The Therapeutic Potential of the Labdane Diterpenoid Forskolin
Abstract
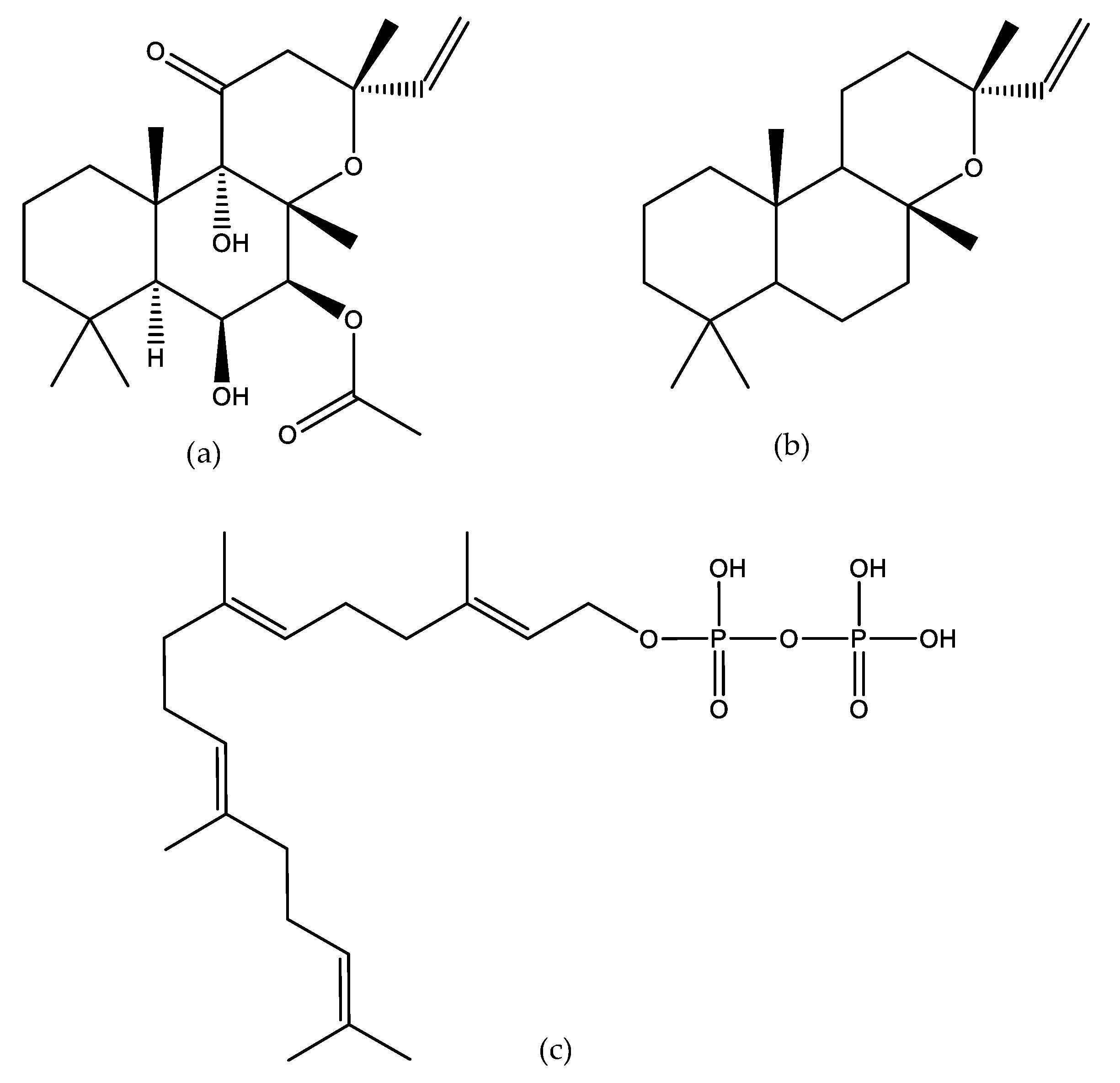
:1. Introduction
2. Pharmacological Activities of Forskolin
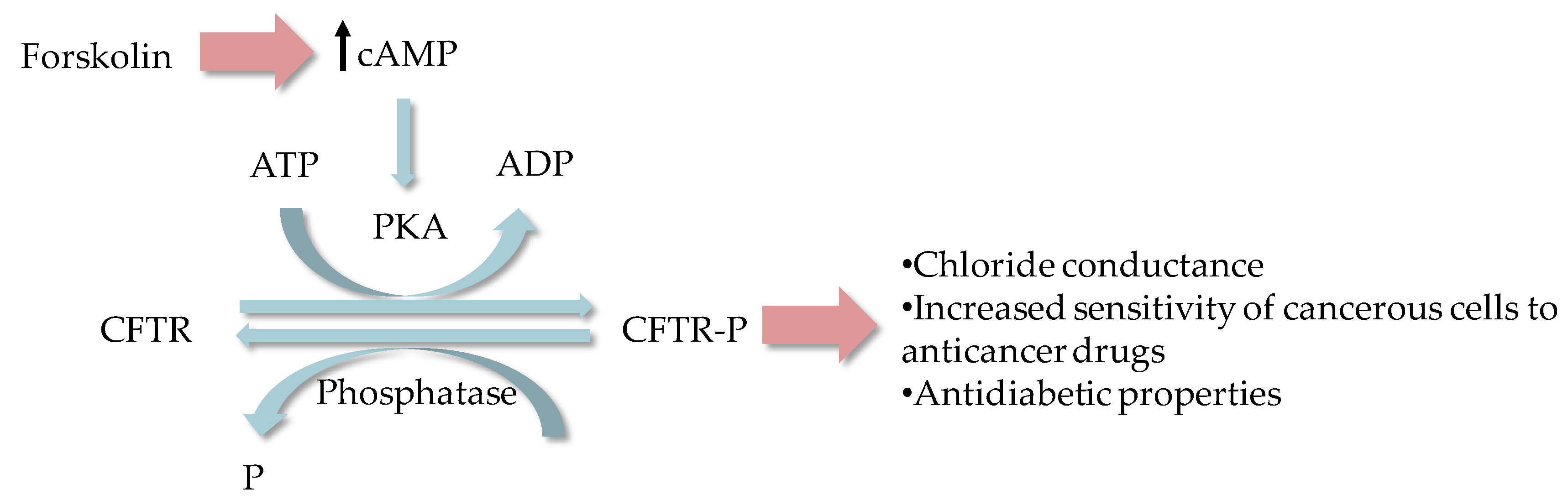
2.1. Cystic Fibrosis
2.2. Cardiovascular Diseases
2.3. Obesity
2.4. Asthma, COPD, and Other Allergies
2.5. Cancer
2.6. Diabetes
2.7. Intraocular Pressure in Glaucoma
2.8. Liver Fibrosis
3. Other Effects
4. Bioavailability of Forskolin
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wink, M. Modes of action of herbal medicines and plant secondary metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Sytar, O.; Dursunoglu, B.; Ozbek, H.; Duman, H.; Guvenalp, Z.; Kılıc, C.S. Antioxidant and anticholinesterase potential of ferulago cassia with farther bio-guided isolation of active coumarin constituents. S. Afr. J. Bot. 2019, 121, 536–542. [Google Scholar] [CrossRef]
- Seigler, D.S. Plant Secondary Metabolism; Kluwer Academic Publishers: Boston, MA, USA, 1995. [Google Scholar]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochemistry 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Sytar, O.; Brestic, M.; Rai, M. Possible ways of fagopyrin biosynthesis and production in buckwheat plants. Fitoterapia 2013, 84, 72–79. [Google Scholar] [CrossRef]
- Pichersky, E.; Noel, J.P.; Dudareva, N. Biosynthesis of plant volatiles: Nature’s diversity and ingenuity. Science 2006, 311, 808–811. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef]
- Jansen, D.J.; Shenvi, R.A. Synthesis of medicinally relevant terpenes: Reducing the cost and time of drug discovery. Future Med. Chem. 2014, 6, 1127–1148. [Google Scholar] [CrossRef]
- Vranova, E.; Coman, D.; Gruissem, W. Structure and dynamics of the isoprenoid pathway network. Mol. Plant 2012, 5, 318–333. [Google Scholar] [CrossRef]
- De Souza, N.J. Industrial development of traditional drugs: The forskolin example. A mini-review. J. Ethnopharmacol. 1993, 38, 177–180. [Google Scholar] [CrossRef]
- Croteau, R.; Ketchum, R.E.B.; Long, R.M.; Kaspera, R.; Wildung, M.R. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006, 5, 75–97. [Google Scholar] [CrossRef]
- Pollier, J.; Moses, T.; Goossens, A. Combinatorial biosynthesis in plants: A (p)review on its potential and future exploitation. Nat. Prod. Rep. 2011, 28, 1897–1916. [Google Scholar] [CrossRef] [PubMed]
- Numonov, S.; Sharopov, F.; Salimov, A.; Sukhrobov, P.; Atolikshoeva, S.; Safarzoda, R.; Habasi, M.; Aisa, H.A. Assessment of artemisinin contents in selected artemisia species from tajikistan (Central Asia). Medicines 2019, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Vanisree, M.; Lee, C.; Lo, S.; Satish, M.; Lin, C.; Tsay, H.S. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot. Bull. Acad. Sin. 2004, 45, 1–22. [Google Scholar]
- Kanne, H.; Burte, N.P.; Prasanna, V.; Gujjula, R. Extraction and elemental analysis of coleus forskohlii extract. Pharmacogn. Res. 2015, 7, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.; Rajamani, K.; Vadivel, E. Coleus forskohlii: A comprehensive review on morphology, phytochemistry and pharmacological aspects. J. Med. Plants Res. 2010, 4, 278–285. [Google Scholar]
- Lakshmanan, G.M.; Manikandan, S. Review on pharmacological effects of Plectranthus forskohli (wild) briq. Int. Lett. Nat. Sci. 2015, 1, 1–9. [Google Scholar]
- Tamboli, E.T.; Chester, K.; Ahmad, S. Quality control aspects of herbs and botanicals in developing countries: Coleus forskohlii briq a case study. J. Pharm. Bioallied Sci. 2015, 7, 254–259. [Google Scholar]
- Bhowal, M.; Mehta, D.M. Coleus forskholii: Phytochemical and pharmacological profile. Int. J. Pharm. Sci. Res. 2017, 8, 3599–3618. [Google Scholar]
- Wagh, V.D.; Patil, P.N.; Surana, S.J.; Wagh, K.V. Forskolin: Upcoming antiglaucoma molecule. J. Postgrad. Med. 2012, 58, 199–202. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Elwia, S.K.; Elnoury, H.A.; Muhammad, M.H. Forskolin effect on foxo1 expression and relationship of foxo1 activation to oxidative stress: From molecular to therapeutic strategy. Biomarkers 2018, 4, 11. [Google Scholar]
- Pateraki, I.; Andersen-Ranberg, J.; Jensen, N.B.; Wubshet, S.G.; Heskes, A.M.; Forman, V.; Hallstrom, B.; Hamberger, B.; Motawia, M.S.; Olsen, C.E.; et al. Total biosynthesis of the cyclic amp booster forskolin from coleus forskohlii. eLife 2017, 6, e23001. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.S.; Desireddy, R.B.; Ciddi, V. A review on forskolin: A cyclic AMP modulator from tissue cultures of Coleus forskohlii. Pharmacogn. Mag. 2005, 1, 85–88. [Google Scholar]
- Doseyici, S.; Mehmetoglu, I.; Toker, A.; Yerlikaya, F.H.; Erbay, E. The effects of forskolin and rolipram on camp, cgmp and free fatty acid levels in diet induced obesity. Biotech. Histochem. 2014, 89, 388–392. [Google Scholar] [CrossRef]
- Gonska, T.; The Hospital for Sick Children. Canadian Observation Trial in cf Patients Undergoing Treatment with Ivacaftor. 2013. Available online: https://ClinicalTrials.gov/show/NCT03390985 (accessed on 31 August 2019).
- Institut National de la Santé Et de la Recherche Médicale; ABCF2. Primary Nasal Cell Culture as a Tool for Personalized Therapy in Cystic Fibrosis. 2010. Available online: https://ClinicalTrials.gov/show/NCT03652090 (accessed on 31 August 2019).
- University of Lincoln; National Health Service, U.K. Association of Physical Activity Levels and Inflammatory Markers Following Pulmonary Rehabilitation. 2018. Available online: https://ClinicalTrials.gov/show/NCT03455153 (accessed on 31 August 2019).
- Olive Lifesciences Pvt Ltd. The Effect of Coleus forskohlii Extract on the Risk Factors of Metabolic Syndrome. 2014. Available online: https://ClinicalTrials.gov/show/NCT02143349 (accessed on 31 August 2019).
- Assistance Publique—Hôpitaux de Paris. Bronchial Trans-Epithelial Transport in Patients with Idiopathic Multiple Dilations of the Bronchi. 2016. Available online: https://ClinicalTrials.gov/show/NCT02586883 (accessed on 31 August 2019).
- Assistance Publique—Hôpitaux de Paris. Validation of Respiratory Epithelial Functional Assessment to Predict Clinical Efficacy of Orkambi®. 2019. Available online: https://ClinicalTrials.gov/show/NCT03894657 (accessed on 31 August 2019).
- University of Roma La Sapienza. Retinal Nerve Fibres Layers Thickness Study in Glaucomatous Patients. Available online: https://ClinicalTrials.gov/show/NCT01254006 (accessed on 31 August 2019).
- Hannover Medical School; Heidelberg University; University of Giessen. ICM to Evaluate the Activation of p.Phe508del-cftr by Lumacaftor in Combination with Ivacaftor. 2016. Available online: https://ClinicalTrials.gov/show/NCT02807415 (accessed on 31 August 2019).
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef]
- Boj, S.F.; Vonk, A.M.; Statia, M.; Su, J.; Dekkers, J.F.; Vries, R.R.; Beekman, J.M.; Clevers, H. Forskolin-induced swelling in intestinal organoids: An in vitro assay for assessing drug response in cystic fibrosis patients. JoVE (J. Vis. Exp.) 2017, 120, e55159. [Google Scholar] [CrossRef]
- Matthews, R.P.; McKnight, G.S. Characterization of the camp response element of the cystic fibrosis transmembrane conductance regulator gene promoter. J. Biol. Chem. 1996, 271, 31869–31877. [Google Scholar] [CrossRef]
- Drumm, M.L.; Wilkinson, D.J.; Smit, L.S.; Worrell, R.T.; Strong, T.V.; Frizzell, R.A.; Dawson, D.C.; Collins, F.S. Chloride conductance expressed by delta f508 and other mutant cftrs in xenopus oocytes. Science 1991, 254, 1797–1799. [Google Scholar] [CrossRef]
- Bristow, M.; Strosberg, A.; Ginsburg, R. Forskolin Activation of Human Myocardial Adenylate-Cyclase, Circulation; AMER HEART ASSOC: Dallas, TX, USA, 1983; p. 60. [Google Scholar]
- Linderer, E.; Metzger, H. The positive inotropic and smooth muscle relaxing effects of forskolin by direct activation of adenylate cyclase. In Proceedings of the International Symposium on Forskolin: Its Chemical Biological and Medical Potential, Bombay, India, 28–29 January 1985; pp. 83–101. [Google Scholar]
- Kramer, W.; Thormann, J.; Kindler, M.; Schlepper, M. Effects of forskolin on left ventricular function in dilated cardiomyopathy. Arzneim.-Forsch. 1987, 37, 364–367. [Google Scholar]
- Schlepper, M.; Thormann, J.; Mitrovic, V. Cardiovascular effects of forskolin and phosphodiesterase-iii inhibitors. In Inotropic Stimulation and Myocardial Energetics; Springer: Berlin/Heidelberg, Germany, 1989; pp. 197–212. [Google Scholar]
- Godard, M.P.; Johnson, B.A.; Richmond, S.R. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes. Res. 2005, 13, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, H.N.; Gopalakrishna, S.; Mariyanna, B.; Thekkoot, M.; Reddy, R.; Tippeswamy, B.S. Effect of Coleus forskohlii extract on cafeteria diet-induced obesity in rats. Pharmacogn. Res. 2014, 6, 42–45. [Google Scholar]
- Loftus, H.; Astell, K.; Mathai, M.; Su, X. Coleus forskohlii extract supplementation in conjunction with a hypocaloric diet reduces the risk factors of metabolic syndrome in overweight and obese subjects: A randomized controlled trial. Nutrients 2015, 7, 9508–9522. [Google Scholar] [CrossRef]
- Häkkinen, K.; Kraemer, W.J.; Pakarinen, A.; Tripleltt-Mcbride, T.; McBride, J.M.; Häkkinen, A.; Alen, M.; McGuigan, M.R.; Bronks, R.; Newton, R.U. Effects of heavy resistance/power training on maximal strength, muscle morphology, and hormonal response patterns in 60-75-year-old men and women. Can. J. Appl. Physiol. 2002, 27, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Hibino, N.; Kawai, A.; Uchikawa, S.; Chikazawa, G.; Kurihara, T.; Kihara, S.; Uebe, K.; Aomi, S.; Nishida, H.; Endo, M. Cardiovascular effects of colforsin daropate hydrochloride for acute heart failure after open heart surgery. Kyobu Geka Jpn. J. Thorac. Surg. 2001, 54, 1016–1019. [Google Scholar]
- Iranami, H.; Okamoto, K.; Kimoto, Y.; Maeda, H.; Kakutani, T.; Hatano, Y. Use of corfolsin dalopate following cardiac surgery in a neonate. Anesthesiol. J. Am. Soc. Anesthesiol. 2002, 97, 503–504. [Google Scholar] [CrossRef]
- Paulson, J.D.; Keller, D.W.; Wiest, W.G.; Warren, J.C. Free testosterone concentration in serum: Elevation is the hallmark of hirsutism. Am. J. Obstet. Gynecol. 1977, 128, 851–857. [Google Scholar] [CrossRef]
- Badmaev, V.; Majeed, M.; Conte, A.A.; Parker, J.E. Diterpene forskolin (coleus forskohlii benth.): A possible new compound for reduction of body weight by increasing lean body mass. NutraCos 2002, 1, 6–7. [Google Scholar]
- Henderson, S.; Magu, B.; Rasmussen, C.; Lancaster, S.; Kerksick, C.; Smith, P.; Melton, C.; Cowan, P.; Greenwood, M.; Earnest, C. Effects of coleus forskohlii supplementation on body composition and hematological profiles in mildly overweight women. J. Int. Soc. Sports Nutr. 2005, 2, 54. [Google Scholar] [CrossRef]
- Tsuguyoshi, A. Clinical Report on Root Extract of Perilla Plant (Coleus Forskohlii) Forslean in Reducing Body Fat; Asano Institute: Tokyo, Japan, 2004. [Google Scholar]
- Yousif, M.H.; Thulesius, O. Forskolin reverses tachyphylaxis to the bronchodilator effects of salbutamol: An in-vitro study on isolated guinea-pig trachea. J. Pharm. Pharmacol. 1999, 51, 181–186. [Google Scholar] [CrossRef]
- Hiramatsu, T.; Kume, H.; Kotlikoff, M.I.; Takagi, K. Role of calcium-activated potassium channels in the relaxation of tracheal smooth muscles by forskolin. Clin. Exp. Pharmacol. Physiol. 1994, 21, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Eleno, N.; Gajate, E.; Macias, J.; Garay, R. Enhancement by reproterol of the ability of disodium cromoglycate to stabilize rat mastocytes. Pulm. Pharmacol. Ther. 1999, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Lindner, E.; Metzger, H. The action of forskolin on muscle cells is modified by hormones, calcium ions and calcium antagonists. Arzneim. Forsch. 1983, 33, 1436–1441. [Google Scholar]
- Seamon, K.; Daly, J. Forskolin: A unique diterpene activator of cyclic AMP-generating systems. J. Cycl. Nucleotide Res. 1981, 7, 201–224. [Google Scholar]
- De Souza, N.J.; Dohadwalla, A.N.; Reden, Ü. Forskolin: A labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med. Res. Rev. 1983, 3, 201–219. [Google Scholar] [CrossRef]
- Tsukawaki, M.; Suzuki, K.; Suzuki, R.; Takagi, K.; Satake, T. Relaxant effects of forskolin on guinea pig tracheal smooth muscle. Lung 1987, 165, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Danahay, H.; Atherton, H.; Jones, G.; Bridges, R.J.; Poll, C.T. Interleukin-13 induces a hypersecretory ion transport phenotype in human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L226–L236. [Google Scholar] [CrossRef] [Green Version]
- Penn, R.B.; Panettieri Jr, R.A.; Benovic, J.L. Mechanisms of acute desensitization of the β2ar–adenylyl cyclase pathway in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 1998, 19, 338–348. [Google Scholar] [CrossRef]
- Tanizawa, M.; Watanabe, T.; Kurne, H.; Yarnaki, K.; Miyamoto, K.; Takagi, K. Phosphodiesterase iv inhibitors synergistically potentiate relaxation induced by forskolin in guinea-pig trachea. Clin. Exp. Pharmacol. Physiol. 1998, 25, 114–119. [Google Scholar] [CrossRef]
- Hidi, R.; Timmermans, S.; Liu, E.; Schudt, C.; Dent, G.; Holgate, S.; Djukanovic, R. Phosphodiesterase and cyclic adenosine monophosphate-dependent inhibition of t-lymphocyte chemotaxis. Eur. Respir. J. 2000, 15, 342–349. [Google Scholar] [CrossRef]
- Hallsworth, M.P.; Twort, C.H.; Lee, T.H.; Hirst, S.J. Β2-adrenoceptor agonists inhibit release of eosinophil-activating cytokines from human airway smooth muscle cells. Br. J. Pharmacol. 2001, 132, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; KNOX, A.J. Regulation of tnf-α-induced eotaxin release from cultured human airway smooth muscle cells by β2-agonists and corticosteroids. FASEB J. 2001, 15, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Staples, K.J.; Bergmann, M.; Tomita, K.; Houslay, M.D.; McPhee, I.; Barnes, P.J.; Giembycz, M.A.; Newton, R. Adenosine 3′,5′-cyclic monophosphate (camp)-dependent inhibition of il-5 from human t lymphocytes is not mediated by the camp-dependent protein kinase a. J. Immunol. 2001, 167, 2074–2080. [Google Scholar] [CrossRef] [PubMed]
- Couve, A.; Thomas, P.; Calver, A.R.; Hirst, W.D.; Pangalos, M.N.; Walsh, F.S.; Smart, T.G.; Moss, S.J. Cyclic amp–dependent protein kinase phosphorylation facilitates gaba b receptor–effector coupling. Nat. Neurosci. 2002, 5, 415. [Google Scholar] [CrossRef]
- Aksoy, M.O.; Mardini, I.A.; Yang, Y.; Bin, W.; Zhou, S.; Kelsen, S.G. Glucocorticoid effects on the β-adrenergic receptor–adenylyl cyclase system of human airway epithelium. J. Allergy Clin. Immunol. 2002, 109, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Shimizu, Y.; Kitaichi, K.; Hiramatsu, K.; Takeuchi, M.; Ito, Y.; Kume, H.; Yamaki, K.; Suzuki, R.; Shibata, E. Differential effect of phosphodiesterase inhibitors on il-13 release from peripheral blood mononuclear cells. Clin. Exp. Immunol. 2001, 126, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, R.; Trujillo, X.; Trujillo-Hernandez, B.; Vásquez, C.; Huerta, M.; Elizalde, A. Forskolin versus sodium cromoglycate for prevention of asthma attacks: A single-blinded clinical trial. J. Int. Med. Res. 2006, 34, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.; Urzua, Z.; Trujillo, X.; Gonzalez-Sanchez, R.; Trujillo-Hernandez, B. Forskolin compared with beclomethasone for prevention of asthma attacks: A single-blind clinical trial. J. Int. Med. Res. 2010, 38, 661–668. [Google Scholar] [CrossRef]
- Bauer, K.; Dietersdorfer, F.; Sertl, K.; Kaik, B.; Kaik, G. Pharmacodynamic effects of inhaled dry powder formulations of fenoterol and colforsin in asthma. Clin. Pharmacol. Ther. 1993, 53, 76–83. [Google Scholar] [CrossRef]
- Kaik, G.; Witte, P.U. Protective effect of forskolin against acetylcholine provocation in healthy volunteers—Comparison of two doses with fenoterol and placebo. Wien. Med. Wochenschr. 1986, 136, 637–641. [Google Scholar]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The natural camp elevating compound forskolin in cancer therapy: Is it time? J. Cell. Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.C.; Parks, R.E., Jr. Forskolin: A potential antimetastatic agent. Int. J. Cancer 1983, 32, 801–804. [Google Scholar] [CrossRef] [PubMed]
- McEwan, D.G.; Brunton, V.G.; Baillie, G.S.; Leslie, N.R.; Houslay, M.D.; Frame, M.C. Chemoresistant km12c colon cancer cells are addicted to low cyclic amp levels in a phosphodiesterase 4–regulated compartment via effects on phosphoinositide 3-kinase. Cancer Res. 2007, 67, 5248–5257. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, D.; Neviani, P. Protein phosphatase 2a (pp2a), a drugable tumor suppressor in ph1(+) leukemias. Cancer Metastasis Rev. 2008, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hikichi, M.; Yukitake, J.; Wakatsuki, T.; Nishio, E.; Utsumi, T.; Harada, N. Forskolin increases the effect of everolimus on aromatase inhibitor-resistant breast cancer cells. Oncotarget 2018, 9, 23451–23461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illiano, M.; Sapio, L.; Salzillo, A.; Capasso, L.; Caiafa, I.; Chiosi, E.; Spina, A.; Naviglio, S. Forskolin improves sensitivity to doxorubicin of triple negative breast cancer cells via Protein Kinase A-mediated ERK1/2 inhibition. Biochem. Pharmacol. 2018, 152, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Holz, G.G. Epac: A new camp-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes 2004, 53, 5–13. [Google Scholar] [CrossRef]
- Ammon, H.; Müller, A. Effect of forskolin on islet cyclic amp, insulin secretion, blood glucose and intravenous glucose tolerance in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1984, 326, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Silva, M.; Trujillo, X.; Trujillo-Hernández, B.; Sánchez-Pastor, E.; Urzúa, Z.; Mancilla, E.; Huerta, M. Effect of chronic administration of forskolin on glycemia and oxidative stress in rats with and without experimental diabetes. Int. J. Med. Sci. 2014, 11, 448–452. [Google Scholar] [CrossRef] [PubMed]
- You, Z.-P.; Xiong, B.; Zhang, Y.-L.; Shi, L.; Shi, K. Forskolin attenuates retinal inflammation in diabetic mice. Mol. Med. Rep. 2018, 17, 2321–2326. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K. A double-blind, randomized clinical trial to evaluate the efficacy and safety of forskolin eye drops 1% in the treatment of open angle glaucoma—A comparative study. J. Clin. Trials. 2014, 4, 1000184. [Google Scholar] [CrossRef]
- National Library of Australia. Available online: https://trove.nla.gov.au/work/18355014?selectedversion=NBD5774557 (accessed on 15 August 1986).
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Kumar, S. A double-blind, randomized clinical trial to evaluate the efficacy and safety of forskolin eye drops 1% in the treatment of open angle glaucoma–a comparative study. J. Clin. Trials 2014, 4, 184. [Google Scholar] [CrossRef]
- Badian, M.; Dabrowski, J.; Grigoleit, H.G.; Lieb, W.; Lindner, E.; Rupp, W. Effect of forskolin-eyedrops on the intraocular pressure of healthy male subjects. Klin. Mon. Augenheilkd. 1984, 185, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Vaidyanathan, P.; Karri, S.K.; Jose, J.A. Efficacy and safety of 1% forskolin eye drops in open angle glaucoma—An open label study. Saudi J. Ophthalmol. 2015, 29, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Philips, G.M.; Chan, I.S.; Swiderska, M.; Schroder, V.T.; Guy, C.; Karaca, G.F.; Moylan, C.; Venkatraman, T.; Feuerlein, S.; Syn, W.-K. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS ONE 2011, 6, e23943. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Omenetti, A.; Syn, W.-K.; Diehl, A.M. The role of hedgehog signaling in fibrogenic liver repair. Int. J. Biochem. Cell Biol. 2011, 43, 238–244. [Google Scholar] [CrossRef]
- El-Agroudy, N.N.; El-Naga, R.N.; El-Razeq, R.A.; El-Demerdash, E. Forskolin, a Hedgehog signalling inhibitor, attenuates carbon tetrachloride-induced liver fibrosis in rats. Br. J. Pharmacol. 2016, 173, 3248–3260. [Google Scholar] [CrossRef]
- Laurberg, P. Forskolin stimulation of thyroid secretion of t4 and t3. FEBS Lett. 1984, 170, 273–276. [Google Scholar] [CrossRef]
- Seamon, K.B.; Daly, J.W. Forskolin: Its biological and chemical properties. Adv. Cycl. Nucleotide Protein Phosphorylation Res. 1986, 20, 1. [Google Scholar]
- Mastan, A.; Bharadwaj, R.; Kushwaha, R.K.; Babu, C.S.V. Functional fungal endophytes in Coleus forskohlii regulate labdane diterpene biosynthesis for elevated forskolin accumulation in roots. Microb. Ecol. 2019. [Google Scholar] [CrossRef]
- Bersudsky, Y.; Kotler, M.; Shifrin, M.; Belmaker, R.H. A preliminary study of possible psychoactive effects of intravenous forskolin in depressed and schizophrenic patients. J. Neural Transm. 1996, 103, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Doorn, J.; Siddappa, R.; Van Blitterswijk, C.A.; De Boer, J. Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.V.; Dohadwalla, A.N.; Bajwa, B.S.; Dadkar, N.K.; Dornauer, H.; de Souza, N.J. The antihypertensive and positive inotropic diterpene forskolin: Effects of structural modifications on its activity. J. Med. Chem. 1983, 26, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Saettone, M.F.; Burgalassi, S.; Giannaccini, B. Preparation and evaluation in rabbits of topical solutions containing forskolin. J. Ocul. Pharmacol. Ther. 2009, 5, 2. [Google Scholar] [CrossRef]
- Gupta, S.; Samanta, M.K.; Raichur, A.M. Dual-Drug Delivery System Based on In Situ Gel-Forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS Pharmscitech 2010, 11, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Samanta, M.K. Design and evaluation of thermoreversible in situ gelling system of forskolin for the treatment of glaucoma. J. Pharm. Dev. Technol. 2010, 15, 386–393. [Google Scholar] [CrossRef]
- Ameeduzzafar, K.N.; Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar]
- Miastkowska, M.; Sikora, E.; Lasoń, E.; Garcia-Celma, M.J.; Escribano-Ferrer, E.; Solans, C.; Llinas, M. Nano-emulsions as vehicles for topical delivery of forskolin. Acta Biochim. Pol. 2017, 64, 713–718. [Google Scholar] [CrossRef]
- Patil, S.; Agarwal, P.; Rojatkar, S.; Mahadik, K. Electrosprayed forskolin cocrystals with enhanced aqueous solubility. Anal. Chem. Lett. 2018, 8, 321–330. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Jiang, D.-B.; Tian, L.-L.; Yin, J.-J.; Huang, J.-M.; Weng, W.-Y. Intestinal permeability of forskolin by in situ single pass perfusion in rats. Planta Med. 2012, 78, 698–702. [Google Scholar] [CrossRef]
- Godugu, D.; Rupula, K.S.; Rao, B. Binding interactions of forskolin with human serum albumin: Insights from in silico and spectroscopic studies. Curr. Chem. Biol. 2016, 10, 127–134. [Google Scholar] [CrossRef]
- Nagati, V.; Nakkka, S.; Yeggoni, D.P.; Subramanyam, R. Forskolin-loaded human serum albumin nanoparticles and its biological importance. J. Biomol. Struct. Dyn. 2019, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Bani, S.; Vaidyanathan, P.; Majeed, S.; Karri, S.K. Investigation of acute, sub-acute, chronic oral toxicity and mutagenicity of coleus forskohlii briq. hydroethanolic extract, standardized for 10% forskolin in experimental animals. Int. J. Pharm. Pharm. Res. 2015, 5, 219–238. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Staniak, M.; Czopek, K.; Stępień, A.; Dua, K.; Wadhwa, R.; Kumar Chellappan, D.; Sytar, O.; Brestic, M.; Ganesh Bhat, N.; et al. The Therapeutic Potential of the Labdane Diterpenoid Forskolin. Appl. Sci. 2019, 9, 4089. https://doi.org/10.3390/app9194089
Salehi B, Staniak M, Czopek K, Stępień A, Dua K, Wadhwa R, Kumar Chellappan D, Sytar O, Brestic M, Ganesh Bhat N, et al. The Therapeutic Potential of the Labdane Diterpenoid Forskolin. Applied Sciences. 2019; 9(19):4089. https://doi.org/10.3390/app9194089
Chicago/Turabian StyleSalehi, Bahare, Mariola Staniak, Katarzyna Czopek, Anna Stępień, Kamal Dua, Ridhima Wadhwa, Dinesh Kumar Chellappan, Oksana Sytar, Marian Brestic, Namrata Ganesh Bhat, and et al. 2019. "The Therapeutic Potential of the Labdane Diterpenoid Forskolin" Applied Sciences 9, no. 19: 4089. https://doi.org/10.3390/app9194089
APA StyleSalehi, B., Staniak, M., Czopek, K., Stępień, A., Dua, K., Wadhwa, R., Kumar Chellappan, D., Sytar, O., Brestic, M., Ganesh Bhat, N., Venkatesh Anil Kumar, N., del Mar Contreras, M., Sharopov, F., C. Cho, W., & Sharifi-Rad, J. (2019). The Therapeutic Potential of the Labdane Diterpenoid Forskolin. Applied Sciences, 9(19), 4089. https://doi.org/10.3390/app9194089











