Abstract
Polyacrylonitrile (PAN)-derived carbon nanofiber mats were fabricated using electrospinning and further carbonization. Scanning electron microscopy (SEM), X-ray diffraction (XRD) and electrochemical characterization were used to investigate the effects of precursor concentration, thermal stabilization and carbonization temperature, addition of multi-walled carbon nanotubes (MWCNTs), activation of nitric acid and sulfuric acid on the morphologies, conductivity, flexibility and electrochemical properties of the fabricated carbon nanofiber mats. The results reveal that the carbon nanofiber mats with uniform fiber diameter of 200 nm and sheet resistance of 154 Ω/sq could be achieved with a PAN mass fraction of 12 wt% and a thermal stabilization and carbonization temperature of 270 °C and 900 °C, respectively. Due to the good conductivity and high strength of the MWCNTs, the sheet resistance of the carbon nanofiber mats decreases to around 60 Ω/sq by adding MWCNTs to precursor, and the mats exhibit excellent bend and fold flexibility. The electrochemical performance of the co-spun carbon nanofiber mats could be further improved by the activation treatment of acids, and the maximum specific capacitance of the carbon mat reaches 113.5 F/g at a current density of 0.1 mA/cm2 in the case of 1:3 HNO3:H2SO4. The investigation provides a reference for improving the performance of spun carbon nanofiber mats, which can be used as the electrodes or current collectors to further load other active materials in the applications of energy storage devices.
1. Introduction
With the development of electronic products, the information industry and industrial manufacturing, the demands for energy have sharply increased in recent years. As a result, many energy conversion and storage devices, such as fuel cells, lithium batteries and supercapacitors, have emerged [1,2,3,4,5,6,7,8,9,10]. Generally, carbon materials such as carbon nanotubes, carbon fibers and graphene, owing to their large specific surface area, good conductivity and high stability, have been widely utilized as the electrodes or as the current collectors to further load other active materials [11,12,13,14,15].
As is well known, the diameter of ultra-long fibers prepared using electrospinning technology can be expediently controlled from a few tens of nanometers to several dozens of micrometers [16,17,18]. Moreover, during the electrospinning process, various materials (metal nanoparticles, metal oxide or metallic salts) could be conveniently added into the spinning precursors to fabricate the composite materials for diverse applications [19,20,21,22,23,24,25]. Therefore, it is advantageous to construct carbon nanofibers with uniform diameter, excellent conductivity and self-supporting flexibility using the electrospinning technology, which can be utilized as the electrodes or current collectors for energy storage devices. For example, Ferraris et al. reported that lignin was blended with polyacrylonitrile (PAN) in different ratios and fabricated into carbon nanofiber electrodes by electrospinning followed by thermal stabilization, carbonization and subsequent activation by CO2 of the carbonized mats. The coin cell supercapacitors employing these electrodes exhibit 128 F/g specific capacitance, 59 Wh/kg energy density and a 15 kW/kg power density when operated at 3.5 V using an ionic liquid electrolyte [26]. Yoon et al. fabricated freestanding and flexible MnO-decorated carbon nanofiber (CNF) composites as lithium-ion battery anode materials with an initial capacity of 1131 mAh/g and a retention capacity of 923 mAh/g after 90 charge-discharge cycles under a current rate of 123 mA/g [1].
In order to apply the spun carbon nanofibers in the flexible energy storage devices with high performances, it is significant to further improve the mechanical, electrical and electrochemical properties of the carbon nanofibers. In this work, we aim to fabricate the spun carbon nanofiber mats followed by thermal stabilization, carbonization, toughening modification and activation to achieve the characteristics of high conductivity, good flexibility and large specific capacitance. The effects of the spinning polymer concentration, thermal stabilization and carbonization temperatures, addition of MWCNTs, as well as acid activation on the performances of the carbon nanofiber mats were carefully investigated.
2. Experimental Sections
2.1. Fabrication of Electrospun Carbon Nanofiber Mats
PAN nanofiber mats were fabricated by a typical electrospinning process at room temperature, as illustrated in Figure 1. Firstly, 4.0–7.5 g PAN powders were dissolved into N,N-Dimethylformamide (DMF) solvent at 80 °C for 6 h to generate a slightly yellow solution with the PAN mass fraction of 8, 10, 12 and 15 wt%, respectively. Following this, the electrospun PAN nanofiber mats were collected using aluminum foil. Then, the carbon nanofiber mats were obtained by heating in a tubular furnace at 220–300 °C in air for a thermal stabilization process and at 800–1000 °C in nitrogen atmosphere for a carbonization process, respectively. The obtained carbon nanofiber mats were further activated by nitric acid and sulfuric acid to improve the electrochemical performances. Finally, by tuning the ratio of nitric acid and sulfuric acid, the carbon nanofiber mat with an optimized performance was achieved.

Figure 1.
Schematic illustration of the fabrication process of polyacrylonitrile (PAN)-derived electrospun carbon nanofiber mats.
2.2. Structure, Morphology and Electrochemical Characterization
The phase compositions of the nanofiber mats were characterized using an X-ray diffractometer (XRD, X’pert Pro MFD, Panalytical, Almelo, The Netherlands) with a Cu Kα radiation (λ = 0.154178 nm). The surface morphologies of the nanofiber mats were obtained from an optical microscope (JZ95MS-60, Powereach, Shanghai, China) and a field emission scanning electron microscope (FESEM, Zeiss Sigma 500, Oberkochen, Germany).
Electrochemical performances of the carbon nanofiber mats were evaluated in a three-electrode mode using cyclic voltammetry (CV), galvanostatic charge-discharge (GCD) and electrical impedance spectroscopy (EIS) on the electrochemical workstation (CHI760E, CH Instruments Inc., Shanghai, China).
3. Results and Discussions
The overall fabrication process for PAN-derived electrospun carbon nanofiber mats is briefly illustrated in Figure 1. PAN was selected as the spinning precursor due to its high carbon content and stable structure during the carbonization process. Generally, as a kind of current collecting material, a carbon nanofiber mat requires both self-supporting flexibility and excellent electrical conductivity. However, there is always a trade-off between flexibility and conductivity during the carbonization process. In order to improve the toughness of carbon nanofibers while maintaining its excellent conductivity, MWCNTs (XFNANO, Nanjing, China) were added into PAN precursor as supporting material. Furthermore, the electrochemical performances of the carbon nanofibers can be further improved by the activation of nitric acid and sulfuric acid. Their strong oxidation abilities not only produce more active groups on the surface but also acquire a rough nanofiber surface, which is beneficial for further deposition of other active materials.
Figure 2 displays optical micrographs of the electrospun PAN nanofibers with PAN mass fraction of 8, 10, 12 and 15 wt%, respectively. It is difficult to form nanofibers with 8 wt% PAN due to a low polymer viscosity. As the mass fraction of PAN increases to 12 wt%, the continuous nanofibers with uniform diameters can be collected. In this work, the fiber yield is determined by the number ratio of fiber to particles in the same field of view. Compared to 15 wt% PAN, a higher yield of nanofibers is gained in the case of 12 wt% PAN. A higher concentration of PAN would cause polymer aggregation in some areas. Hence, the precursor concentration is one of the key factors in spinning continuous and uniform nanofibers. In the following investigation, we chose 12 wt% PAN as the spinning precursor.

Figure 2.
Optical micrographs of PAN nanofiber films electrospun with PAN mass fraction of (a) 8 wt%; (b) 10 wt%; (c) 12 wt% and (d) 15 wt%, respectively. The scale bar is 100 µm.
It is well known that after carbonization of electrospun PAN nanofibers in a nitrogen atmosphere, elements such as hydrogen (H), oxygen (O) and nitrogen (N) in the fibers can be removed, excluding the element carbon (C). However, the carbonization process is prone to destroying the internal structure of the nanofibers and making the nanofibers fragile. Therefore, it is necessary to conduct a thermal stabilization process to ensure the structure stability before the nanofiber carbonization. In this work, the influence of thermal stabilization and carbonization temperatures on the morphology, conductivity and flexibility of carbon nanofiber mats were explored. The thermal stabilization and carbonization temperature rise rates were controlled in the range of 1.5–2 °C/min and 4–6 °C/min, respectively.
The nanofiber mats with 12 wt% PAN were heat-treated with a thermal stabilization temperature of 220, 250, 270 and 300 °C, and a carbonization temperature of 800 °C. The carbonized nanofiber mats were correspondingly named as samples C-1, C-2, C-3 and C-4, respectively. Their sheet resistances and average diameters are listed in Table 1. Samples C-1 and C-2 present shrinking and a corrugated surface, indicating that the nanofibers are not stable enough to adapt to the stress release during the carbonization process at a low thermal stabilization temperature. With the thermal stabilization temperature elevated to 270 and 300 °C, the samples C-3 and C-4 show a deep black color and large, smooth surface. The carbonization temperature of the samples C-5 and C-6 is further increased to 900 and 1000 °C, while the thermal stabilization temperature is maintained at 270 °C. The sheet resistance of six samples is decreased from 665 to 116 Ω/sq. The conductivity enhancement is mainly attributed to the increase of carbonization temperature, leading to the relatively higher carbonization degree. However, with the increase of carbonization temperature and the reduction of other components except carbon in the nanofibers, the nanofiber structure would become fragile, resulting in poor flexibility (the flexibility of the nanofiber mats is determined by bending and folding the mats for several times), especially in the case of 1000 °C carbonization.

Table 1.
Sheet resistance and average diameter of the PAN-derived carbon nanofibers with 12 wt% PAN treated at various thermal stabilization and carbonization temperatures.
Figure 3a–f display the SEM images of samples C-1, C-2, C-3, C-4 C-5 and C-6, respectively, demonstrating that the morphologies and average diameters of the obtained carbon nanofiber mats were distinctly influenced by the thermal stabilization and carbonization temperature. The fiber average diameters are determined by measuring the diameters of 30–50 fibers in the SEM images. Figure 3a shows that sample C-1 is composed of cross-linked nanofibers forming a three-dimensional porous carbon structure with pore size ranging from tens of nanometers to several micrometers. A magnified SEM image of sample C-1 is provided in Figure S1 contained in the supplementary. As the thermal stabilization temperature increased, the crosslinking phenomenon gradually disappeared. The average diameter of the carbon nanofibers is around 200 nm for samples C-2 and C-3, but 300 nm for sample C-4, as shown in Figure 3b–d. The magnified SEM images of samples C-2, C-3 and C-4 are displayed in Figure S2a–c contained in the supplementary. It is supposed that further reaction happens with a thermal stabilization temperature of 300 °C, leading to a decrease of structure stability, and the nanofibers might melt during the carbonization process. Thus, the average diameter of the carbon nanofibers increases from 200 to 300 nm. Figure 3e,f shows that even if the carbonization temperature is elevated to 900 and 1000 °C, the average diameters of the carbon nanofibers for the samples C-5 and C-6 remain approximately 200 nm (higher magnification of SEM images are presented in Figure S3a,b contained in the supplementary), demonstrating that the nanofiber diameter is mainly dependent on the thermal stabilization temperature. However, a higher carbonization temperature makes the nanofibers crack, as shown in Figure 3f.
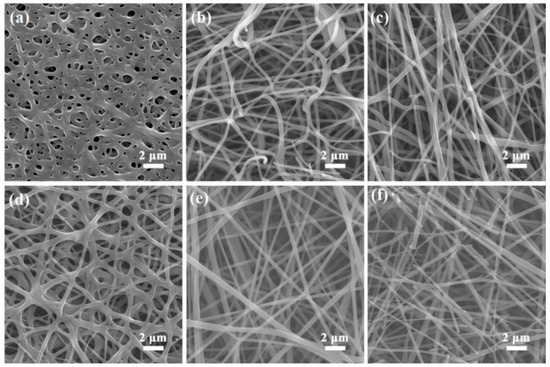
Figure 3.
Scanning electron microscopy (SEM) images of PAN-derived carbon nanofiber mats of (a) C-1, (b) C-2, (c) C-3, and (d) C-4; SEM images of PAN-derived carbon nanofibers of (e) C-5 and (f) C-6.
Using the above analysis, with a thermal stabilization temperature of 270 °C and a carbonization temperature of 900 °C, the nanofibers show a uniform average diameter of around 200 nm, low resistance of 154 Ω/sq and good flexibility. Thus, these temperatures are regarded as the optimized experimental factors in the following investigation.
XRD patterns of the carbon nanofiber mats at various thermal stabilization and carbonization temperatures were obtained to elucidate the structures. Figure 4 suggests that there are two diffraction peaks located at 2θ = 16.2° and 22.6° in all patterns of the six samples, which respectively refers to the lateral packing of crystalline regions in PAN linear macromolecules and the conjugated ladder polymer structure along the chain axis [27,28,29]. The relative intensity ratios of 2θ = 16.2° to 2θ = 22.6° for the samples C-1, C-2, C-3, C-4, C-5 and C-6 are correspondingly 1.19, 1.06, 1.03, 1.09, 0.98 and 1.12, demonstrating that sample C-5 has the largest relative intensity ratio. When the nanofibers were carbonized at a certain temperature, the chemical structure was transformed, ascribed to chemical reactions such as cyclization, dehydrogenation, aromatization and oxidation, which could result in the formation of the conjugated ladder structure [27]. Thus, sample C-5 exhibits the most stable structure among the six samples. Furthermore, a weak and wide peak can be observed at 42.7°, corresponding to (100) reflections of carbon materials, which generally appears in the carbon nanofibers with a carbonization temperature over 1000 °C [29]. Thus, the result indicates that the spun nanofibers are more likely to be completely carbonized at a relatively low carbonization temperature as undergoing a thermal stabilization process.
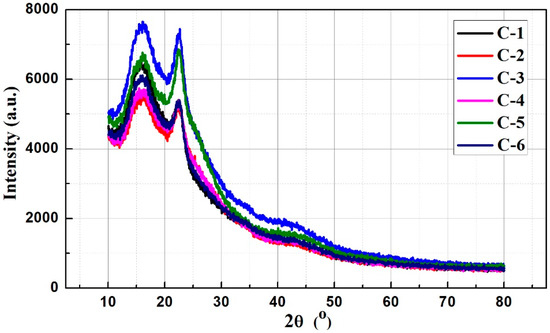
Figure 4.
X-ray diffraction (XRD) patterns of the PAN-derived carbon nanofiber mats treated at various thermal stabilization and carbonization temperatures.
Electrochemical performances of the PAN-derived carbon nanofiber mats were also investigated. Figure 5a displays CV curves of sample C-5 with the scan rate of 5-600 mV/s and a potential window of −0.8~0 V. There is no redox peak in the curves under various scanning speeds, revealing the absence of defects or impurities in the carbon nanofibers. The shape of the curve is closer to the rectangle at a low scan rate. With the scan rate enhanced, the deviation in shape is gradually increased. The GCD curve of sample C-5 at the current density of 0.1 mA/cm2 is plotted in Figure 5b. The IR drop (where I and R represents the current and resistance, respectively) of the carbon nanofiber is 0.22 V. Furthermore, the specific capacitance of the carbon nanofiber mat is calculated to be 25.0 F/g from the GCD curve. To understand the diffusion effect on the electrochemical performances of the carbon nanofiber mat, the EIS spectrum was carried out in a frequency range from 1 MHz to 0.01 Hz, as shown in Figure 5c. The impedance spectrum consists of two parts with a semicircle in the high frequency region and a straight slope in the low frequency region. The internal resistance value of the carbon nanofiber mat is around 100 Ω, and the large radius of the semicircle is mainly attributed to the relatively low conductivity of nanofiber mats.
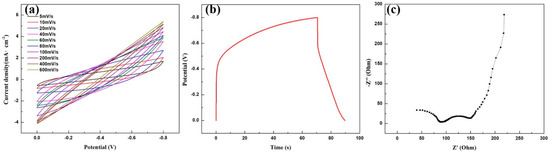
Figure 5.
Electrochemical performance of the sample C-5: (a) cyclic voltammetry (CV) curves at a scan rate of 5-600 mV/s in potential window of −0.8~0 V; (b) galvanostatic charge-discharge (GCD) curve at the current density of 0.1 mA/cm2; (c) Nyquist impedance spectrum.
Based on the above analysis, the electrochemical performance of the PAN-derived carbon nanofiber mats should be further improved in the application of energy storage devices. In order to avoid any side reaction or introducing other elements, we added MWCNTs into PAN precursor to co-spin the nanofibers due to the high intensity and good conductivity of MWCNTs. The effects of MWCNT addition on the average diameter and sheet resistance of the carbon nanofiber mats are listed in Table 2. The added quantity of MWCNTs is 0.05, 0.10 and 0.15 g, corresponding to the samples PMC-1, PMC-2 and PMC-3, respectively. The resultant mass fraction of MWCNTs is 0.7 wt%, 1.5 wt% and 2.4 wt%. The co-spun nanofibers were treated at a thermal stabilization temperature of 270 °C and carbonization temperature of 900 °C. By comparison, the conductivity of the carbon nanofiber mats was greatly modified by adding MWCNTs. The sheet resistances are decreased to 66, 59 and 60 Ω/sq for the three samples.

Table 2.
Sheet resistance and average diameter of the carbon nanofiber mats fabricated with 12 wt% PAN with the addition of various amounts of mult-walled cabon nanotubes (MWCNTs).
Figure 6a–c display SEM images of the samples PMC-1, PMC-2 and PMC-3 after carbonization. The average diameter of the carbon nanofibers for three samples is increased from 140 to 240 nm, as the mass fraction of MWCNTs enhanced from 0.7 to 2.4 wt%. Moreover, the surface of the carbon nanofibers would be rougher, and there are some bulges on the nanofiber surface (Figure 6c). It might be ascribed to the exposure of several MWCNTs to the nanofiber surface as the MWCNT addition increased. In addition, the flexibility of the nanofiber mats is also improved by the addition of MWCNTs. The photographs of the co-spun carbon nanofiber mats, which can be easily bended and folded, demonstrating excellent flexibility, are shown in the insets of Figure 6c.
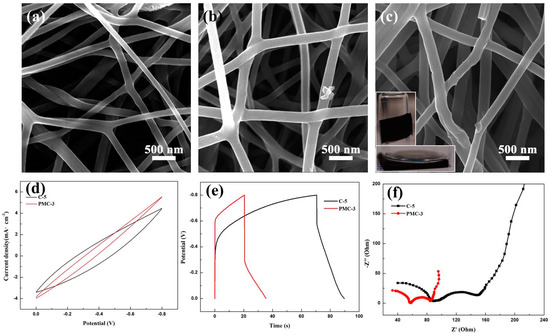
Figure 6.
SEM images of the carbon nanofibers with an MWCNT mass fraction of 0.7 wt% (a), 1.5 wt% (b) and 2.4 wt% (c), respectively; electrochemical performance comparison of the sample C-5 and PMC-3: (d) CV curves at a scan rate of 5 mV/s in potential window of −0.8~0 V; (e) GCD curves at the current density of 0.1 mA/cm2; (f) Nyquist impedance spectra.
Figure 6d–f shows electrochemical performance comparisons of samples C-5 and PMC-3. By comparing with the CV curves of two samples, it can be seen that sample PMC-3 has a similar potential window as sample C-5 at a low scanning rate, as depicted in Figure 6d. The specific capacitance of sample PMC-3 is calculated to be 18.8 F/g from Figure 6e, which is smaller than that of sample C-5. However, the electrical conductivity of the carbon nanofibers is improved by adding the MWCNTs. The impedance spectrum of the sample PMC-3 shows that the internal resistance value is about 56 Ω, which also exhibits the semicircle with a smaller radius than that of sample C-5.
Although the MWCNT addition can improve the mechanical flexibility and conductivity of the PAN-derived carbon nanofiber mat, the specific capacitance is reduced. Therefore, it is necessary to further increase the electrochemical performances using another activation treatment. The activation methods and treatment conditions are listed in Table 3.

Table 3.
Activation methods and treatment conditions of the PAN-derived carbon nanofiber mats.
Figure 7a–c illustrate SEM images of sample PMC-3 activated by 20 wt% HNO3, 1:3 HNO3:H2SO4 and 1:1 HNO3:H2SO4, respectively. Figure 7a shows that sample PMC-3 activated by 20 wt% HNO3 possesses a rough surface in several areas. After treating with 1:3 HNO3:H2SO4, the nanofibers reveal a rougher surface, as displayed in Figure 7b. Figure 7c shows that there are obvious cracks appearing on the nanofiber surfaces after the activation of 1:1 HNO3:H2SO4. Figure 7d,e depict the CV curves at a scan rate of 5 mV/s and GCD curves at a current density of 0.1 mA/cm2 of sample PMC-3 activated using various methods. Compared with the untreated sample PMC-3, the areas of CV curves are all improved after the activations, indicating that the activation treatment might increase the active groups on the surfaces of the carbon nanofiber mats to provide more active sites and promote charge adsorption and desorption. Resultantly, the electrochemical performances of the carbon nanofiber mats are improved. Calculated from GCD curves, a maximum specific capacitance of 113.5 F/g of the carbon nanofiber mat was obtained in the case of 1:3 HNO3:H2SO4. Therefore, the electrochemical performances of the carbon nanofiber mats can be further improved by choosing a suitable activation method.
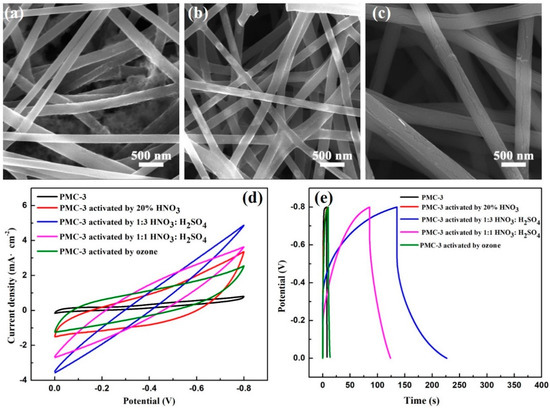
Figure 7.
SEM images of the sample PMC-3 activated by 20 wt% HNO3 (a), 1:3 HNO3: H2SO4 (b) and 1:1 HNO3: H2SO4 (c), respectively; electrochemical performance comparison of the activated carbon nanofiber mats: (d) CV curves at a scan rate of 5 mV/s in potential window of −0.8~0 V; (e) GCD curves at a current density of 0.1 mA/cm2.
4. Conclusions
Uniform PAN polymer nanofiber mats were fabricated using the electrospinning method. We investigated the effects of precursor concentration of PAN, thermal stabilization and carbonization temperature on the diameter, surface morphology, conductivity and flexibility of the carbon nanofibers. It is found that a precursor concentration of 12 wt% PAN, a thermal stabilization temperature of 270 °C and a carbonization temperature of 900 °C are the optimized experimental factors in this work. Furthermore, the electrical and mechanical properties of the carbon nanofibers could be improved by the addition of MWCNTs, most of which are embedded in the nanofibers. The optimal mass fraction of MWCNTs is 2.4 wt%, leading to a decrease in sheet resistance of the co-spun nanofiber mat to 60 Ω/sq. Finally, different activation strategies were explored to improve the electrochemical performances of the carbon nanofiber mats. The results suggest that the specific capacitance of the carbon nanofibers could be greatly increased from 18.8 F/g to 113.5 F/g with an activation treatment of 1:3 HNO3:H2SO4.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/9/18/3683/s1, Figure S1: SEM image of PAN-derived carbon nanofiber mats of the sample C-1 with a high magnification, Figure S2: SEM images of PAN-derived carbon nanofiber mats of the sample C-2 (a), C-3 (b) and C-4 (c) with a high magnification, Figure S3: SEM images of PAN-derived carbon nanofiber mats of the sample C-5 (a) and C-6 (b) with a high magnification.
Author Contributions
Conceptualization, X.H.; Data curation, Q.L.; Formal analysis, S.Z. and M.C.; Funding acquisition, X.H.; Investigation, J.L. and B.C.; Methodology, C.Z.; Writing—original draft, X.H.; Writing—review & editing, C.Z. and M.S.
Funding
This research was funded by Science Foundation for Young Teachers Projects of Wuyi University (2018td03); Science and Technology Projects of Jiangmen ((2017)307, (2017)149, (2018)352, (2018)359); Innovative Leading Talents of Jiangmen (Jiangmen(2019)7); Cooperative education platform of Guangdong Province ((2016)31); Key Laboratory of Optoelectronic Materials and Applications in Guangdong Higher Education (2017KSYS011); College Students Maker Space Project of Wuyi University (18KWL01).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Samuel, E.; Jo, H.S.; Joshi, B.; An, S.; Park, H.G.; Kim, Y.; Yoon, W.Y.; Yoon, S.S. Decoration of MnO nanocrystals on flexible freestanding carbon nanofibers for lithium ion battery anodes. Electrochim. Acta 2017, 231, 582–589. [Google Scholar] [CrossRef]
- Joshi, B.N.; An, S.; Kim, Y.I.; Samuel, E.P.; Song, K.Y.; Seong, I.W.; Al-Deyab, S.S.; Swihart, M.T.; Yoon, W.Y.; Yoon, S.S. Flexible freestanding Fe2O3-SnO-carbon nanofiber composites for Li ion battery anodes. J. Alloys Comp. 2017, 700, 259–266. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Wang, Y.; Dong, Y.; Qiu, J. Freestanding flexible Li2S paper electrode with high mass and capacity loading for high-energy Li-S batteries. Adv. Energy Mater. 2017, 7, 1700018. [Google Scholar] [CrossRef]
- Ding, X.; Zhou, H.; Wang, M.; Chen, M.; Li, L.; Yang, Z.; Wang, X. High rate performance and long cycle stability of lithium manganate nanofibers by tuned pre-oxidation treatment. J. Alloys Comp. 2017, 724, 975–980. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Wang, Y.; Shen, X.; Wang, F.; Li, H.; Hwang, B.J.; Zhao, J. Directly coating a multifunctional interlayer on the cathode via electrospinning for advanced lithium-sulfur batteries. ACS App. Mater. Interfaces 2017, 9, 29804–29811. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Guo, X.; Li, J.; Sun, H.; Zhang, H.; Wang, W. Electrospinning preparation and dye adsorption capacity of TiO2@carbon flexible fiber. Ceram. Int. 2019, 45, 11856–11860. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Huang, J.; Zhang, W.; Ji, W.; Hui, Y.; Yao, X. Electrospinning synthesis of porous carbon fiber supported Pt-SnO2 anode catalyst for direct ethanol fuel cell. Solid State Sci. 2019, 94, 64–69. [Google Scholar] [CrossRef]
- Yun, S.I.; Kim, S.H.; Kim, D.W.; Kim, Y.A.; Kim, B.H. Facile preparation and capacitive properties of low-cost carbon nanofibers with ZnO derived from lignin and pitch as supercapacitor electrodes. Carbon 2019, 149, 637–645. [Google Scholar] [CrossRef]
- Dong, X.; Guo, Z.; Song, Y.; Hou, M.; Wang, J.; Wang, Y.; Xia, Y. Flexible and wire-shaped micro-supercapacitor based on Ni(OH)2-nanowire and ordered mesoporous carbon electrodes. Adv. Funct. Mater. 2014, 24, 3405–3412. [Google Scholar] [CrossRef]
- Ng, C.H.; Lim, H.N.; Lim, Y.S.; Chee, W.K.; Huang, N.M. Fabrication of flexible polypyrrole/graphene oxide/manganese oxide supercapacitor. Int. J. Energy Res. 2015, 39, 344–355. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef] [PubMed]
- Couly, C.; Alhabeb, M.; Van Aken, K.L.; Kurra, N.; Gomes, L.; Navarro-Suárez, A.M.; Anasori, B.; Alshareef, H.N.; Gogotsi, Y. Asymmetric flexible MXene-reduced graphene oxide micro-supercapacitor. Adv. Electron. Mat. 2018, 4, 1700339. [Google Scholar] [CrossRef]
- Souza, V.H.R.; Oliveira, M.M.; Zarbin, A.J.G. Bottom-up synthesis of graphene/polyaniline nanocomposites for flexible and transparent energy storage devices. J. Power Sources 2017, 348, 87–93. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, Y.; Sun, N.; Wen, Z.; Cheng, P.; Xie, X.; Shao, H.; Shen, Q.; Chen, X.; Liu, Y.; et al. Flexible self-charging power units for portable electronics based on folded carbon paper. Nano Res. 2018, 11, 4313–4322. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A potential material for energy conversion and storage. Prog. Energy Combust. Sci. 2018, 64, 219–253. [Google Scholar] [CrossRef]
- Hu, Y.; Guo, P.; Thompson, J.; Wang, Z. Rational design of mesoporous LiFePO4@C nanofibers as cathode materials for energy storage. Ceram. Int. 2017, 43, 10201–10206. [Google Scholar] [CrossRef]
- Wang, X.; Xi, M.; Wang, X.; Fong, H.; Zhu, Z. Flexible composite felt of electrospun TiO2 and SiO2 nanofibers infused with TiO2 nanoparticles for lithium ion battery anode. Electrochim. Acta 2016, 190, 811–816. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, Y.; Ding, Y.; Shende, A.R.; Fong, H.; Shende, R.V. Mechanically flexible electrospun carbon nanofiber mats derived from biochar and polyacrylonitrile. Mater. Lett. 2017, 205, 206–210. [Google Scholar] [CrossRef]
- Ning, H.; Xie, H.; Zhao, Q.; Liu, J.; Tian, W.; Wang, Y.; Wu, M. Electrospinning ZnO/carbon nanofiber as binder-free and self-supported anode for Li-ion batteries. J. Alloys Compd. 2017, 722, 716–720. [Google Scholar] [CrossRef]
- Vijayan, B.L.; Krishnan, S.G.; Zain, N.K.M.; Harilal, M.; Yar, A.; Misnon, I.I.; Dennis, J.O.; Yusoff, M.M.; Jose, R. Large scale synthesis of binary composite nanowires in the Mn2O3-SnO2 system with improved charge storage capabilities. Chem. Eng. J. 2017, 327, 962–972. [Google Scholar] [CrossRef]
- Qin, R.; Shao, G.; Hou, J.; Zheng, Z.; Zhai, T.; Li, H. One-pot synthesis of Li3VO4@C nanofibers by electrospinning with enhanced electrochemical performance for lithium-ion batteries. Sci. Bull. 2017, 62, 1081–1088. [Google Scholar] [CrossRef]
- Mao, M.; Yan, F.; Cui, C.; Ma, J.; Zhang, M.; Wang, T.; Wang, C. Pipe-wire TiO2-Sn@carbon nanofibers paper anodes for lithium and sodium ion batteries. Nano Lett. 2017, 17, 3830–3836. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, Y.; Si, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun polyvinylidene fluoride/SiO2 nanofibrous membranes with enhanced electret property for efficient air filtration. Compos. Commun. 2019, 13, 57–62. [Google Scholar] [CrossRef]
- Khatti, T.; Naderi-Manesh, H.; Kalantar, S.M. Application of ANN and RSM techniques for modeling electrospinning process of polycaprolactone. Neural Comput. Appl. 2017, 31, 239–248. [Google Scholar] [CrossRef]
- Wang, Z.; Song, G.; Xu, J.; Fu, Q.; Pan, C. Electrospun titania fibers by incorporating graphene/Ag hybrids for the improved visible-light photocatalysis. Front. Mat. Sci. 2018, 12, 379–391. [Google Scholar] [CrossRef]
- Perera Jayawickramage, R.A.; Balkus, K.J.; Ferraris, J.P. Binder free carbon nanofiber electrodes derived from polyacrylonitrile-lignin blends for high performance supercapacitors. Nanotechnology 2019, 30, 355402. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Wang, B.; Wang, H. Effects of stabilization temperature on structures and properties of polyacrylonitrile (PAN)-based stabilized electrospun nanofiber mats. J. Macromol. Sci. Part B 2012, 51, 2428–2437. [Google Scholar] [CrossRef]
- Lei, S.; Cao, W.; Fu, Z.; Xu, L. The conjugated plane formed in polyacrylonitrile during thermal stabilization. J. Appl. Polym. Sci. 2016, 133, 43890. [Google Scholar] [CrossRef]
- Song, C.; Wang, T.; Qiu, Y.; Qiu, J.; Cheng, H. Effect of carbonization atmosphere on the structure changes of PAN carbon membranes. J. Porous Mater. 2009, 16, 197–203. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).