Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification
Abstract
:1. Introduction
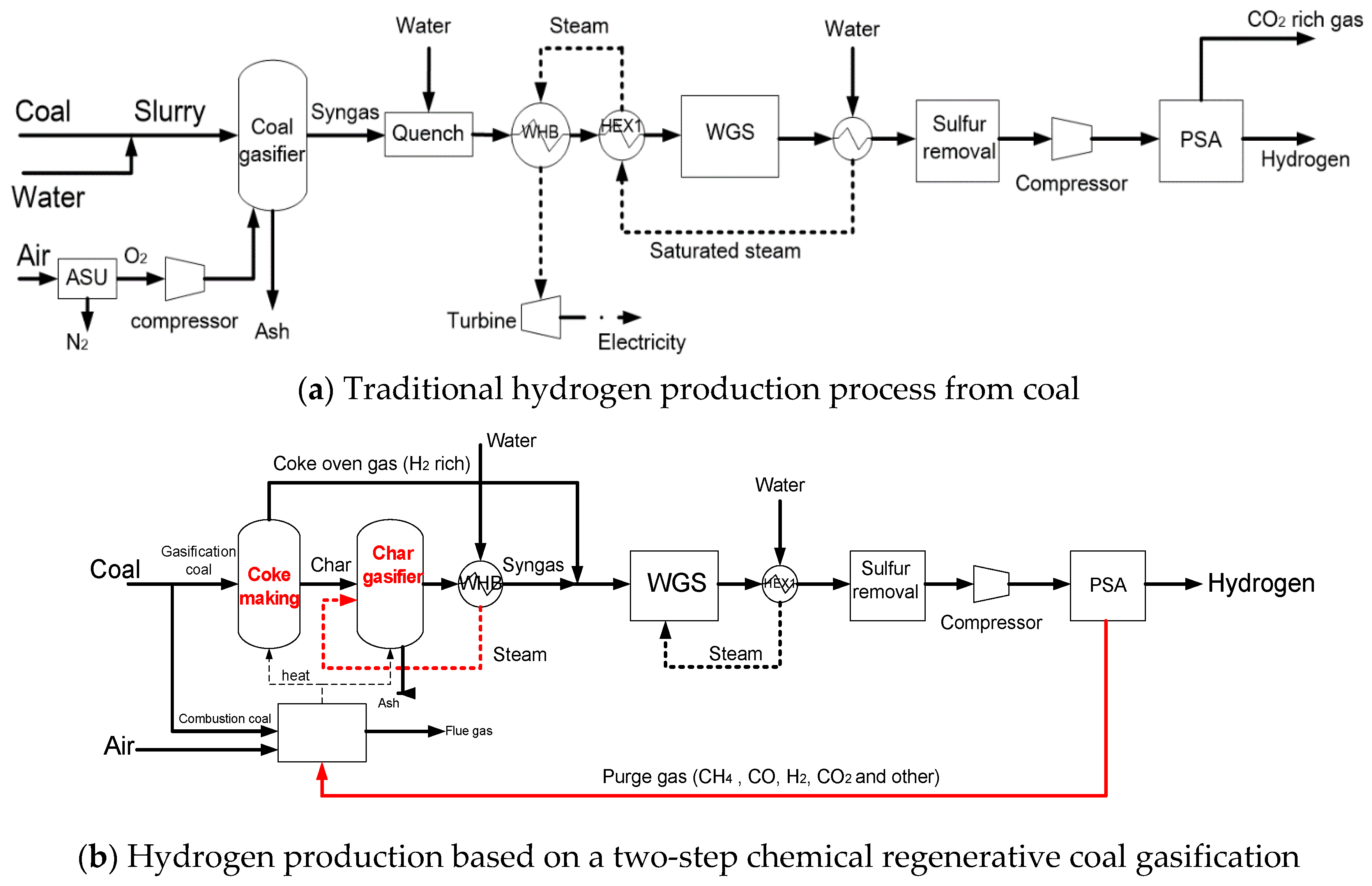
2. Hydrogen Production Based on a Two-Step Chemical Regenerative Coal Gasification
3. Experimental Setup and Results
3.1. Experimental Setup
3.2. Typical Results of Char Making
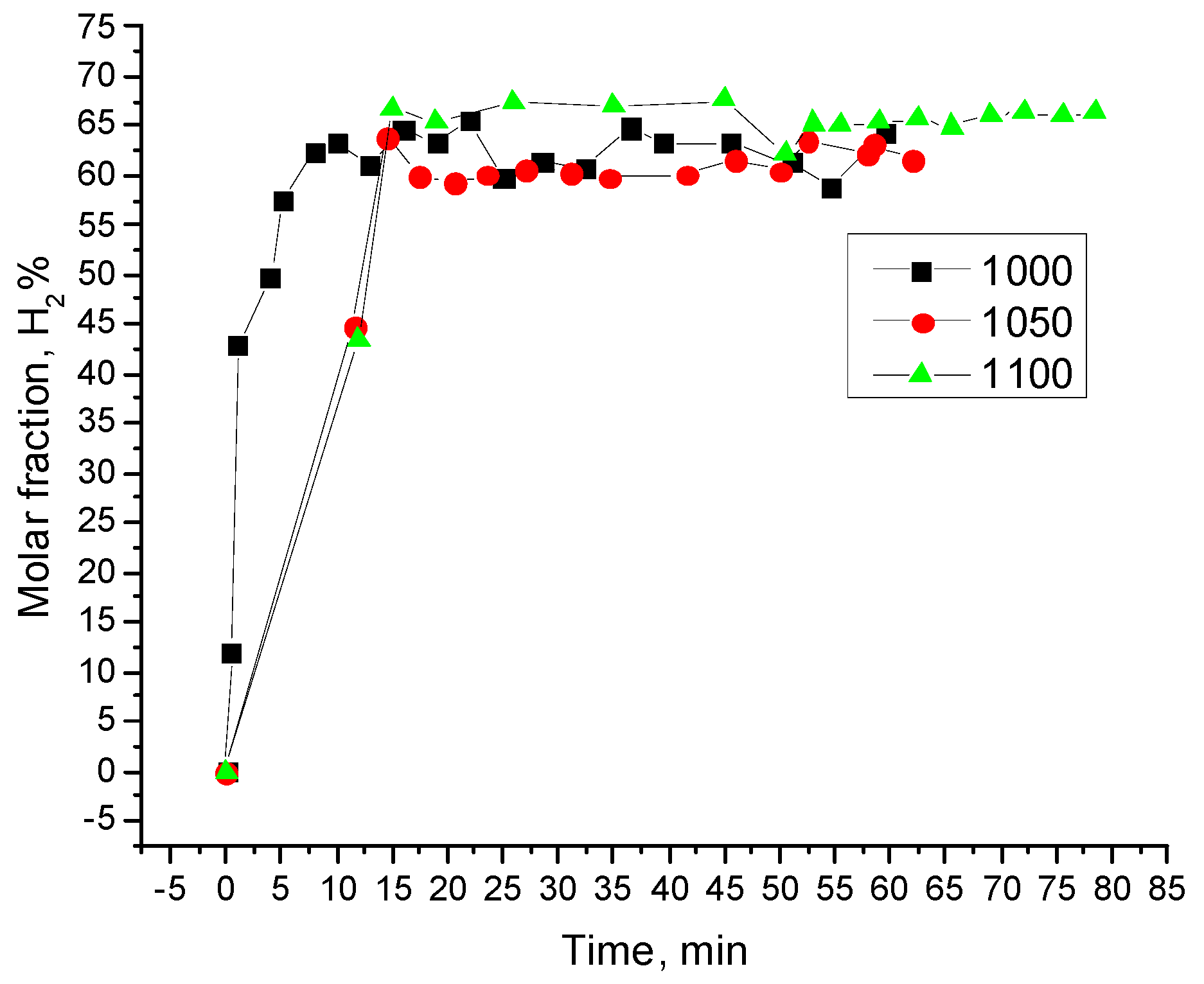
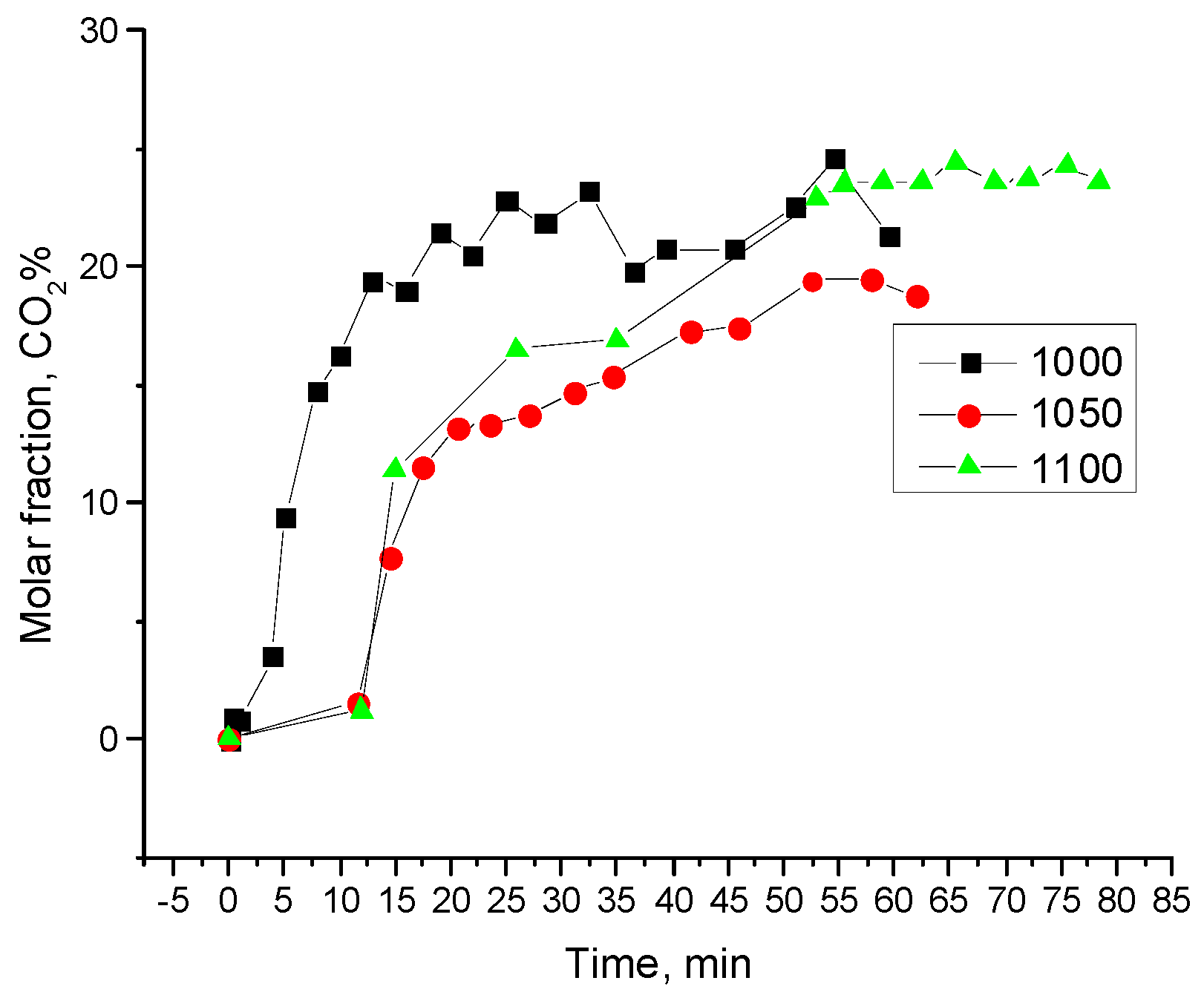
3.3. Typical Results of Char-Steam Gasification
4. Thermodynamic Performance Comparison and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vaiano, V.; Iervolino, G. Photocatalytic Hydrogen Production from Glycerol Aqueous Solution Using Cu-Doped ZnO under Visible Light Irradiation. Appl. Sci. 2019, 9, 2741. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen. Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Herdem, M.S.; Farhad, S.; Dincer, I.; Hamdullahpur, F. Thermodynamic modeling and assessment of a combined coal gasification and alkaline water electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 3061–3071. [Google Scholar] [CrossRef]
- Jin, H.; Chen, Y.; Ge, Z.; Liu, S.; Ren, C.; Guo, L. Hydrogen production by Zhundong coal gasification in supercritical water. Int. J. Hydrogen Energy 2015, 40, 16096–16103. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Irfan, M.F.; Usman, M.R.; Kusakabe, K. Coal gasification in CO2 atmosphere and its kinetics since 1948: A brief review. Energy 2011, 36, 12–40. [Google Scholar] [CrossRef]
- Nicoletti, G. The hydrogen option for energy: A review of technical, environmental and economic aspects. Int. J. Hydrogen Energy 1995, 20, 759–765. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield-A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Jiao, Y.; Wang, X. Gasification Kinetics of Bituminous Coal Char in the Mixture of CO2, H2O, CO, and H2. Energies 2019, 12, 496. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J. From coal to biomass gasification: Comparison of thermodynamic efficiency. Energy 2007, 32, 1248–1259. [Google Scholar] [CrossRef]
- Seyitoglu, S.S.; Dincer, I.; Kilicarslan, A. Energy and exergy analyses of hydrogen production by coal gasification. Int. J. Hydrogen Energy 2017, 42, 2592–2600. [Google Scholar] [CrossRef]
- Bargigli, S.; Raugei, M.; Ulgiati, S. Comparison of thermodynamic and environmental indexes of natural gas, syngas and hydrogen production processes. Energy 2004, 29, 2145–2159. [Google Scholar] [CrossRef]
- Zhuang, Q.; Biondi, M.; Yan, S.; Bhagat, K.; Vansickle, R.; Chen, C.; Tan, H.; Zhu, Y.; You, W.; Xia, W. TRIG™: An advanced gasification technology to utilize low rank coals for power. Fuel 2015, 152, 103–109. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Xu, S.; Paterson, N.; Dugwell, D.R.; Kandiyoti, R. Performance of Chinese Coals under Conditions Simulating Entrained-Flow Gasification. Energy Fuels 2005, 195, 2006–2013. [Google Scholar] [CrossRef]
- Jin, H.; Lu, Y.; Liao, B.; Guo, L.; Zhang, X. Hydrogen production by coal gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2010, 35, 7151–7160. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; Zhang, X.; Jin, H.; Lu, Y. Hydrogen production from coal gasification in supercritical water with a continuous flowing system. Int. J. Hydrogen Energy 2010, 35, 3036–3045. [Google Scholar] [CrossRef]
- Guo, L.; Jin, H. Boiling coal in water: Hydrogen production and power generation system with zero net CO2 emission based on coal and supercritical water gasification. Int. J. Hydrogen Energy 2013, 38, 12953–12967. [Google Scholar] [CrossRef]
- Yilmaz, F.; Ozturk, M.; Selbas, R. Design and thermodynamic analysis of coal-gasification assisted multigeneration system with hydrogen production and liquefaction. Energy Convers. Manag. 2019, 186, 229–240. [Google Scholar] [CrossRef]
- Bai, L.; Kudo, S.; Norinaga, K.; Wang, Y.G.; Hayashi, J.I. Kinetics and Mechanism of Steam Gasification of Char from Hydrothermally Treated Woody Biomass. Energy Fuels 2014, 28, 7133–7139. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Zhou, H.; Gao, Y.; Xu, D.; Bai, L.; Zhang, S. Effects of Water Content and Particle Size on Yield and Reactivity of Lignite Chars Derived from Pyrolysis and Gasification. Molecules 2018, 23, 2717. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Qiao, Y.; Bai, L.; Liu, F.; Tian, Y.; Xie, K. Separation and structural characterization of groups from a high-volatile bituminous coal based on multiple techniques. Fuel Process. Technol. 2017, 159, 386–395. [Google Scholar] [CrossRef]
- Tian, B.; Qiao, Y.; Fan, J.; Bai, L.; Tian, Y. Coupling Pyrolysis and Gasification Processes for Methane-Rich Syngas Production: Fundamental Studies on Pyrolysis Behavior and Kinetics of a Calcium-Rich High-Volatile Bituminous Coal. Energy Fuels 2017, 31, 10665–10673. [Google Scholar] [CrossRef]
- Wang, D.; Li, S.; He, S.; Gao, L. Coal to substitute natural gas based on combined coal-steam gasification and one-step methanation. Appl. Energy 2019, 240, 851–859. [Google Scholar] [CrossRef]


| C | H | O | N | S | ASH | LHV, KJ/KG |
|---|---|---|---|---|---|---|
| 74.3% | 4.7% | 10.7% | 0.87% | 0.33% | 9.1% | 26,835.8 |
| Component | COG |
|---|---|
| CO | 21.9% |
| H2 | 51.7% |
| O2 | 1.7% |
| N2 | 9.0% |
| CH4 | 11.6% |
| H2O | - |
| H2S | 0.1% |
| CO2 | 3.5% |
| C2H4 | 0.5% |
| Volume Per kg Coal | 563.85 L |
| Component | Char |
|---|---|
| C | 94.94% |
| H | 0.55% |
| O | 0.39% |
| N | - |
| S | - |
| Ash | 4.12% |
| Char Yield, kg Per kg Coal | 59.33% |
| Item | Hydrogen Production Based on Chemical Regenerative Gasification | Hydrogen Production Based on GE Gasification | ||
|---|---|---|---|---|
| Total System Input | ||||
| Energy Input, kJ | ||||
| Coal input for gasification (including to gasifier and combustion unit) | 25,842.9 | 96.3% | 24,071.7 | 89.7% |
| Coal input for auxiliary power generation | 992.9 | 3.7% | 2764.1 | 10.3% |
| subtotal | 26,835.8 | 100.0% | 26,835.8 | 100.0% |
| Gasification Island | ||||
| Energy Input, kJ | ||||
| Coal input for gasification | 25,842.9 | - | 24,071.7 | - |
| LHV of purge gas recovered from PSA | 4588.9 | - | - | - |
| Subtotal | 30,431.8 | 24,071.7 | ||
| Gas output from gasifier and COG, kJ | 25,333.0 | 18,677.7 | - | |
| Cold gas efficiency of gasification | 83.2% | 77.6% | ||
| WGS and PSA Unit | ||||
| Energy Output, kJ | ||||
| Hydrogen | 19,214.4 | 15,806.3 | ||
| LHV of purge gas | 4588.9 | - | ||
| Coal to H2 Efficiency | ||||
| Coal to H2 efficiency | 71.6% | 58.9% | ||
| Energy consumption for unit hydrogen production, MJ/kg | 151.2 | 183.8 | ||
| Item | Hydrogen Production Based on Chemical Regenerative Gasification | Hydrogen Production Based on GE Gasification |
|---|---|---|
| Power Produced, kJ | ||
| Power from auxiliary power generation | 397.2 | 1105.6 |
| Power from heat recovered | - | 358.4 |
| subtotal | 397.2 | 1464.0 |
| Power Consumption in Each Unit, kJ | ||
| ASU | - | 898.2 |
| Oxygen compression | - | 434.8 |
| PSA | 397.2 | 131.0 |
| Subtotal | 397.2 | 1464.0 |
| Item | Hydrogen Production Based on Chemical Regenerative Gasification | Hydrogen Production Based on GE Gasification | ||
|---|---|---|---|---|
| Total System Input | ||||
| Exergy Input, kJ | ||||
| Coal input for gasification (including to gasifier and combustion unit) | 26,876.6 | 96.3% | 25,034.6 | 89.7% |
| Coal input for auxiliary power generation | 1032.6 | 3.7% | 2874.6 | 10.3% |
| subtotal | 27,909.2 | 100.0% | 27,909.2 | 100.0% |
| Exergy Destruction | ||||
| Air separation and oxygen compression | - | - | 865.2 | 3.1% |
| Coal gasification (including char making and char gasification) | 3070.0 | 11.0% | 4270.1 | 15.3% |
| Quench or heat recovery of syngas | 753.5 | 2.7% | 1535.0 | 5.5% |
| WGS | 669.8 | 2.4% | 641.9 | 2.3% |
| Shifted gas cooling | 195.4 | 0.7% | 725.6 | 2.6% |
| PSA unit | 697.7 | 2.5% | 614.0 | 2.2% |
| Auxiliary power generation | 614.0 | 2.2% | 1255.9 | 4.5% |
| Subtotal | 6000.5 | 21.5% | 9879.9 | 35.4% |
| Exergy Output | ||||
| Exergy Output, kJ | ||||
| Hydrogen | 21,908.7 | 78.5% | 18,029.3 | 64.6% |
| Exergy Efficiency of Coal to H2 | ||||
| Exergy efficiency of coal to H2 | 78.5% | 64.6% | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; He, S.; Li, S. Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification. Appl. Sci. 2019, 9, 3035. https://doi.org/10.3390/app9153035
Li W, He S, Li S. Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification. Applied Sciences. 2019; 9(15):3035. https://doi.org/10.3390/app9153035
Chicago/Turabian StyleLi, Wei, Song He, and Sheng Li. 2019. "Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification" Applied Sciences 9, no. 15: 3035. https://doi.org/10.3390/app9153035
APA StyleLi, W., He, S., & Li, S. (2019). Experimental Study and Thermodynamic Analysis of Hydrogen Production through a Two-Step Chemical Regenerative Coal Gasification. Applied Sciences, 9(15), 3035. https://doi.org/10.3390/app9153035





