Wood Bark as Valuable Raw Material for Compounds with a Bioregulator Effect in Lemon Balm (Melissa officinalis L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction Method
2.3. Characterization of the Extracts
2.4. Working Protocol
2.5. Biological Tests on Lemon Balm Seeds
2.6. Determination of Photosynthetic Pigment Concentrations
2.7. Histo-Anatomical Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Extracts Characterization
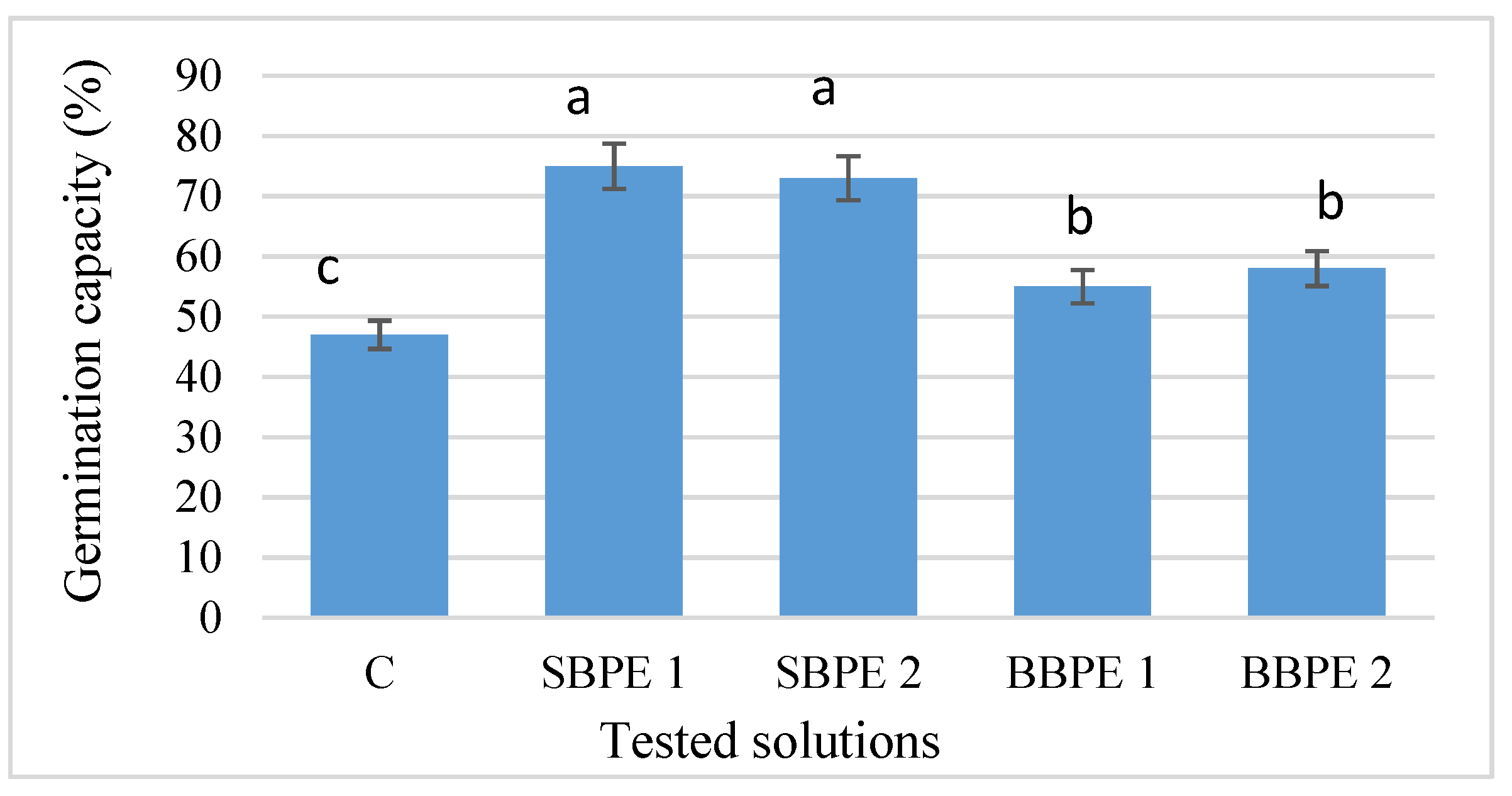
3.2. Seed Germination
3.3. Growth and Development of the Vegetative Organs
3.3.1. Biomass Accumulation in Vegetative Organs
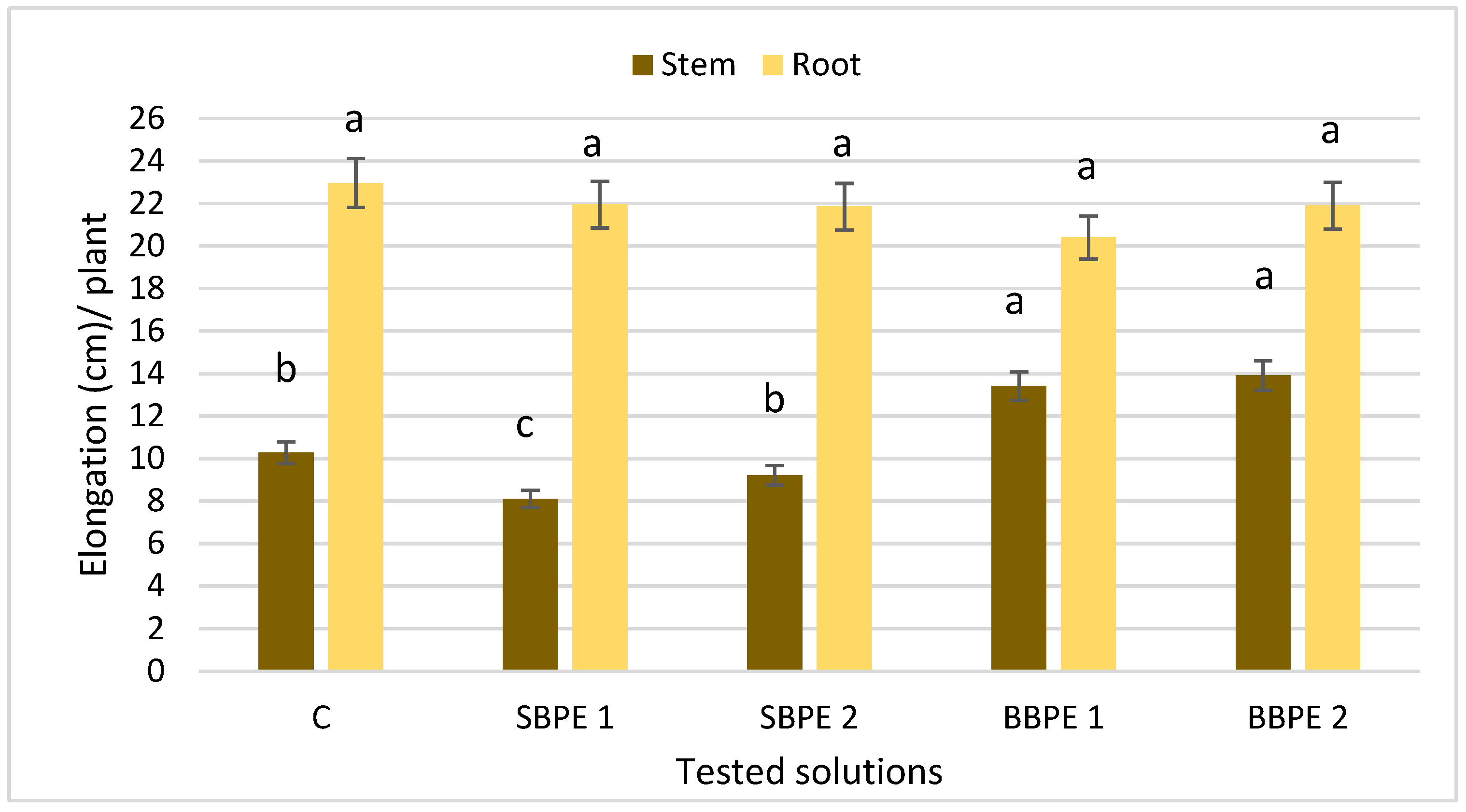
3.3.2. Elongation of the Vegetative Organs
3.3.3. Photo-Assimilating Pigment Content in Lemon Balm Primary Leaves
3.4. Histo-Anatomical Aspects of the Lemon Balm
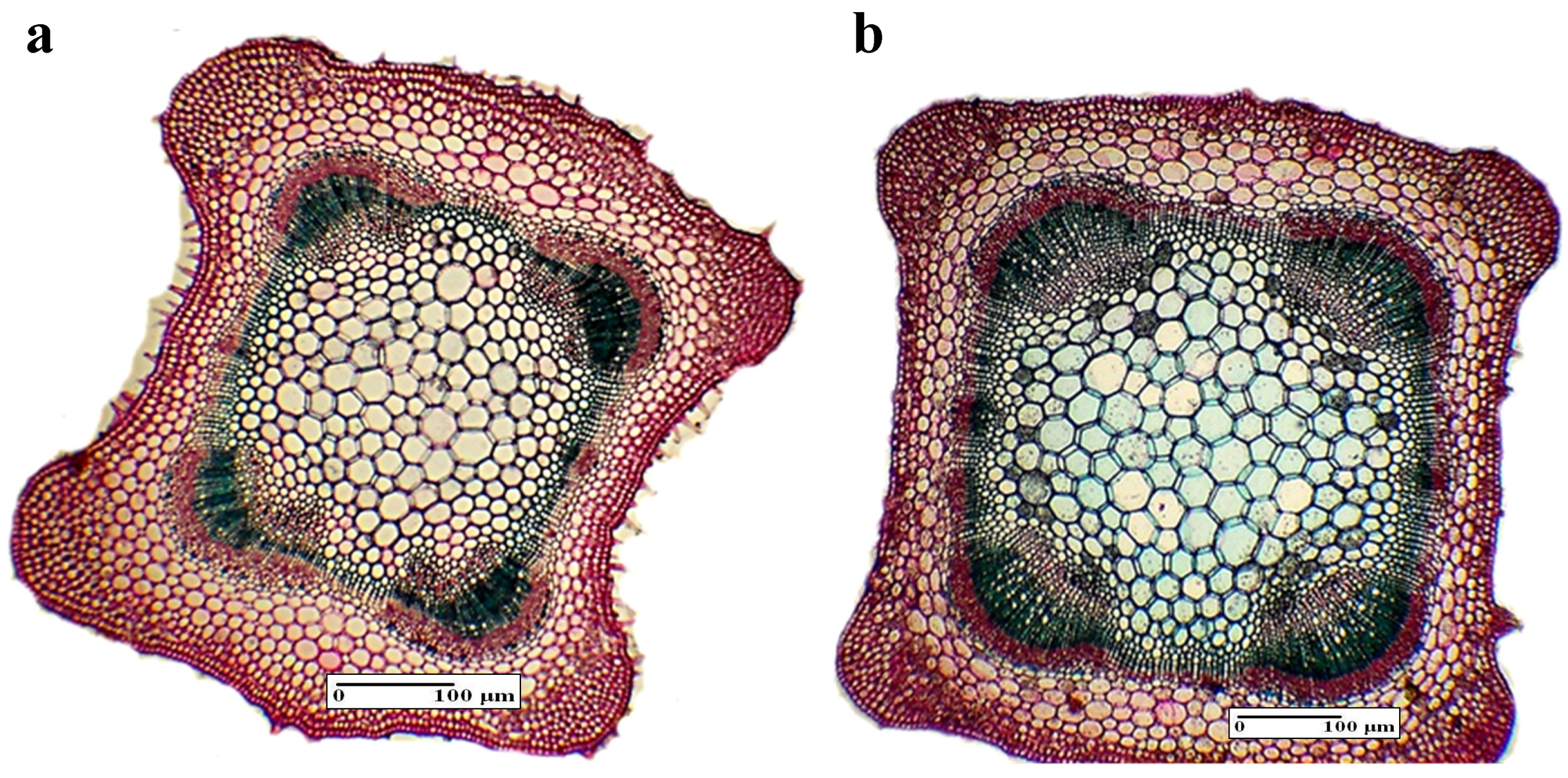
3.4.1. Internal Structure of the Root
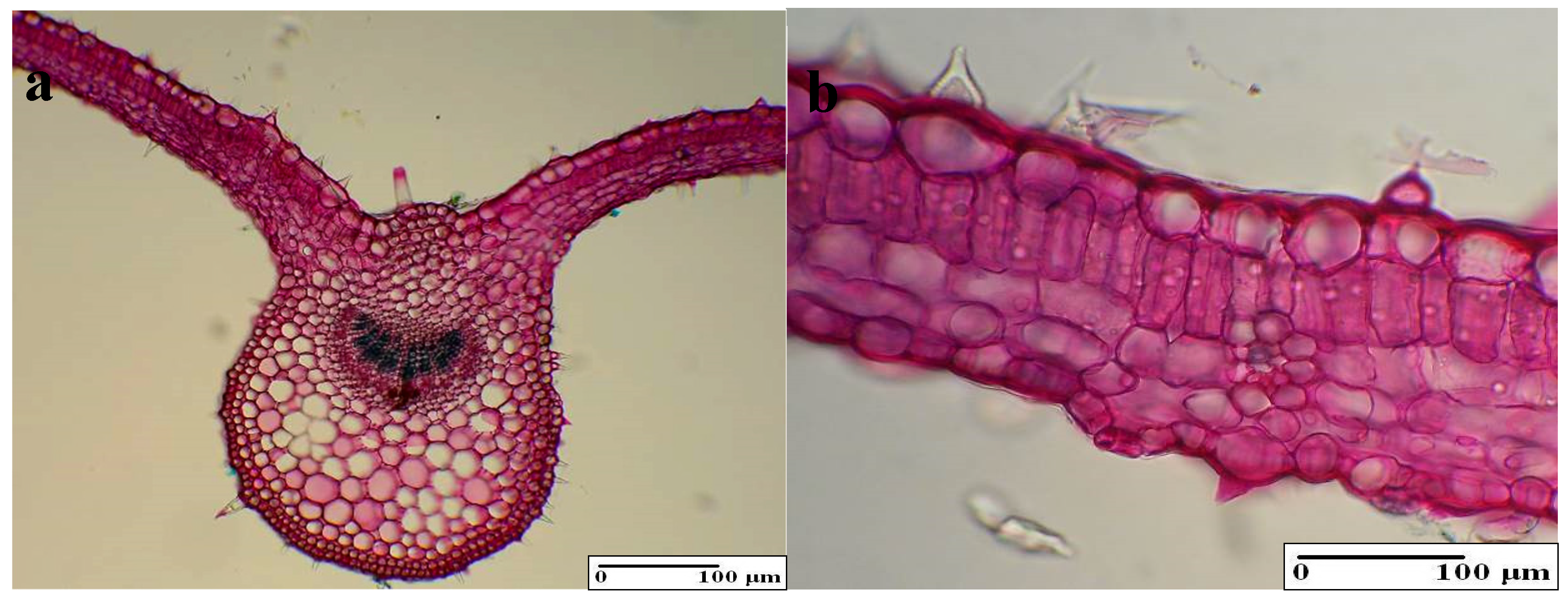
3.4.2. Internal Structure of the Stem
3.4.3. Internal Structure of the Leaves
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Javid, A.Z.; Haybar, H.; Dehghan, P.; Haghighizadeh, M.H.; Mohaghegh, S.M.; Ravanbakhsh, M.; Mohammadzadeh, A.; Bahrololumi, S.S. The effects of Melissa officinalis on echocardiography, exercise test, serum biomarkers, and blood pressure in patients with chronic stable angina. J. Herb. Med. 2018, 11, 24–29. [Google Scholar] [CrossRef]
- Saeb, K.; Gholamrezaee, S. Variation of essential oil composition of Melissa officinalis L. leaves during different stages of plant growth. Asian Pac. J. Trop. Biomed. 2012, 2 (Suppl. 2), S547–S549. [Google Scholar] [CrossRef]
- Boneza, M.M.; Niemeyer, E.D. Cultivar affects the phenolic composition and antioxidant properties of commercially available lemon balm (Melissa officinalis L.) varieties. Ind. Crops Prod. 2018, 112, 783–789. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Łożykowska, K.; Bocianowski, J.; Król, D. The evaluation of the variability of morphological and chemical traits of the selected lemon balm (Melissa officinalis L.) genotypes. Ind. Crops Prod. 2013, 49, 515–520. [Google Scholar] [CrossRef]
- Jalal, Z.; El Atki, Y.; Lyoussi, B.; Abdellaoui, A. Phytochemistry of the essential oil of Melissa officinalis L. growing wild in morocco: Preventive approach against nosocomial infections. Asian Pac. J. Trop. Biomed. 2015, 5, 458–461. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Popa, V.I. Histo-anatomic aspects on Zea mays L. influenced by spruce bark polyphenolic extract. Romanian Biotechnol. Lett. 2016, 21, 11238–11245. [Google Scholar]
- García-Pérez, M.-E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Lazar, L.; Talmaciu, A.I.; Volf, I.; Popa, V.I. Kinetic modeling of the ultrasound-assisted extraction of polyphenols from Picea abies bark. Ultrason. Sonochem. 2016, 32, 191–197. [Google Scholar] [CrossRef]
- Tanase, C.; Domokos, E.; Cosarca, S.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C.A. Study of the ultrasound-assisted extraction of polyphenols from beech (Fagus sylvatica L.) bark. Bioresources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Gligorić, E.; Igić, R.; Suvajdžić, L.; Grujić-Letić, N. Species of the Genus Salix L.: Biochemical Screening and Molecular Docking Approach to Potential Acetylcholinesterase Inhibitors. Appl. Sci. 2019, 9, 1842. [Google Scholar] [CrossRef]
- Venturi, F.; Bartolini, S.; Sanmartin, C.; Orlando, M.; Taglieri, I.; Macaluso, M.; Lucchesini, M.; Trivellini, A.; Zinnai, A.; Mensuali, A. Potato Peels as a Source of Novel Green Extracts Suitable as Antioxidant Additives for Fresh-Cut Fruits. Appl. Sci. 2019, 9, 2431. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. Physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus L.) plants. Ind. Crops Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Popa, V.I.; Agache, C.; Beleca, C.; Popa, M. Polyphenols from spruce bark as plant growth regulator. Crop Res. 2002, 24, 398–406. [Google Scholar]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.-F.; Mérillon, J.-M. Stilbenes from common spruce (Picea abies) bark as natural antifungal agent against downy mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crops Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Hofmann, T.; Nebehaj, E.; Stefanovits-Bányai, É.; Albert, L. Antioxidant capacity and total phenol content of beech (Fagus sylvatica L.) bark extracts. Ind. Crops Prod. 2015, 77, 375–381. [Google Scholar] [CrossRef]
- Cosarca, S.-L.; Moaca, E.-A.; Tanase, C.; Muntean, D.L.; Pavel, I.Z.; Dehelean, C.A. Spruce and beech bark aqueous extracts: Source of polyphenols, tannins and antioxidants correlated to in vitro antitumor potential on two different cell lines. Wood Sci. Technol. 2019, 53, 313–333. [Google Scholar] [CrossRef]
- Bargali, K.; Bargali, S.S. Germination capacity of seeds of leguminous plants under water deficit conditions: Implication for restoration of degraded lands in Kumaun Himalaya. Trop. Ecol. 2016, 57, 445–453. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus verticillium dahliae as revealed by RNA-seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- O’Brien, T.P. Further observations on hydrolysis of the cell wall in the xylem. Protoplasma 1970, 69, 1–14. [Google Scholar] [CrossRef]



| Experimental Variant | Chl a mg/g | Chl b mg/g | Chl a + Chl b | Chl a/Chl b | Carotens mg/g |
|---|---|---|---|---|---|
| Control | 0.77 ± 0.08 c | 0.18 ± 0.08 c | 0.95 | 4.10 | 0.19 ± 0.02 c |
| SBPE 1 | 0.88 ± 0.10 c | 0.25 ± 0.09 b | 1.13 | 3.49 | 0.23 ± 0.08 c |
| SBPE 2 | 0.62 ± 0.06 d | 0.15 ± 0.03 c | 0.77 | 4.21 | 0.18 ± 0.05 c |
| BBPE 1 | 1.09 ± 0.10 b | 0.30 ± 0.07 a | 1.39 | 3.60 | 0.26 ± 0.04 c |
| BBPE 2 | 1.33 ± 0.12 a | 0.35 ± 0.08 a | 1.68 | 3.84 | 0.47 ± 0.07 a,b |
| Vegetative Organs | Microscopic Characteristics | Treated Plants (Mean ± SD) | Control Plants (Mean ± SD) | |||
|---|---|---|---|---|---|---|
| SBPE1 | SBPE2 | BBPE1 | BBPE2 | |||
| Root | Secondary xylem area (%) | 44.22 ± 4.51 b | 45.88 ± 6.41 b | 49.84 ± 5.12 a | 46.82 ± 5.74 b | 43.04 ± 5.12 b |
| Stem | Vascular bundles area (%) | 13.81 ± 2.02 b | 13.54 ± 1.44 b | 15.9 ± 1.51 a | 13.1 ± 1.12 b | 12.72 ± 1.74 b |
| Sclerenchyma cap area (%) | 1.11 ± 0.17 a | 0.85 ± 0.09 b | 0.89 ± 0.11 b | 0.88 ± 0.11 b | 0.91 ± 0.09 b | |
| Colenchim area (%) | 6.95 ± 0.94 a | 6.21 ± 0.82 b | 5.81 ± 1.18 b | 5.71 ± 1.04 b | 5.74 ± 1.11 b | |
| Number of vascular bundles | 5.45 ± 1.42 b | 5.54 ± 1.19 b | 6.85 ± 1.04 a | 5.21 ± 1.55 b | 5.01 ± 1.41 b | |
| Leaf | Leaf lamina thickness (mm) | 0.851 ± 0.054 a | 0.842 ± 0.047 a | 0.872 ± 0.027 a | 0.79 ± 0.023 a | 0.82 ± 0.042 a |
| Vascular bundles area in the main string (%) | 15.24 ± 1.56 b | 16.12 ± 1.09 b | 17.42 ± 1.47 a | 15.87 ± 2.15 b | 14.98 ± 1.72 b | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanase, C.; Nișca, A.; Mirica, A.; Milan, A.; Boz, I. Wood Bark as Valuable Raw Material for Compounds with a Bioregulator Effect in Lemon Balm (Melissa officinalis L.) Plants. Appl. Sci. 2019, 9, 3148. https://doi.org/10.3390/app9153148
Tanase C, Nișca A, Mirica A, Milan A, Boz I. Wood Bark as Valuable Raw Material for Compounds with a Bioregulator Effect in Lemon Balm (Melissa officinalis L.) Plants. Applied Sciences. 2019; 9(15):3148. https://doi.org/10.3390/app9153148
Chicago/Turabian StyleTanase, Corneliu, Adrian Nișca, Anca Mirica, Andreea Milan, and Irina Boz. 2019. "Wood Bark as Valuable Raw Material for Compounds with a Bioregulator Effect in Lemon Balm (Melissa officinalis L.) Plants" Applied Sciences 9, no. 15: 3148. https://doi.org/10.3390/app9153148
APA StyleTanase, C., Nișca, A., Mirica, A., Milan, A., & Boz, I. (2019). Wood Bark as Valuable Raw Material for Compounds with a Bioregulator Effect in Lemon Balm (Melissa officinalis L.) Plants. Applied Sciences, 9(15), 3148. https://doi.org/10.3390/app9153148





