Forced Circulation of Nitrogen Gas for Accelerated and Eco-Friendly Cooling of Metallic Parts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
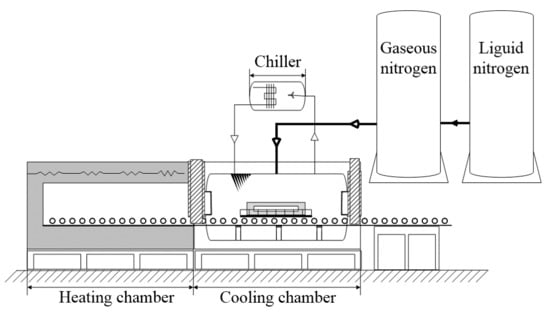
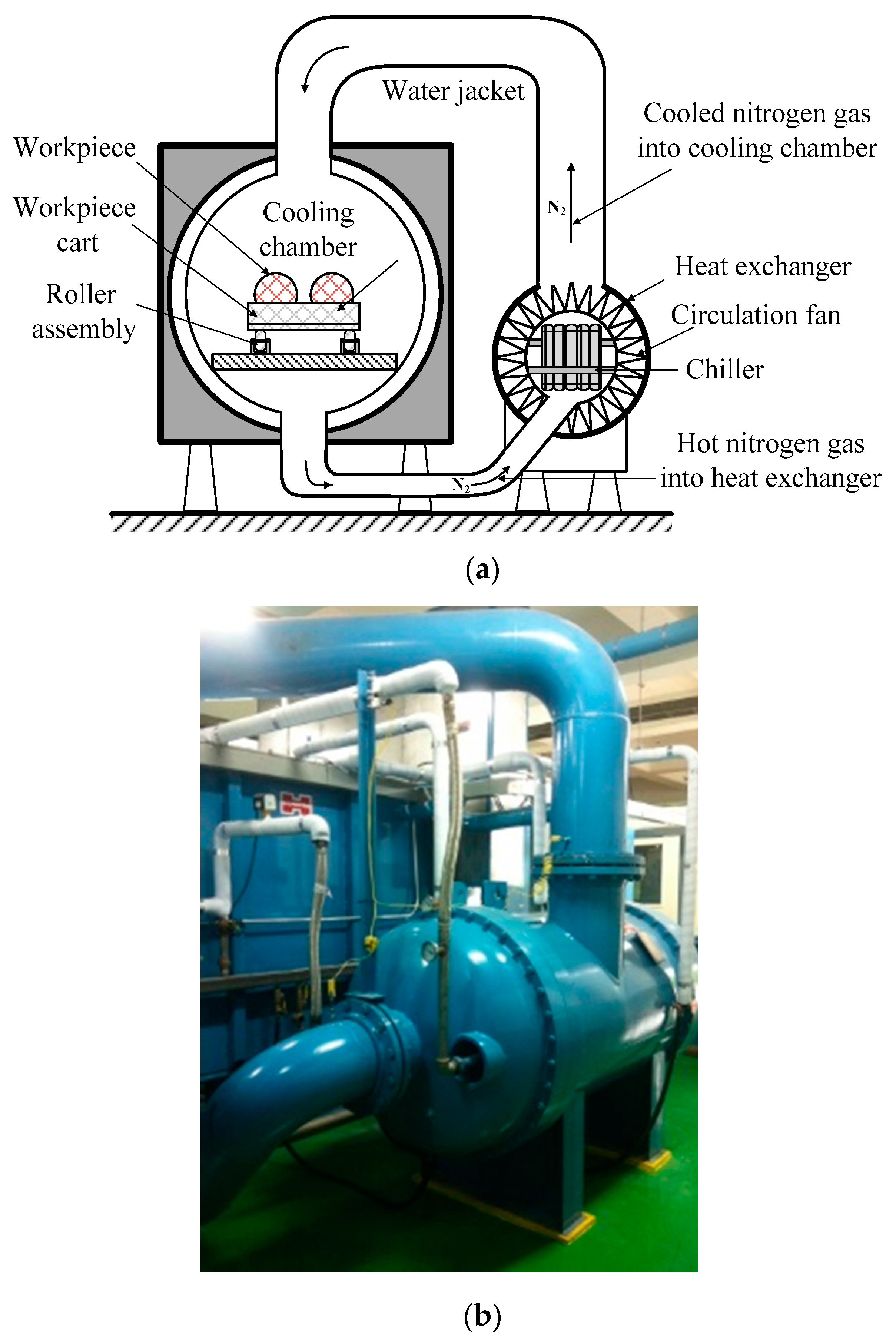
2.2.1. Design of the Dry Nitrogen Cooling System
2.2.2. Application of the Nitrogen Cooling System to Cool the Sintered THA
3. Results and Discussion
3.1. Surface Cleanliness
3.2. Thermal Distortion
3.3. Mechanical Properties
3.4. Feasibility of Nitrogen Cooling
4. Conclusions and Future Work
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cp | Specific heat, J kg−1 °C−1 |
| Di | Inner diameter of the tube, m |
| Do | Outer diameter of the tube, m |
| et | Total elongation, % |
| h | Heat transfer coefficient, W m−2 °C −1 |
| k | Thermal conductivity, W m−1 °C−1 |
| L | Length of the tube, m |
| (Greek symbols) | |
| ρ | Density, kg m−3 |
| σt | Tensile strength, kg mm−2 |
References
- Lior, N. The cooling process in gas quenching. J. Mater. Process. Technol. 2004, 155, 1881–1888. [Google Scholar] [CrossRef]
- Narazaki, M.; Kogawara, M.; MING, Q.; Watanabe, Y. Measurement and construction of heat transfer coefficients of gas quenching. Stroj. Vest. 2009, 55, 167–173. [Google Scholar]
- Stratton, P.; Shedletsky, I.; Lee, M. Gas quenching with helium. Solid State Phenom. 2006, 118, 221–226. [Google Scholar] [CrossRef]
- Lin, M. Gas Quenching with Air Products’ Rapid Gas Quenching Gas Mixture. 2007. Available online: http://www.airproducts.com/metals (accessed on 17 May 2019).
- Baxter, W.J.; Kilhefner, P.T.; Baukal, C.E., Jr. Rapid Gas Quenching Process. U.S. Patent No.5173124, 22 December 1992. [Google Scholar]
- Wieser, M.E.; Holden, N.; Coplen, T.B.; Böhlke, J.K.; Berglund, M.; Brand, W.A.; Bièvre, P.F.; Gröning, M.; Loss, R.D.; Meija, J.; et al. Atomic weights of the elements 2011 (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 1047–1078. [Google Scholar] [CrossRef]
- Yoder, C.H. Ionic Compounds: Applications of Chemistry to Mineralogy; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Ji, H.; Li, Y.; Su, H.; Cheng, W.; Wu, X. Experimental investigation on the cooling and inerting effects of liquid nitrogen injected into a confined space. Symmetry 2019, 11, 579. [Google Scholar] [CrossRef]
- Seo, D.M.; Hwang, T.W.; Moon, Y.H. Carbonitriding of Ti-6Al-4V alloy via laser irradiation of pure graphite powder in nitrogen environment. Surf. Coat. Technol. 2019, 363, 244–254. [Google Scholar] [CrossRef]
- Hollman, J.O. Heat Transfer, 7th ed.; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Kumar, M.P.; Ahmed, L.S. Drilling of AISI 304 stainless steel under liquid nitrogen cooling: A comparison with flood cooling. Mater. Today 2017, 4, 1518–1524. [Google Scholar] [CrossRef]
- Dhananchezian, M. Study the machinability characteristics of Nicked based Hastelloy C-276 under cryogenic cooling. Measurement 2019, 136, 694–702. [Google Scholar] [CrossRef]
- Reggiani, B.; Donati, L. Prediction of liquid nitrogen die cooling effect on the extrusion process parameters by means of FE simulations and experimental validation. J. Manuf. Process. 2019, 41, 231–241. [Google Scholar] [CrossRef]
- Wang, J.; Gu, J.; Shan, X.; Hao, X.; Chen, N.; Zhang, W. Numerical simulation of high pressure gas quenching of H13 steel. J. Mater. Process. Technol. 2008, 202, 188–194. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Shi, H.X.; Wang, W.X.; Xu, B.S. Laser surface melting AZ31B magnesium alloy with liquid nitrogen-assisted cooling. Trans. Nonferrous Met. Soc. China 2015, 25, 1446–1453. [Google Scholar] [CrossRef]
- Manikandan, S.G.K.; Sivakumar, D.; Rao, K.P.; Kamaraj, M. Microstructural characterization of liquid nitrogen cooled Alloy 718 fusion zone. J. Mater. Process. Technol. 2014, 214, 3141–3149. [Google Scholar] [CrossRef]
- Cai, C.; Li, G.; Huang, Z.; Shen, Z.; Tian, S.; Wei, J. Experimental study of the effect of liquid nitrogen cooling on rock pore structure. J. Nat. Gas Sci. Eng. 2014, 21, 507–517. [Google Scholar] [CrossRef]
- Gao, F.; Cai, C.; Yang, Y. Experimental research on rock fracture failure characteristics under liquid nitrogen cooling conditions. Results Phys. 2018, 9, 252–262. [Google Scholar] [CrossRef]
- Atraszkiewicz, R.; Januszewicz, B.; Kaczmarek, L.; Stachurski, W.; Dybowski, K.; Rzepkowski, A. High pressure gas quenching: Distortion analysis in gears after heat treatment. Mater. Sci. Eng. 2012, 558, 550–557. [Google Scholar] [CrossRef]
- Elkatatny, I.; Morsi, Y.; Blicblau, A.S.; Das, S.; Doyle, E.D. Numerical analysis and experimental validation of high pressure gas quenching. Int. J. Therm. Sci. 2003, 42, 417–423. [Google Scholar] [CrossRef]
- Senthilnathan, N.; Annamalai, A.R.; Venkatachalam, G. Sintering of tungsten and tungsten heavy alloys of W–Ni–Fe and W–Ni–Cu: A review. Trans. Indian Inst. Met. 2017, 70, 1161–1176. [Google Scholar] [CrossRef]
- Muresan, R.; Riti-Mihoc, E.; Prica, C.; Bodea, M. Improving mechanical properties of sintered wolfram based alloy with liquid phase trough controlled cooling parameters. Arch. Metall. Mater. 2012, 57, 87–92. [Google Scholar] [CrossRef]
- Baek, W.H.; Hong, M.H.; Kim, E.P.; Noh, J.W.; Lee, S.; Song, H.S.; Lee, S.H. Heat treatment behavior of tungsten heavy alloy. Solid State Phenom. 2006, 118, 35–40. [Google Scholar] [CrossRef]
- Das, J.; Kiran, U.R.; Chakraborty, A.; Prasad, N.E. Hardness and tensile properties of tungsten based heavy alloys prepared by liquid phase sintering technique. Int. J. Refract. Met. Hard Mater. 2009, 27, 577–583. [Google Scholar] [CrossRef]
- Dinçer, O.; Pehlivanoğlu, M.K.; Çalişkan, N.K.; Karakaya, İ.; Kalkanli, A. Processing and microstructural characterization of liquid phase sintered tungsten–nickel–cobalt heavy alloys. Int. J. Refract. Met. Hard Mater. 2015, 50, 106–112. [Google Scholar] [CrossRef]
- German, R.M.; Suri, P.; Park, S.J. Review: Liquid phase sintering. J. Mater. Sci. 2009, 44, 1–39. [Google Scholar] [CrossRef]
- Liu, J.; German, R.M. Microstructural parameters related to liquid-phase sintering. Metall. Mater. Trans. 2000, 31, 2607–2614. [Google Scholar] [CrossRef]
- Wang, H.F.; Han, J.T.; Hao, Q.L. Influence of mandrel on the performance of titanium tube with cold rotary swaging. Mater. Manuf. Process. 2015, 30, 1251–1255. [Google Scholar] [CrossRef]
- Al-Khazraji, H.; El-Danaf, E.; Wollmann, M.; Wagner, L. Microstructure, mechanical, and fatigue strength of Ti-54M processed by rotary swaging. J. Mater. Eng. Perform. 2015, 24, 2074–2084. [Google Scholar] [CrossRef]
- Simonetto, E.; Ghiotti, A.; Bruschi, S. Dynamic detection of tubes wrinkling in three roll push bending. Procedia Eng. 2017, 207, 2316–2321. [Google Scholar] [CrossRef]
- Kim, S.Y.; Joo, B.D.; Shin, S.; Van Tyne, C.J.; Moon, Y.H. Discrete layer hydroforming of three-Layered tubes. Int. J. Mach. Tools Manuf. 2013, 68, 56–62. [Google Scholar] [CrossRef]
- Han, S.W.; Woo, Y.Y.; Hwang, T.W.; Oh, I.Y.; Moon, Y.H. Tailor layered tube hydroforming for fabricating tubular parts with dissimilar thickness. Int. J. Mach. Tools Manuf. 2019, 138, 51–65. [Google Scholar] [CrossRef]
- Park, J.Y.; Han, S.W.; Jeong, H.S.; Cho, J.R.; Moon, Y.H. Advanced sealing system to prevent leakage in hydroforming. J. Mater. Process. Technol. 2017, 247, 103–110. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.; Lee, J.; Kim, S.J.; Lee, Y.; Moon, Y.H. Effect of an aluminum driver sheet on the electromagnetic forming of DP780 steel sheet. J. Mater. Process. Technol. 2016, 235, 158–170. [Google Scholar] [CrossRef]
- Hwang, T.W.; Woo, Y.Y.; Han, S.W.; Moon, Y.H. Functionally graded properties in directed-Energy-Deposition titanium parts. Opt. Laser Technol. 2018, 105, 80–88. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Hao, X.; Yin, W. A measurement system for time constant of thermocouple sensor based on high temperature furnace. Appl. Sci. 2018, 8, 2585. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, C.G.; Moon, Y.H. Induction Heating of Semisolid Billet and Control of Globular Microstructure to Prevent Coarsening Phenomena. J. Mater. Eng. Perform. 2000, 9, 12–23. [Google Scholar] [CrossRef]
- Kil, T.D.; Lee, J.M.; Moon, Y.H. Quantitative formability estimation of ring rolling process by using deformation processing map. J. Mater. Process. Technol. 2015, 220, 224–230. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, J. Study of the electromagnetic properties of nano (MxZn1−x) Fe2O4 (M = Cu, Ni) as a function of the sintering temperature. Appl. Sci. 2018, 8, 605. [Google Scholar] [CrossRef]
- Kim, D.K.; Woo, Y.Y.; Park, K.S.; Sim, W.J.; Moon, Y.H. Advanced induction heating system for hot stamping. Int. J. Adv. Manuf. Technol. 2018, 99, 583–593. [Google Scholar] [CrossRef]
- Hauke, K.; Kehren, J.; Böhme, N.; Zimmer, S.; Geisler, T. In situ hyperspectral raman imaging: A new method to investigate sintering processes of ceramic material at high-Temperature. Appl. Sci. 2019, 9, 1310. [Google Scholar] [CrossRef]
- Song, M.C.; Moon, Y.H. Coupled electromagnetic and thermal analysis of induction heating for the forging of marine crankshafts. Appl. Therm. Eng. 2016, 98, 98–109. [Google Scholar] [CrossRef]
- Joo, B.D.; Jang, J.H.; Lee, J.H.; Son, Y.M.; Moon, Y.H. Selective laser melting of Fe-Ni-Cr layer on AISI H13 tool steel. Trans. Nonferrous Met. Soc. China 2009, 19, 921–924. [Google Scholar] [CrossRef]
- Jang, J.H.; Joo, B.D.; Van Tyne, C.J.; Moon, Y.H. Characterization of deposited layer fabricated by direct laser melting process. Met. Mater. Int. 2013, 19, 497–506. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, J.H.; Joo, B.D.; Moon, Y.H. Flow characteristics of aluminum coated boron steel in hot press forming. Trans. Nonferrous Met. Soc. China 2009, 19, 913–916. [Google Scholar] [CrossRef]
- ABAQUS, Abaqus Documentation 6.13, Dassault Systems, Providence, RI, USA. 2013. Available online: http://dsk.ippt.pan.pl/docs/abaqus/v6.13/index.html (accessed on 15 May 2019).
- Ferrari, J.; Lior, N.; Slycke, J. An evaluation of gas quenching of steel rings by multiple-Jet impingement. J. Mater. Process. Technol. 2003, 136, 190–201. [Google Scholar] [CrossRef]
- Hsieh, W.H.; Wu, J.Y.; Shih, W.H.; Chiu, W.C. Experimental investigation of heat-Transfer characteristics of aluminum-Foam heat sinks. Int. J. Heat Mass Transf. 2004, 47, 5149–5157. [Google Scholar] [CrossRef]
- Jeon, C.H.; Han, S.W.; Joo, B.D.; VanTyne, C.J.; Moon, Y.H. Deformation analysis for cold rolling of Al-Cu double layered sheet by the physical modeling and finite element method. Met. Mater. Int. 2013, 19, 1069–1076. [Google Scholar] [CrossRef]
- Li, M.; Ruprecht, D.; Kracker, G.; Höschen, T.; Neu, R. Impact of heat treatment on tensile properties of 97W-2Ni-1Fe heavy alloy. J. Nucl. Mater. 2018, 512, 1–7. [Google Scholar] [CrossRef]












| Heat Treatment | Tensile Properties | Hardness (HRC) | |
|---|---|---|---|
| σt (kg/mm2) | et (%) | ||
| Sintering | 80.4 | 5.3 | 26.5 |
| Sintering + water cooling | 96.6 | 22.9 | 29.7 |
| Sintering + nitrogen cooling | 95.1 | 24.8 | 27.9 |
| Item | Nitrogen Cooling (NC) | Water Cooling (WC) | Remarks |
|---|---|---|---|
| Equipment cost | Intermediate | Low | NC: needs vacuum chamber |
| Floor space | Low | Intermediate | WC: needs water reservoir |
| Operation cycle | Fast | Slow | - |
| Installation cost | Low | Very High | WC: water-proof design |
| Rearrangement cost | Low | Very High | WC: needs pit reconstruction |
| Eco friendly | High | Low | WC: contaminated sludge |
| Operating cost | Low | Low | |
| Maintenance cost | Very Low | Intermediate | WC: plumbing, leakage, water pumping |
| Post machining | Low | High | WC: low circularity |
| Yield ratio | High | Low | WC: large machining loss |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Z.S.; Kim, J.; Woo, Y.Y.; Lee, H.; Kim, J.H.; Moon, Y.H. Forced Circulation of Nitrogen Gas for Accelerated and Eco-Friendly Cooling of Metallic Parts. Appl. Sci. 2019, 9, 3679. https://doi.org/10.3390/app9183679
Park ZS, Kim J, Woo YY, Lee H, Kim JH, Moon YH. Forced Circulation of Nitrogen Gas for Accelerated and Eco-Friendly Cooling of Metallic Parts. Applied Sciences. 2019; 9(18):3679. https://doi.org/10.3390/app9183679
Chicago/Turabian StylePark, Zu Seong, Jeong Kim, Young Yun Woo, Habeom Lee, Ji Hoon Kim, and Young Hoon Moon. 2019. "Forced Circulation of Nitrogen Gas for Accelerated and Eco-Friendly Cooling of Metallic Parts" Applied Sciences 9, no. 18: 3679. https://doi.org/10.3390/app9183679
APA StylePark, Z. S., Kim, J., Woo, Y. Y., Lee, H., Kim, J. H., & Moon, Y. H. (2019). Forced Circulation of Nitrogen Gas for Accelerated and Eco-Friendly Cooling of Metallic Parts. Applied Sciences, 9(18), 3679. https://doi.org/10.3390/app9183679