Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass and Extracellular Enzyme Activities—A Case Study in Mu Us Sandy Land in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Experimental Design and Soil Sampling
2.3. Analysis of Soil Physicochemical Properties and Microbial Biomass
2.4. Analysis of Soil Extracellular Enzyme Activities
2.5. Statistical Analyses
3. Results
3.1. Effects of Mixing Feldspathic Sandstone and Sand on Soil Physicochemical Properties
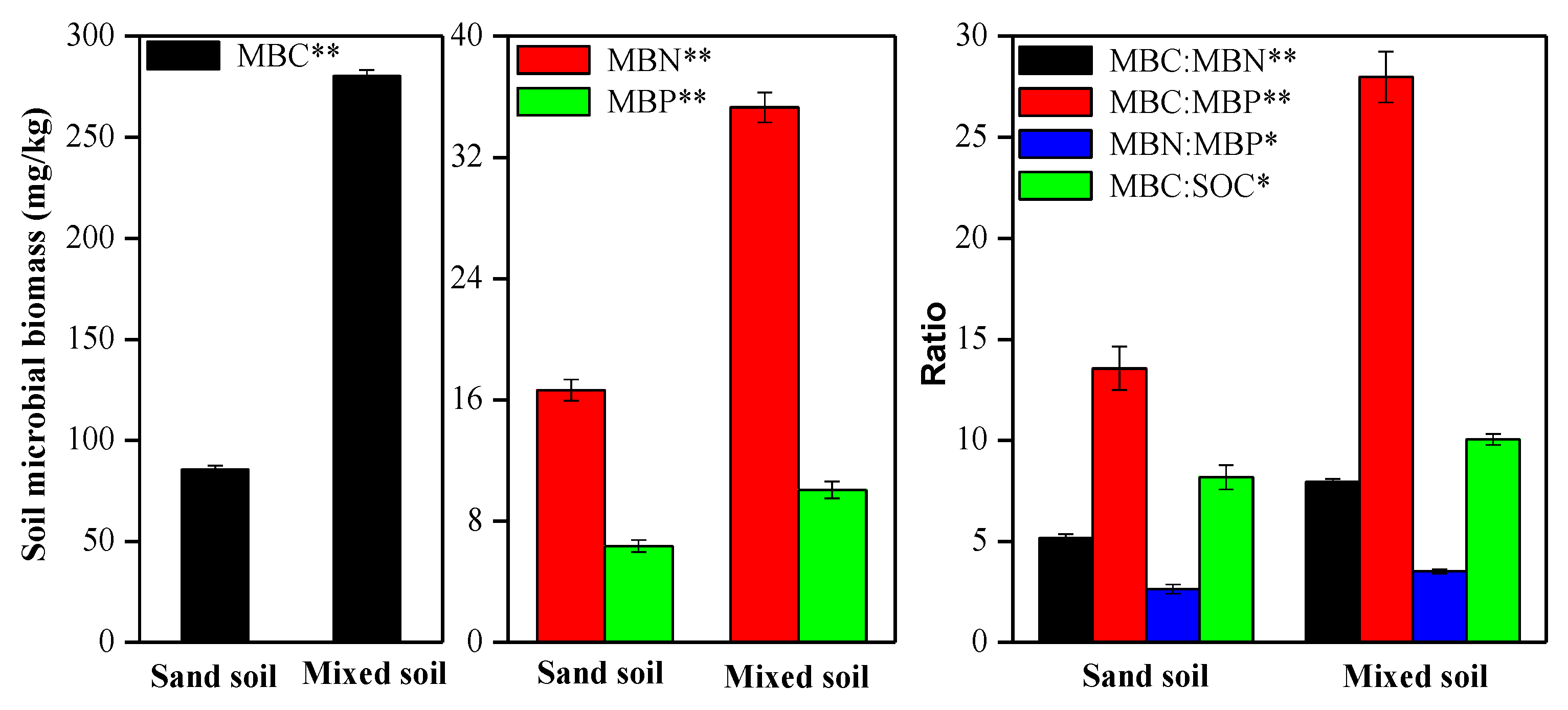
3.2. Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass Indices
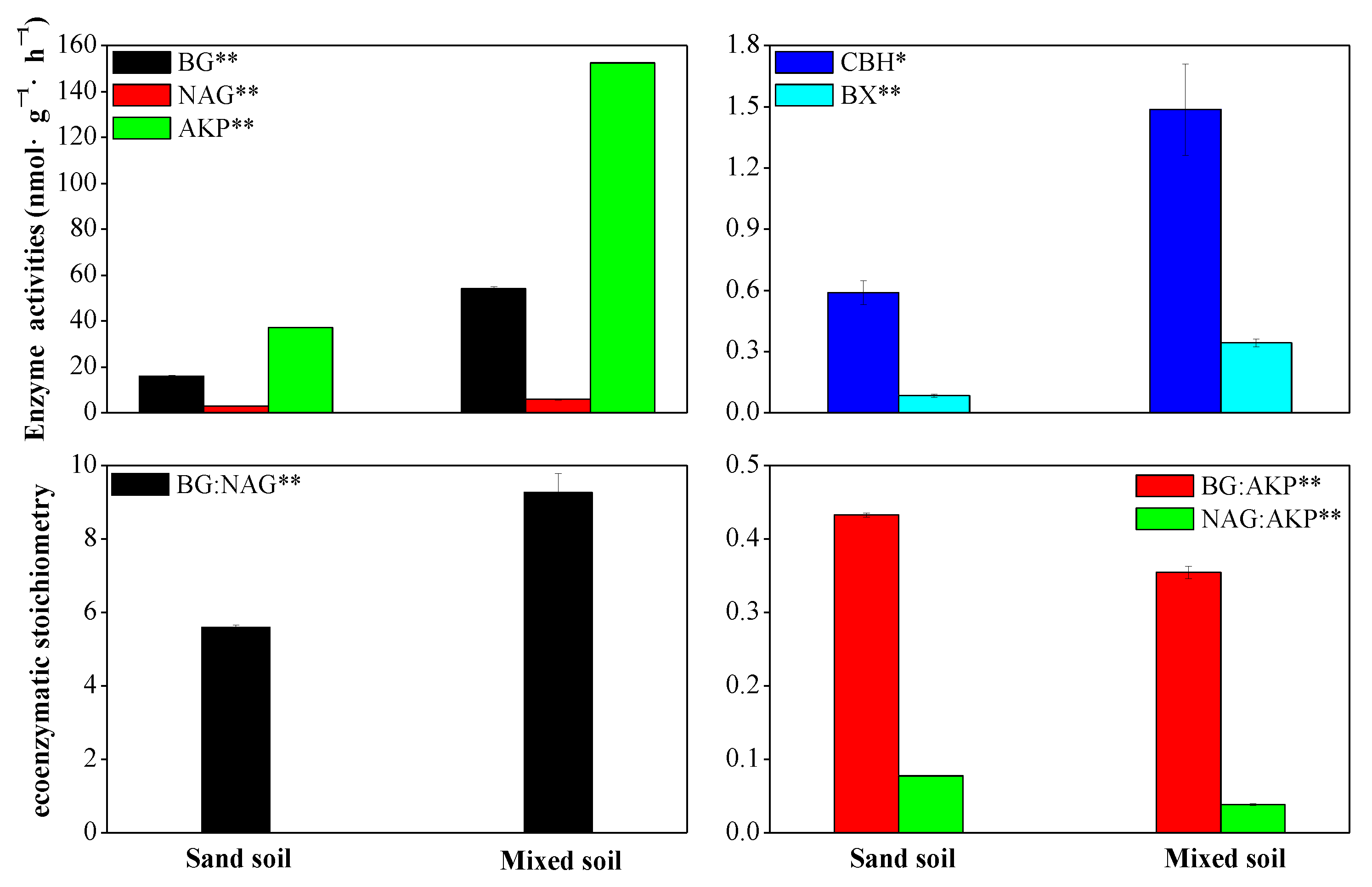
3.3. Effects of Mixing Feldspathic Sandstone and Sand on Soil Extracellular Enzymes Activities and Its Stoichiometry
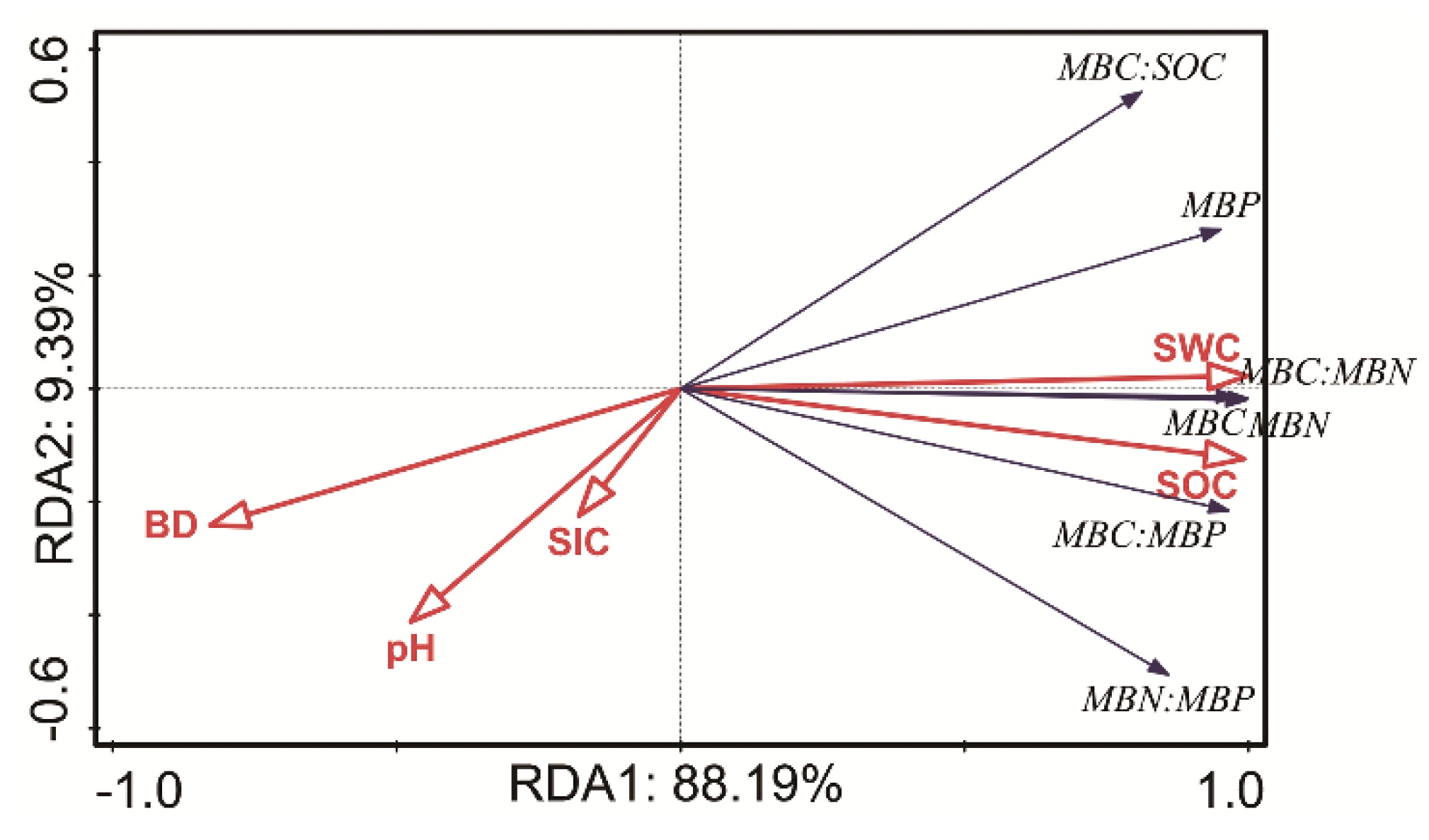
3.4. Relationships among Soil Physiochemical Properties, Microbial Biomass and Enzyme Activity
4. Discussion
4.1. Explaining Variance in Soil Physiochemical Properties in Sand vs. a Mix of Sand and Feldspathic Sandstone
4.2. Explaining Variance in Soil Microbial Biomass Indices in Sand vs. a Mix of Sand and Feldspathic Sandstone
4.3. Explaining Variance in Soil Extracellular Enzyme Activity Metrics in Sand vs. a Mix of Sand and Feldspathic Sandstone
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Mixed soil | a mix of sand and feldspathic sandstone |
| RDA | redundancy analysis |
| Soil characteristics | |
| EC | soil electrical conductivity |
| BD | soil bulk density |
| STC | soil total carbon |
| SOC | soil organic carbon |
| SIC | soil inorganic carbon |
| SWC | soil water content |
| FC | field capacity |
| Soil microbial biomass characteristics | |
| MBC | microbial biomass carbon |
| MBN | microbial biomass nitrogen |
| MBP | microbial biomass phosphorus |
| MBC:SOC | microbial quotient |
| Extracellular enzyme activity characteristics | |
| BG | β-1,4-glucosidase |
| BX | β-1,4-xylosidase |
| CBH | Cellobiohydrolase |
| NAG | β-N-acetylglucosaminidase |
| AKP | Alkaline phosphatase |
| VL | vector length |
| VA | vector angle |
References
- Wang, F.; Pan, X.; Wang, D.; Shen, C.; Lu, Q. Combating desertification in China: Past, present and future. Land Use Policy 2013, 31, 311–313. [Google Scholar] [CrossRef]
- Wang, N.; Xie, J.; Han, J. A Sand Control and Development Model in Sandy Land Based on Mixed Experiments of Arsenic Sandstone and Sand: A Case Study in Mu Us Sandy Land in China. Chin. Geogr. Sci. 2013, 23, 700–707. [Google Scholar] [CrossRef]
- Tapia-Torres, Y.; Elser, J.J.; Souza, V.; Garcia-Oliva, F. Ecoenzymatic stoichiometry at the extremes: How microbes cope in an ultra-oligotrophic desert soil. Soil Biol. Biochem. 2015, 87, 34–42. [Google Scholar] [CrossRef]
- Campbell, A.; Miles, L.; Lysenko, I.; Huges, A.; Gibbs, H. Carbon Storage in Protected Areas; Technical Report; UNEP World Conservation Monitoring Center: Cambridge, UK, 2008. [Google Scholar]
- Zhang, L.; Ban, J. Analyzing the Sand-fixing Effect of Feldspathic Sandstone from the Texture Characteristics. In Proceedings of the Iop Conference Series: Earth & Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; p. 032039. [Google Scholar]
- Wang, N.; Xie, J.; Han, J.; Luo, L. A comprehensive framework on land-water resources development in Mu Us Sandy Land. Land Use Policy 2014, 40, 69–73. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Li, Y.K.; Li, F.M.; Ma, Q.; Zhang, P.L.; Yin, P. Changes in soil organic carbon, nutrients and aggregation after conversion of native desert soil into irrigated arable land. Soil Tillage Res. 2009, 104, 263–269. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, L.-Y.; Peng, Y.-F.; Ding, J.-Z.; Li, F.; Yang, G.-B.; Kou, D.; Liu, L.; Fang, K.; Zhang, B.-B.; et al. Linking microbial C:N:P stoichiometry to microbial community and abiotic factors along a 3500-km grassland transect on the Tibetan Plateau. Glob. Ecol. Biogeogr. 2016, 25, 1416–1427. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Ren, C.J.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of soil microbial and enzyme activities are linked to soil C, N and P stoichiometry in afforested ecosystems. For. Ecol. Manag. 2018, 427, 289–295. [Google Scholar] [CrossRef]
- Hu, N.; Li, H.; Tang, Z.; Li, Z.; Li, G.; Jiang, Y.; Hu, X.; Lou, Y. Community size, activity and C:N stoichiometry of soil microorganisms following reforestation in a Karst region. Eur. J. Soil Biol. 2016, 73, 77–83. [Google Scholar] [CrossRef]
- Paul, E.A.; Clark, F.E. Soil Microbiology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996; p. 340. [Google Scholar]
- Elser, J.J.; Acharya, K.; Kyle, M.; Cotner, J.; Makino, W.; Markow, T.; Watts, T.; Hobbie, S.; Fagan, W.; Schade, J.; et al. Growth rate-stoichiometry couplings in diverse biota. Ecol. Lett. 2003, 6, 936–943. [Google Scholar] [CrossRef]
- Schimel, J.P.; Weintraub, M.N. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: A theoretical model. Soil Biol. Biochem. 2003, 35, 549–563. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Treseder, K.K. Soil extracellular enzyme activities correspond with abiotic factors more than fungal community composition. Biogeochemistry 2014, 117, 23–37. [Google Scholar] [CrossRef]
- Chapin, F.S.I.; Matson, P.A.; Mooney, H.A. Terrestrial Nutrient Cycling. In Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Peng, X.; Wang, W. Stoichiometry of soil extracellular enzyme activity along a climatic transect in temperate grasslands of northern China. Soil Biol. Biochem. 2016, 98, 74–84. [Google Scholar] [CrossRef]
- Han, J.; Xie, J.; Zhang, Y. Potential role of feldspathic sandstone as a natural water retaining agent in Mu Us Sandy Land, Northwest China. Chin. Geogr. Sci. 2012, 22, 550–555. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Zhang, Y. Sand stabilization effect of feldspathic sandstone during the fallow period in Mu Us Sandy Land. J. Geogr. Sci. 2015, 25, 428–436. [Google Scholar] [CrossRef]
- Nelson, D.W. Total carbon, organic carbon and organic matter. Methods Soil Anal. 1996, 9, 961–1010. [Google Scholar]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, J.; Lammers, P.S.; Damerow, L.; Hueging, H.; Zhang, H.; Sun, W. Predicting surface porosity using a fine-scale index of roughness in a cultivated field. Soil Tillage Res. 2009, 103, 57–64. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Brookes, P.C.; Powlson, D.S.; Jenkinson, D.S. Measurement of microbial biomass phosphorus in soil. Soil Biol. Biochem. 1982, 14, 319–329. [Google Scholar] [CrossRef]
- Tischer, A.; Potthast, K.; Hamer, U. Land-use and soil depth affect resource and microbial stoichiometry in a tropical mountain rainforest region of southern Ecuador. Oecologia 2014, 175, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme-based decomposition models. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Carreiro, M.M.; Repert, D.A. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Silver, W.L.; Neff, J.; McGroddy, M.; Veldkamp, E.; Keller, M.; Cosme, R. Effects of soil texture on belowground carbon and nutrient storage in a lowland Amazonian forest ecosystem. Ecosystems 2000, 3, 193–209. [Google Scholar] [CrossRef]
- Li, J.; Han, J.C.; Zhang, Y.; Lei, G.Y.; Wang, H.Y.; Zhu, D.W. Effects of composite soil with feldspathic sandstone and sand on soil aggregates and organic carbon. In Proceedings of the 2nd International Conference on Agricultural and Biological Sciences, Shanghai, China, 23–26 July 2016; Golabi, M.H., Ed.; IOP Publishing: London, UK, 2016; Volume 41, p. 012002. [Google Scholar]
- Xu, X.; Thornton, P.E.; Post, W.M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems. Glob. Ecol. Biogeogr. 2013, 22, 737–749. [Google Scholar] [CrossRef]
- Lou, Y.; Liang, W.; Xu, M.; He, X.; Wang, Y.; Zhao, K. Straw coverage alleviates seasonal variability of the topsoil microbial biomass and activity. Catena 2011, 86, 117–120. [Google Scholar] [CrossRef]
- Mcgill, W.B.; Cannon, K.R.; Robertson, J.A.; Cook, F.D. Dynamics of Soil Microbial Biomass and Water-Soluble Organic C in Breton L After 50 Years of Cropping to Two Rotations. Can. J. Soil Sci. 1986, 66, 1–19. [Google Scholar] [CrossRef]
- Marinari, S.; Masciandaro, G.; Ceccanti, B.; Grego, S. Influence of organic and mineral fertilisers on soil biological and physical properties. Bioresour. Technol. 2000, 72, 9–17. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, W.; Zhong, Z.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass, diversity, and compositions to altitudinal gradients depend on plant and soil characteristics. Sci. Total Environ. 2018, 610, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, A.; Pandey, H.N. Ecosystem restoration of jhum fallows in northeast India: Microbial C and N along altitudinal and successional gradients. Restor. Ecol. 2003, 11, 168–173. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Yang, L.; Zheng, H.; Liu, Y.; Yang, W.; Zhang, J. Effects of simulated climate warming on soil microbial biomass carbon, nitrogen and phosphorus of alpine forest. Chin. J. Appl. Environ. Biol. 2016, 22, 599–605. [Google Scholar] [CrossRef]
- Jia, G.M.; Cao, J.; Wang, C.Y.; Wang, G. Microbial biomass and nutrients in soil at the different stages of secondary forest succession in Ziwulin, northwest China. For. Ecol. Manag. 2005, 217, 117–125. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: The growth rate hypothesis in reverse. Biogeochemistry 2011, 102, 31–43. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem. 1989, 21, 471–479. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J. Differential Nutrient Limitation of Soil Microbial Biomass and Metabolic Quotients (qCO(2)): Is There a Biological Stoichiometry of Soil Microbes? PLoS ONE 2013, 8, e57127. [Google Scholar] [CrossRef] [PubMed]
- Junhua, L.I.; Qirong, S.; Guixin, C.H.U.; Changzhou, W.E.I.; Xu, Q.; Xingming, Y. Effects of Application Amino Acid Fertilizer on Soil Enzyme Activity and Available Nutrients in Cotton Rhizosphere and Bulk Soils. Soils 2011, 43, 277–284. [Google Scholar]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry and ecological theory. In Annual Review of Ecology, Evolution, and Systematics; Futuyma, D.J., Ed.; Annual Reviews: Palo Alto, CA, USA, 2012; Volume 43, pp. 313–343. [Google Scholar]
- Khalili-Rad, M.; Nourbakhsh, F.; Jalalian, A.; Eghbal, M.K. The Effects of Slope Position on Soil Biological Properties in an Eroded Toposequence. Arid Land Res. Manag. 2011, 25, 308–312. [Google Scholar] [CrossRef]
- Bell, C.W.; Tissue, D.T.; Loik, M.E.; Wallenstein, M.D.; Acosta-Martinez, V.; Erickson, R.A.; Zak, J.C. Soil microbial and nutrient responses to 7 years of seasonally altered precipitation in a Chihuahuan Desert grassland. Glob. Chang. Biol. 2014, 20, 1657–1673. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.-H.; Min, H.; Lu, Z.-H.; Yuan, H.-P. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource Limitation in Plants—An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Snajdr, J.; Valaskova, V.; Merhautova, V.; Herinkova, J.; Cajthaml, T.; Baldrian, P. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008, 40, 2068–2075. [Google Scholar] [CrossRef]
| Enzyme | EC | Abbr. | Substrate | Function |
|---|---|---|---|---|
| β-1,4-glucosidase | EC 3.2.1.21 | BG | 4-MUB-β-D-glucoside | Hydrolysis of cellobiose to glucose during carbon cycling |
| β-1,4-xylosidase | EC 3.2.1.37 | BX | 4-MUB-β-D-xylopyranoside | Hydrolysis of cellulose to form hemicellulose during carbon cycle |
| Cellobiohydrolase | EC 3.2.1.91 | CBH | 4-MUB-β-D-cellobioside | Hydrolysis of cellulose to produce sucrose during carbon cycle |
| β-N-acetylglucosaminidase | EC 3.2.1.14 | NAG | 4-MUB-N-acetyl-β-D-glucosaminide | Participation in the hydrolysis of chitin during the carbon-nitrogen cycle |
| Alkaline phosphatase | EC 3.1.3.1 | AKP | 4-MUB-phosphate | Hydrolyses phosphate from phosphosaccarides and phospholipids during the phosphorus cycle |
| Characteristics | Sand Soil | Mixed Soil | F (1, 5) | p |
|---|---|---|---|---|
| Texture | sand | sandy loam | / | / |
| pH | 8.34 ± 0.07 | 8.16 ± 0.17 | 1.07 | 0.36 |
| EC (μs/cm) | 181.73 ± 14.98 | 201.37 ± 35.52 | 0.26 | 0.64 |
| BD (g/cm3) | 1.63 ± 0.05 | 1.47 ± 0.02 | 9.13 | 0.04 * |
| SWC (%) | 2.66 ± 0.08 | 4.61 ± 0.04 | 479.31 | <0.01 ** |
| STC (g/kg) | 1.61 ± 0.21 | 3.25 ± 0.24 | 26.17 | <0.01 ** |
| SOC (g/kg) | 1.06 ± 0.09 | 2.79 ± 0.06 | 252.95 | <0.01 ** |
| SIC (g/kg) | 0.55 ± 0.18 | 0.46 ± 0.18 | 0.13 | 0.74 |
| Porosity (%) | 25.31 | 33.46 | 17.03 | 0.02 * |
| Field capacity (%) | 6.88 | 16.51 | 216.39 | <0.01 ** |
| Metric | Sand Soil | Mixed Soil | F (1, 5) | p |
|---|---|---|---|---|
| BG/(BG + AKP) | 0.302 ± 0.001 | 0.262 ± 0.005 | 67.09 | <0.01 ** |
| BG/(BG + NAG) | 0.85 ± 0.01 | 0.90 ± 0.01 | 116.41 | <0.01 ** |
| Vector length | 0.90 ± 0.01 | 0.94 ± 0.01 | 39.91 | <0.01 ** |
| Vector angle | 70.41 ± 0.06 | 73.82 ± 0.20 | 278.87 | <0.01 ** |
| (a) | Edaphic Abiotic Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | EC | BD | SWC | STC | SOC | SIC | Porosity | FC | |
| BG | −0.421 | 0.260 | −0.845 * | 0.993 ** | 0.936 ** | 0.994 ** | −0.171 | 0.886 * | 0.992 ** |
| NAG | −0.574 | 0.350 | −0.790 | 0.987 ** | 0.877 * | 0.972 ** | −0.296 | 0.906 * | 0.967 ** |
| AKP | −0.476 | 0.276 | −0.829 * | 0.996 ** | 0.923 ** | 0.991 ** | −0.204 | 0.900 * | 0.989 ** |
| CBH | −0.575 | −0.013 | −0.587 | 0.896 * | 0.858 * | 0.890 * | −0.084 | 0.944 ** | 0.857 * |
| BX | −0.432 | 0.376 | −0.815 * | 0.975 ** | 0.913 * | 0.977 ** | −0.187 | 0.832 * | 0.970 ** |
| BG:NAG | −0.229 | 0.140 | −0.867 * | 0.951 ** | 0.956 ** | 0.971 ** | −0.027 | 0.827 * | 0.971 ** |
| BG:AKP | 0.609 | −0.311 | 0.731 | −0.973 ** | −0.873 * | −0.958 ** | 0.260 | −0.913 * | −0.944 ** |
| NAG:AKP | 0.416 | −0.220 | 0.846 * | −0.994 ** | −0.941 ** | −0.995 ** | 0.154 | −0.897 * | −0.993 ** |
| BG/(BG + AKP) | 0.618 | −0.312 | 0.729 | −0.972 ** | −0.868 * | −0.955 ** | 0.268 | −0.914 * | −0.942 ** |
| BG/(BG + NAG) | −0.325 | 0.171 | −0.872 * | 0.977 ** | 0.948 ** | 0.986 ** | −0.100 | 0.869 * | 0.989 ** |
| Vector length | −0.226 | 0.121 | −0.887 * | 0.946 ** | 0.940 ** | 0.961 ** | −0.048 | 0.826 * | 0.970 ** |
| Vector angle | −0.543 | 0.277 | −0.786 | 0.992 ** | 0.907 * | 0.982 ** | −0.225 | 0.917 ** | 0.974 ** |
| (b) | Edaphic Biotic Factors | ||||||||
| MBC | MBN | MBP | MBC:MBN | MBC:MBP | MBN:MBP | MBC:SOC | |||
| BG | 0.996 ** | 0.986 ** | 0.921 ** | 0.986 ** | 0.983 ** | 0.884 * | 0.794 | ||
| NAG | 0.988 ** | 0.992 ** | 0.942 ** | 0.955 ** | 0.951 ** | 0.864 * | 0.818 * | ||
| AKP | 0.999 ** | 0.993 ** | 0.938 ** | 0.982 ** | 0.975 ** | 0.875 * | 0.812 * | ||
| CBH | 0.903 * | 0.924 ** | 0.930 ** | 0.844 * | 0.815 * | 0.731 | 0.725 | ||
| BX | 0.985 ** | 0.971 ** | 0.896 * | 0.984 ** | 0.982 ** | 0.886 * | 0.806 | ||
| BG:NAG | 0.955 ** | 0.931 ** | 0.853 * | 0.970 ** | 0.970 ** | 0.864 * | 0.722 | ||
| BG:AKP | −0.979 ** | −0.988 ** | −0.952 ** | −0.940 ** | −0.927 ** | −0.839 * | −0.822 * | ||
| NAG:AKP | −0.996 ** | −0.986 ** | −0.928 ** | −0.985 ** | −0.979 ** | −0.876 * | −0.794 | ||
| BG/(BG + AKP) | −0.977 ** | −0.986 ** | −0.953 ** | −0.937 ** | −0.923 ** | −0.834 * | −0.824 * | ||
| BG/(BG + NAG) | 0.978 ** | 0.961 ** | 0.896 * | 0.980 ** | 0.975 ** | 0.867 * | 0.764 | ||
| Vector length | 0.945 ** | 0.921 ** | 0.849 * | 0.960 ** | 0.957 ** | 0.847 * | 0.720 | ||
| Vector angle | 0.996 ** | 0.997 ** | 0.954 ** | 0.967 ** | 0.956 ** | 0.859 * | 0.822 * | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Zhang, L.; Zhao, F.; Bai, H.; Doughty, R. Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass and Extracellular Enzyme Activities—A Case Study in Mu Us Sandy Land in China. Appl. Sci. 2019, 9, 3963. https://doi.org/10.3390/app9193963
Feng X, Zhang L, Zhao F, Bai H, Doughty R. Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass and Extracellular Enzyme Activities—A Case Study in Mu Us Sandy Land in China. Applied Sciences. 2019; 9(19):3963. https://doi.org/10.3390/app9193963
Chicago/Turabian StyleFeng, Xiuxiu, Lu Zhang, Fazhu Zhao, Hongying Bai, and Russell Doughty. 2019. "Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass and Extracellular Enzyme Activities—A Case Study in Mu Us Sandy Land in China" Applied Sciences 9, no. 19: 3963. https://doi.org/10.3390/app9193963
APA StyleFeng, X., Zhang, L., Zhao, F., Bai, H., & Doughty, R. (2019). Effects of Mixing Feldspathic Sandstone and Sand on Soil Microbial Biomass and Extracellular Enzyme Activities—A Case Study in Mu Us Sandy Land in China. Applied Sciences, 9(19), 3963. https://doi.org/10.3390/app9193963






