Liao, Q.; Bai, R.; Guo, W.; Chen, P.; Zhang, Y.; Zhang, H. Promising transport and high-selective separation of Li(I) from Na(I) and K(I) by a functional polymer inclusion membrane (PIM) system. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membrane: concept and application. Procedia Eng. 2012, 44, 681–682. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415–416, 9–23. [Google Scholar] [CrossRef]
- Ling, Y.Y.; Suah, F.B.M. Extraction of malachite green from wastewater by using polymer inclusion membrane. J. Environ. Chem. Eng. 2017, 5, 785–794. [Google Scholar] [CrossRef]
- Meng, X.; Wang, C.; Ren, T.; Wang, L.; Wang, X. Electrodriven transport of chromium (VI) using 1-octanol/PVC in polymer inclusion membrane under low voltage. Chem. Eng. 2018, 346, 506–514. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Selective Extraction of Vanadium(V) from Sulfate Solutions into a Polymer Inclusion Membrane Composed of Poly(vinylidenefluoride-cohexafluoropropylene) and Cyphos IL 101. J. Membr. Sci. 2018, 545, 57–65. [Google Scholar] [CrossRef]
- Malankowska, M.; Juliana, I.; Pellejero, I.; Rho, H.S.; Schlautmann, S.; Tiggelaar, R.M.; Pina, M.P.; Gardeniers, H.J.G.E.; Mallada, R. Understanding blood oxygenation in a microfluidic meander double side membrane contactor. Sens. Act. B Chem. 2019, 288, 414–424. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier. Membranes 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Benavente, J.; Romero, V.; Vázquez, M.I.; Anticó, E.; Fontàs, C. Electrochemical Characterization of a Polymer Inclusion Membrane Made of Cellulose Triacetate and Aliquat 336 and Its Application to Sulfonamides Separation. Separations 2018, 5, 5. [Google Scholar] [CrossRef]
- Vera, R.; Anticó, E.; Fontàs, C. The Use of a Polymer Inclusion Membrane for Arsenate Determination in Groundwater. Water 2018, 10, 1093. [Google Scholar] [CrossRef]
- Witt, K.; Radzyminska-Lenarcik, E.; Kosciuszko, A.; Gierszewska, M.; Ziuziakowski, K. The Influence of the Morphology and Mechanical Properties of Polymer Inclusion Membranes (PIMs) on Zinc Ion Separation from Aqueous Solutions. Polymers 2018, 10, 134. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Liu, R.; She, Z.; Tan, M.; Mao, D.; Fu, R.; Zhang, Y. Polymer inclusion membrane (PIM) containing ionic liquid as a proton blocker to improve waste acid recovery efficiency in electrodialysis process. J. Membr. Sci. 2019, 581, 18–27. [Google Scholar] [CrossRef]
- Wionczyk, B.; Apostoluk, W.; Prochaska, K.; Kozlowski, C.A. Properties of 4-(10-n-tridecyl)pyridine N-oxide in the extraction and polymer inclusion membrane transport of Cr(VI). Anal. Chim. Acta 2001, 428, 89–101. [Google Scholar] [CrossRef]
- Scindia, Y.M.; Pandey, A.K.; Reddy, A.V.R. Coupled-diffusion transport of Cr(VI) across anion-exchange membranes prepared by physical and chemical immobilization methods. J. Membr. Sci. 2005, 249, 143–152. [Google Scholar] [CrossRef]
- Cezary, A.; Kozlowski, C.A.; Walkowiak, W. Applicability of liquid membranes in chromium(VI) transport with amines as ion carriers. J. Membr. Sci. 2005, 266, 143–150. [Google Scholar]
- Kebiche-Senhadji, O.; Mansouri, L.; Tingry, S.; Seta, P.; Benamor, M. Facilitated Cd(II) transport across CTA polymer inclusion membrane using anion (Aliquat 336) and cation (D2EHPA) metal carriers. J. Membr. Sci. 2008, 310, 438–445. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. J. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Jacyna, J.; Kordalewska, M.; Markuszewski, M.J. Design of Experiments in metabolomics-related studies: An overview. J. Pharm. Biom. Anal. 2019, 164, 598–606. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.M. Efficient online model-based design of experiments via parameter subset selection for batch dynamical systems. J. Comp. Chem. Eng. 2019, 121, 646–653. [Google Scholar] [CrossRef]
- Nasser, I.I.; Amor, F.I.E.H.; Donato, L.; Algieri, C.; Garofalo, A.; Drioli, E.; Ahmed, C. Removal and recovery of Ag(CN)-2 from synthetic electroplating baths by polymer inclusion membrane containing Aliquat 336 as a carrier. Chem. Eng. 2016, 295, 207–217. [Google Scholar] [CrossRef]
- Gherasim, C.-V.; Bourceanu, G.; Olariu, R.-I.; Arsene, C. A novel polymer inclusion membrane applied in chromium (VI) separation from aqueous solutions. J. Hazard. Mater. 2011, 197, 244–253. [Google Scholar] [CrossRef]
- Ranjbar, S.; Tanhaei, B.; Ayati, A.; Sillapää, M. Novel Aliquat-336 impregnated chitosan beads for adsoptive removal of of anionic azo dyes. Int. J. Biol. Macrom. 2019, 125, 989–998. [Google Scholar] [CrossRef]
- Vázquez, M.I.; Romero, V.; Fontàs, C.; Anticó, E.; Benavente, J. Polymer inclusion membranes (PIMs) with the ionic liquid (IL) Aliquat 336 as extractant: Effect of base polymer and IL concentration on their physical–chemical and elastic characteristics. J. Membr. Sci. 2014, 455, 312–319. [Google Scholar] [CrossRef]
- Anupam, K.; Dutta, S.; Bhattacharjee, C.; Datta, S. Adsorptive removal of chromium (VI) from aqueous solution over powdered activated carbon: Optimisation through response surface methodology. Chem. Eng. J. 2011, 173, 135–143. [Google Scholar] [CrossRef]
- Ben Khalifa, E.; Rzig, B.; Chakroun, R.; Nouagui, H.; Hamrouni, B. Application of response surface methodology for chromium removal by adsorption on low-cost biosorbent. Chem. Intell. Lab. Syst. 2019, 189, 18–26. [Google Scholar] [CrossRef]
- Rajasimman, M.; Karthic, P. Application of response surface methodology for the extraction of chromium (VI) by emulsion liquid membrane. J. Taiwan Inst. Chem. Eng. 2010, 41, 105–110. [Google Scholar] [CrossRef]
- Rajasimman, M.; Sangeetha, R. Optimization of process parameters for the extraction of chromium (VI) by emulsion liquid membrane using response surface methodology. J. Hazard. Mater. 2009, 168, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Hashim, M.A.; Nosrati, S.; Jayakumar, N.S. Extraction performance of chromium (VI) with emulsion liquid membrane by Cyanex 923 as carrier using response surface methodology. Desalination 2011, 266, 286–290. [Google Scholar]
- Mondal, S.K.; Saha, P. Separation of hexavalent chromium from industrial effluent through liquid membrane using environmentally benign solvent: A study of experimental optimization through response surface methodology. Chem. Eng. Res. Des. 2018, 132, 564–583. [Google Scholar] [CrossRef]
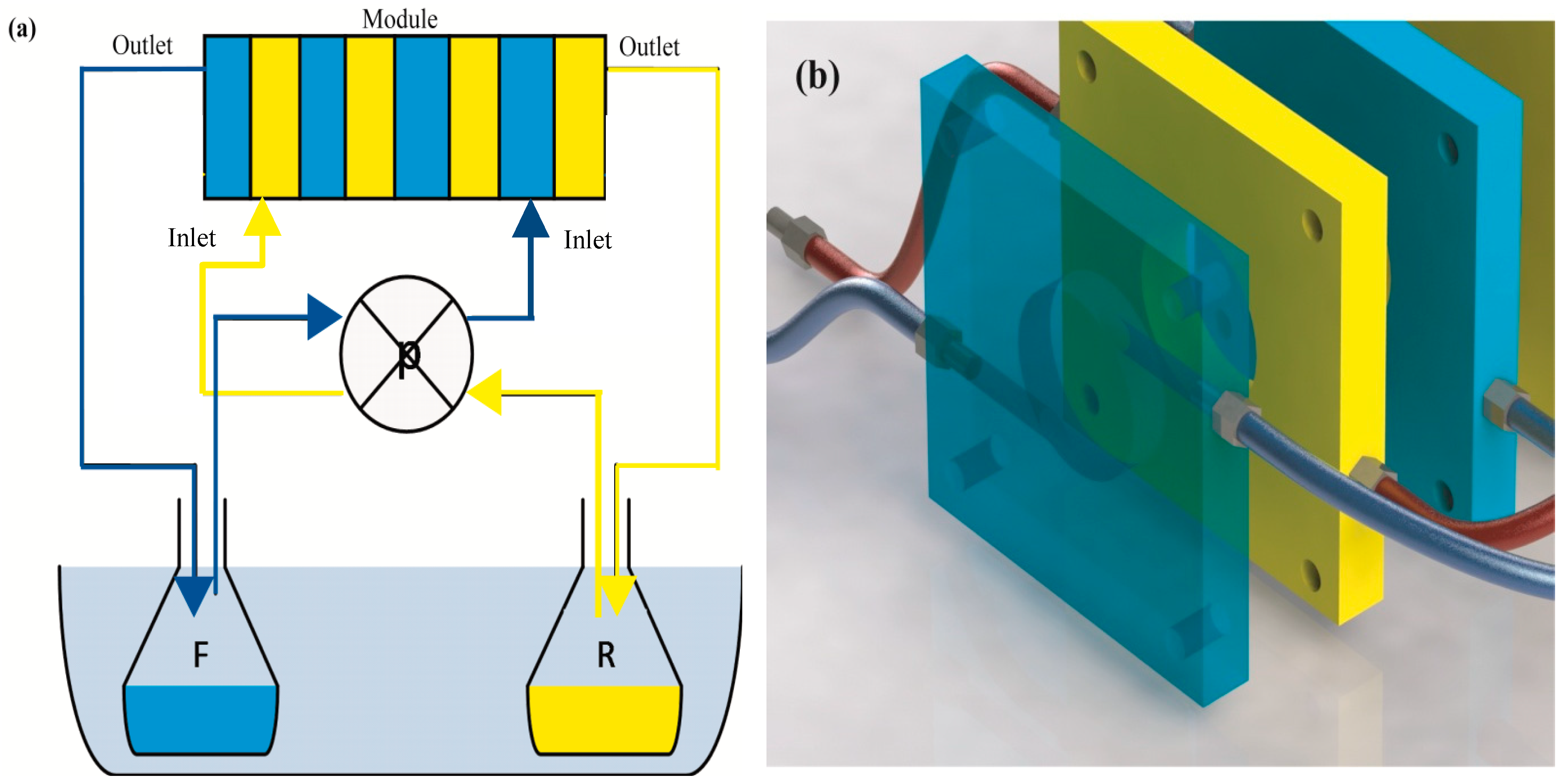


| Number of Frames | Number of Membranes | Type of Membranes | Feed Phase Composition | Receiving Phase Composition | ∆P (bar) | Temperature (°C) | Flow (mL/s) |
|---|---|---|---|---|---|---|---|
| 8 | 7 | Polyvinyl chloride (PVC)/Aliquat-336 | Cr(VI) | NaOH-NaCl | 0 | 20, 30, 50 | 0.3 |
| Factor | Names | Low Level | High Level |
|---|---|---|---|
| [Cr(VI)] (ppm) | A | 10 | 50 |
| Temperature (°C) | B | 20 | 50 |
| [Aliquat-336] (%) | C | 50 | 100 |
| Factor | Names | Low Level | High Level |
|---|---|---|---|
| Temperature (°C) | A | 20 | 50 |
| Aliquat-336 (%) | B | 50 | 100 |
| [NaCl] (M) | C | 1 | 2 |
| Run Order | Center Pt | [Cr(VI)] | [Aliquat-336] | T | Yield S Experimental (%) | Yield S Predicted (%) | Residuals |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 88.38 | 87.03 | 1.34 |
| 2 | 1 | −1 | −1 | 1 | 75.08 | 75.64 | −0.55 |
| 3 | 1 | 1 | 1 | 1 | 89.02 | 89.58 | −0.55 |
| 4 | 1 | −1 | −1 | −1 | 92.47 | 93.03 | −0.55 |
| 5 | 0 | 0 | 0 | 0 | 87.84 | 87.03 | 0.81 |
| 6 | 0 | 0 | 0 | 0 | 87.74 | 87.03 | 0.71 |
| 7 | 1 | 1 | 1 | −1 | 89.62 | 89.62 | 90.18 |
| 8 | 1 | −1 | 1 | 1 | 89.58 | 89.58 | 90.13 |
| 9 | 0 | 0 | 0 | 0 | 87.83 | 87.03 | 0.80 |
| 10 | 1 | 1 | −1 | −1 | 89.49 | 90.04 | −0.55 |
| 11 | 0 | 0 | 0 | 0 | 87.79 | 87.03 | 0.76 |
| 12 | 1 | 1 | −1 | 1 | 88.34 | 88.89 | −0.55 |
| 13 | 1 | −1 | 1 | −1 | 78.18 | 78.73 | −0.55 |
| Run Order | Center Pt | [NaCl] | [Aliquat-336] | T | Yield R Experimental (%) | Yield R Predicted (%) | Residuals |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 40.76 | 43.87 | −3.10 |
| 2 | 0 | 0 | 0 | 0 | 42.66 | 4387 | −1.20 |
| 3 | 1 | 1 | −1 | 1 | 50.48 | 49.60 | 0.87 |
| 4 | 0 | 0 | 0 | 0 | 43.17 | 43.87 | −0.70 |
| 5 | 1 | 1 | −1 | −1 | 44.68 | 43.80 | 0.87 |
| 6 | 1 | −1 | 1 | −1 | 28.71 | 27.84 | 0.87 |
| 7 | 0 | 0 | 0 | 0 | 42.86 | 43.87 | −1.0 |
| 8 | 1 | −1 | −1 | 1 | 48.08 | 47.20 | 0.87 |
| 9 | 1 | −1 | −1 | −1 | 28.88 | 28.00 | 0.87 |
| 10 | 1 | −1 | 1 | 1 | 41.53 | 40.65 | 0.87 |
| 11 | 0 | 0 | 0 | 0 | 42.87 | 43.87 | −1.0 |
| 12 | 1 | 1 | 1 | −1 | 55.40 | 54.52 | 0.87 |
| 13 | 1 | 1 | 1 | 1 | 60,21 | 59.34 | 0.87 |
| Term | Effect | Coefficient |
|---|---|---|
| Constant | 87.033 | |
| [Cr(VI)] | 5.289 | 2.645 |
| [Aliquat-336] | 0.257 | 0.129 |
| T | −1.935 | −0.967 |
| [Cr(VI)] * [Aliquat-336] | 0.155 | 0.077 |
| [Cr(VI)] * T | 1.059 | 0.530 |
| [Aliquat-336] * T | 7.333 | 3.667 |
| [Cr(VI)] * [Aliquat-336] * T | −7.060 | −3.530 |
| Source | DF 1 | Adj SS 2 | Adj MS 3 | F-Value | P-Value |
|---|---|---|---|---|---|
| Regression | 7 | 273.116 | 39.017 | 29.14 | 0.001 |
| [Cr(VI)] | 1 | 55.956 | 55.956 | 41.80 | 0.001 |
| [Aliquat-336] | 1 | 0.132 | 0.132 | 0.10 | 0.766 |
| T | 1 | 7.487 | 7.487 | 5.59 | 0.064 |
| [Cr(VI)] * [Aliquat-336] | 1 | 0.048 | 0.048 | 0.04 | 0.858 |
| [Cr(VI)] * T | 1 | 2.243 | 2.243 | 1.68 | 0.252 |
| [Aliquat-336] * T | 1 | 107.552 | 107.552 | 80.34 | 0.000 |
| [Cr(VI)] * [Aliquat-336] * T | 1 | 99.698 | 99.698 | 74.47 | 0.000 |
| Term | Effect | Coefficient |
|---|---|---|
| Constant | 43.872 | |
| [NaCl] | 15.891 | 7.945 |
| [Aliquat-336] | 3.435 | 1.717 |
| T | 10.658 | 5.329 |
| [NaCl] * [Aliquat-336] | 6.794 | 3.397 |
| [NaCl] * T | −5.348 | −2.674 |
| [Alqiaut336] * T | −1.842 | −0.921 |
| [NaCl] * [Aliquat-336] * T | 1.351 | 0.676 |
| Source | DF 1 | Adj SS 2 | Adj MS 3 | F-Value | P-Value |
|---|---|---|---|---|---|
| Regression | 7 | 915.747 | 130.821 | 33.08 | 0.001 |
| [NaCl] | 1 | 505.018 | 505.018 | 127.68 | 0.000 |
| [Aliquat-336] | 1 | 23.597 | 23.597 | 5.97 | 0.058 |
| T | 1 | 227.185 | 227.185 | 57.44 | 0.001 |
| [NaCl] * [Aliquat-336] | 1 | 92.314 | 92.314 | 23.34 | 0.005 |
| [NaCl] * T | 1 | 57.195 | 57.195 | 14.46 | 0.013 |
| [Aliquat-336] * T | 1 | 6.787 | 6.787 | 1.72 | 0.247 |
| [NaCl] * [Aliquat-336] * T | 1 | 3.561 | 3.651 | 0.92 | 0.381 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semghouni, H.; Bey, S.; Figoli, A.; Criscuoli, A.; Russo, F.; Mohamed, B.; Drioli, E. Chromium(VI) Removal by Polyvinyl Chloride (PVC)/Aliquat-336 Polymeric Inclusion Membranes in a Multiframe Flat Sheet Membrane Module. Appl. Sci. 2019, 9, 2994. https://doi.org/10.3390/app9152994
Semghouni H, Bey S, Figoli A, Criscuoli A, Russo F, Mohamed B, Drioli E. Chromium(VI) Removal by Polyvinyl Chloride (PVC)/Aliquat-336 Polymeric Inclusion Membranes in a Multiframe Flat Sheet Membrane Module. Applied Sciences. 2019; 9(15):2994. https://doi.org/10.3390/app9152994
Chicago/Turabian StyleSemghouni, Hassina, Said Bey, Alberto Figoli, Alessandra Criscuoli, Francesca Russo, Benamor Mohamed, and Enrico Drioli. 2019. "Chromium(VI) Removal by Polyvinyl Chloride (PVC)/Aliquat-336 Polymeric Inclusion Membranes in a Multiframe Flat Sheet Membrane Module" Applied Sciences 9, no. 15: 2994. https://doi.org/10.3390/app9152994







