Mechanochemical-Assisted Extraction and Pharmacological Study of Triterpenoids from Antrodia Camphorata
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Samples
2.2.1. Mechanochemical-Assisted Extraction Method (MAEM)
2.2.2. Ethanol Hot Reflux Extraction Method (EHRE)
2.3. Analysis of TAEM and TAEE by HPLC and LC-MS/MS
2.4. Antioxidant Experiment In Vitro
2.4.1. Scavenging Effect on DPPH Radicals
2.4.2. Scavenging Effect on ABTS Radicals
2.4.3. Scavenging Effect on Hydroxyl Radicals (·OH)
2.4.4. Reducing Power
2.5. Anticancer Activity of TAEM and TAEE
2.5.1. Cell Culture
2.5.2. Cell Viability Assay (MTT Assay)
2.6. Anti-Inflammatory Activity and Mechanism of TAEM and TAEE
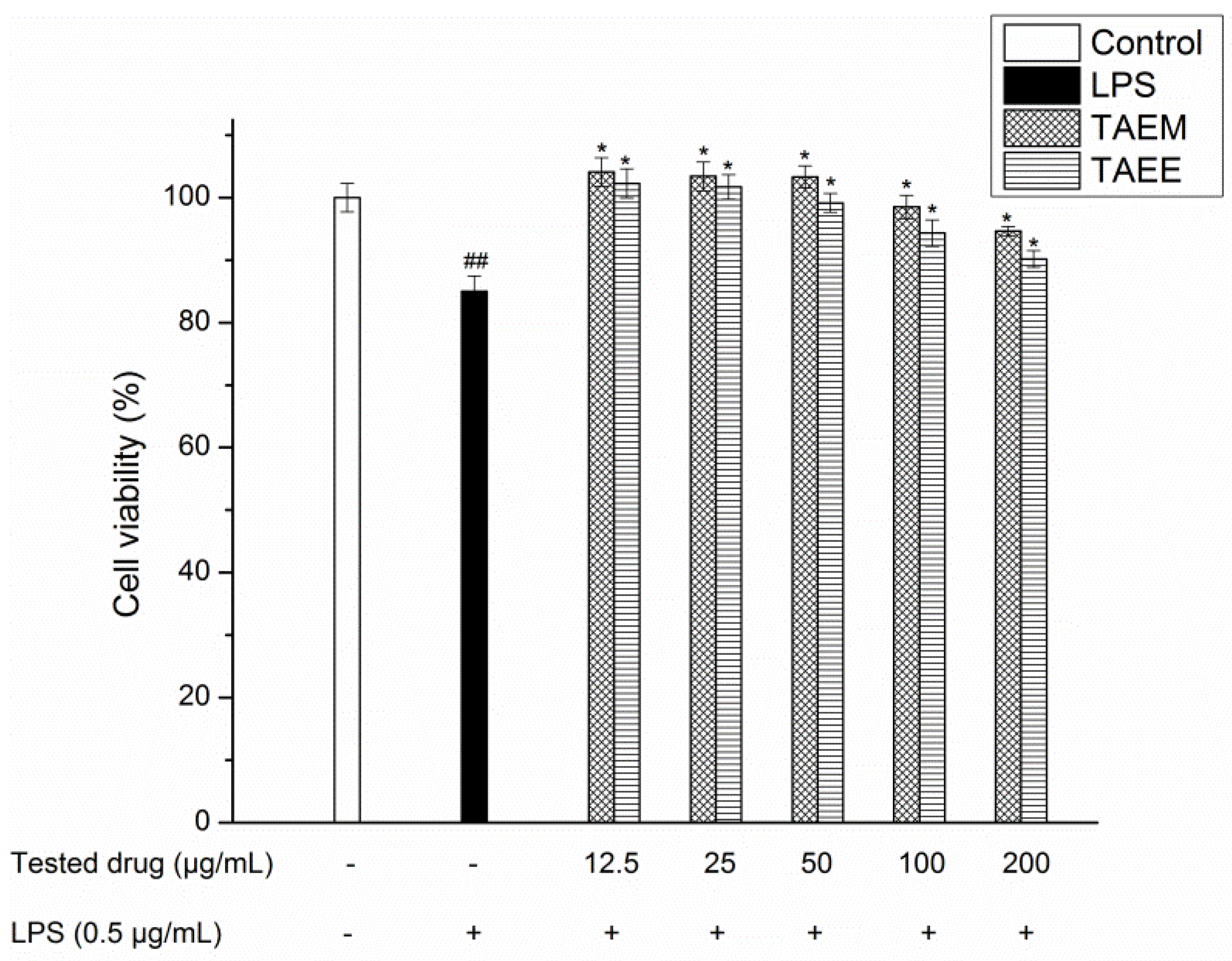
2.6.1. Toxicity Test
2.6.2. Measurement of Nitric Oxide (NO) Production
2.6.3. Measurement of iNOS Protein Induced by LPS in RAW 264.7 (BCA Assay)
2.7. Splenocyte Proliferation Assay
2.7.1. Preparation of Spleen Cell Suspension
2.7.2. Induction of Rat Spleen Lymphocyte Proliferation Response of TAEM and TAEE
2.8. Statistical Analysis
3. Results and Discussion
3.1. HPLC Analysis of TAEM and TAEE
3.2. Chemical Analysis of Compounds by LC-MS
3.3. Antioxidant Activity of TAEM and TAEE
3.4. Anticancer Activity of TAEM and TAEE
3.5. Anti-Inflammatory Activity of TAEM and TAEE
3.6. Immunomodulatory Activity of TAEM and TAEE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, S.S.; Wang, S.Y.; Lin, Y.Y.; Kuo, Y.H.; Wang, G.J.; Lee, T.H. New Constituents with iNOS Inhibitory Activity from Mycelium of Antrodia camphorata. Planta Med. 2009, 75, 512–516. [Google Scholar] [CrossRef]
- Tzeng, Y.M.; Geethangili, M. Review of pharmacological effects of antrodia camphorata and its bioactive compounds. Evidence-based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar]
- Wang, Y.J.; Lee, S.C.; Hsu, C.H.; Kuo, Y.H.; Yang, C.C.; Lin, F.J. Antcins, triterpenoids from Antrodia cinnamomea, as new agonists for peroxisome proliferator-activated receptor α. J. Food Drug Anal. 2019, 27, 295–304. [Google Scholar] [CrossRef]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Lin, Y.S.; Lin, Y.Y.; Yang, Y.H.; Lin, C.L.; Kuan, F.C.; Lu, C.N.; Chang, G.H.; Tsai, M.S.; Hsu, C.M.; Yeh, R.A.; et al. Antrodia cinnamomea extract inhibits the proliferation of tamoxifen-resistant breast cancer cells through apoptosis and skp2/microRNAs pathway. BMC Complement. Altern. Med. 2018, 18, 152. [Google Scholar] [CrossRef]
- Lee, M.T.; Lin, W.C.; Wang, S.Y.; Lin, L.J.; Yu, B.; Lee, T.T. Evaluation of potential antioxidant and anti-inflammatory effects of Antrodia cinnamomea powder and the underlying molecular mechanisms via Nrf2- and NF-κB-dominated pathways in broiler chickens. Poult. Sci. 2018, 97, 2419–2434. [Google Scholar] [CrossRef]
- Wang, P.; Tang, J. Solvent-free mechanochemical extraction of chondroitin sulfate from shark cartilage. Chem. Eng. Process. Process. Intensif. 2009, 48, 1187–1191. [Google Scholar] [CrossRef]
- Su, W.; Zhu, X.; Chen, X.; Xie, J.; Wang, P. Mechanochemical-assisted extraction and antioxidant activity of polysaccharides from Ganoderma lucidum spores. Int. J. Food Sci. Technol. 2012, 47, 927–932. [Google Scholar]
- Wu, K.; Ju, T.; Deng, Y.; Xi, J. Mechanochemical assisted extraction: A novel, efficient, eco-friendly technology. Trends Food Sci. Technol. 2017, 66, 166–175. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubàa, M.; Sant’Ana, A.S.; Orlien, V.; Sant’Ana, A.D.S. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liu, C.; Ren, J.; Zhao, X.; Sun, R.; Wu, A. An efficient pretreatment for the selectively hydrothermal conversion of corncob into furfural: The combined mixed ball milling and ultrasonic pretreatments. Ind. Crop. Prod. 2016, 94, 721–728. [Google Scholar] [CrossRef]
- Xie, J.; Lin, Y.S.; Shi, X.J.; Zhu, X.Y.; Su, W.K.; Wang, P. Mechanochemical-assisted extraction of flavonoids from bamboo (Phyllostachys edulis) leaves. Ind. Crop. Prod. 2013, 43, 276–282. [Google Scholar] [CrossRef]
- Wang, M.; Bi, W.; Huang, X.; Chen, D.D.Y. Ball mill assisted rapid mechanochemical extraction method for natural products from plants. J. Chromatogr. A 2016, 1449, 8–16. [Google Scholar] [CrossRef]
- Ma, T.W.; Lai, Y.; Yang, F.C. Enhanced production of triterpenoid in submerged cultures of Antrodia cinnamomea with the addition of citrus peel extract. Bioprocess Biosyst. Eng. 2014, 37, 2251–2261. [Google Scholar] [CrossRef]
- He, R.; Zhao, Y.; Zhao, R.; Sun, P. Antioxidant and antitumor activities in vitro of polysaccharides from E. sipunculoides. Int. J. Boil. Macromol. 2015, 78, 56–61. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Liu, Z.Q. Free-Radical-Scavenging Effect of Carbazole Derivatives on DPPH and ABTS Radicals. J. Am. Oil Chem. Soc. 2007, 84, 1095–1100. [Google Scholar] [CrossRef]
- He, R.; Ye, J.; Zhao, Y.; Su, W. Partial characterization, antioxidant and antitumor activities of polysaccharides from Philomycusbilineatus. Int. J. Boil. Macromol. 2014, 65, 573–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Wang, Z.; Zang, W.; Rao, P.; Liang, Y.; Mei, Y. Alpha-terpineol affects synthesis and antitumor activity of triterpenoids from Antrodia cinnamomea mycelia in solid-state culture. Food Funct. 2018, 9, 6517–6525. [Google Scholar] [CrossRef]
- Thulin, M.; Moore, A.J.; El-Seedi, H.; Larsson, A.; Christin, P.A.; Edwards, E.J. Phylogeny and generic delimitation in Molluginaceae, new pigment data in Caryophyllales, and the new family Corbichoniaceae. Taxon 2016, 65, 775–793. [Google Scholar] [CrossRef]
- Huang, H.S.; Hsu, J.L.; Yu, C.C.; Chang, L.C.; Chen, C.R.; Huang, T.C.; Chang, C.I. Qualitative and quantitative analysis of seven signature components in the fruiting body of Antrodia cinnamomea by HPLC—ESI—MS/MS. Acta Chromatogr. 2015, 28, 387–402. [Google Scholar] [CrossRef]
- Hsieh, Y.L.; Wu, S.P.; Fang, L.W.; Hwang, T.S. Effects of Antrodia camphorata extracts on anti-oxidation, anti-mutagenesis and protection of DNA against hydroxyl radical damage. BMC Complement. Altern. Med. 2015, 15, 237. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, C.C.; Lee, C.L.; Hsieh, S.L.; Chen, J.B.; Lee, C.I. Antimelanogenic, Antioxidant and Antiproliferative Effects of Antrodia camphorata Fruiting Bodies on B16-F0 Melanoma Cells. PLoS ONE 2017, 12, 0170924. [Google Scholar] [CrossRef]
- Zhang, F.; Cai, H.; Liu, X.; Chen, F.; Yang, N.; Hou, Y.; Ma, Y.; Luo, Y.; Liu, J. Simultaneous determination of 19 fatty acids in Antrodia camphorata by derivatized GC–MS and evaluation of antioxidant activity of Antrodia camphorata crude oil. Arch. Pharmacal Res. 2015, 38, 1–8. [Google Scholar] [CrossRef]
- Song, T.Y.; Yen, G.C. Antioxidant properties of Antrodia camphorata in submerged culture. J. Agric. Food Chem. 2002, 50, 3322. [Google Scholar] [CrossRef]
- Lee, Y.P.; Tsai, W.C.; Ko, C.J.; Rao, Y.K.; Yang, C.R.; Chen, D.R.; Yang, M.H.; Yang, C.C.; Tzeng, Y.M. Anticancer effects of eleven triterpenoids derived from Antrodia camphorata. Anticancer. Res. 2012, 32, 2727–2734. [Google Scholar]
- Zhu, P.L.; Fu, X.Q.; Li, J.K.; Tse, A.K.W.; Guo, H.; Yin, C.L.; Chou, J.Y.; Wang, Y.P.; Liu, Y.X.; Chen, Y.J.; et al. Antrodia camphorata Mycelia Exert Anti-liver Cancer Effects and Inhibit STAT3 Signaling in vitro and in vivo. Front. Pharmacol. 2018, 9, 1449. [Google Scholar] [CrossRef]
- Du, Y.C.; Wu, T.Y.; Chang, F.R.; Lin, W.Y.; Hsu, Y.M.; Cheng, F.T.; Lu, C.Y.; Yen, M.H.; Tsui, Y.T.; Chen, H.L.; et al. Chemical profiling of the cytotoxic triterpenoid-concentrating fraction and characterization of ergostane stereo-isomer ingredients from Antrodia camphorata. J. Pharm. Biomed. Anal. 2012, 58, 182–192. [Google Scholar] [CrossRef]
- Huang, G.J.; Deng, J.S.; Huang, S.S.; Lee, C.Y.; Hou, W.C.; Wang, S.Y.; Sung, P.J.; Kuo, Y.H. Hepatoprotective effects of eburicoic acid and dehydroeburicoic acid from Antrodia camphorata in a mouse model of acute hepatic injury. Food Chem. 2013, 141, 3020–3027. [Google Scholar] [CrossRef]
- Li, Z.W.; Kuang, Y.; Tang, S.N.; Li, K.; Huang, Y.; Qiao, X.; Yu, S.W.; Tzeng, Y.M.; Lo, J.Y.; Ye, M. Hepatoprotective activities of Antrodia camphorata and its triterpenoid compounds against CCl 4 -induced liver injury in mice. J. Ethnopharmacol. 2017, 206, 31–39. [Google Scholar] [CrossRef]

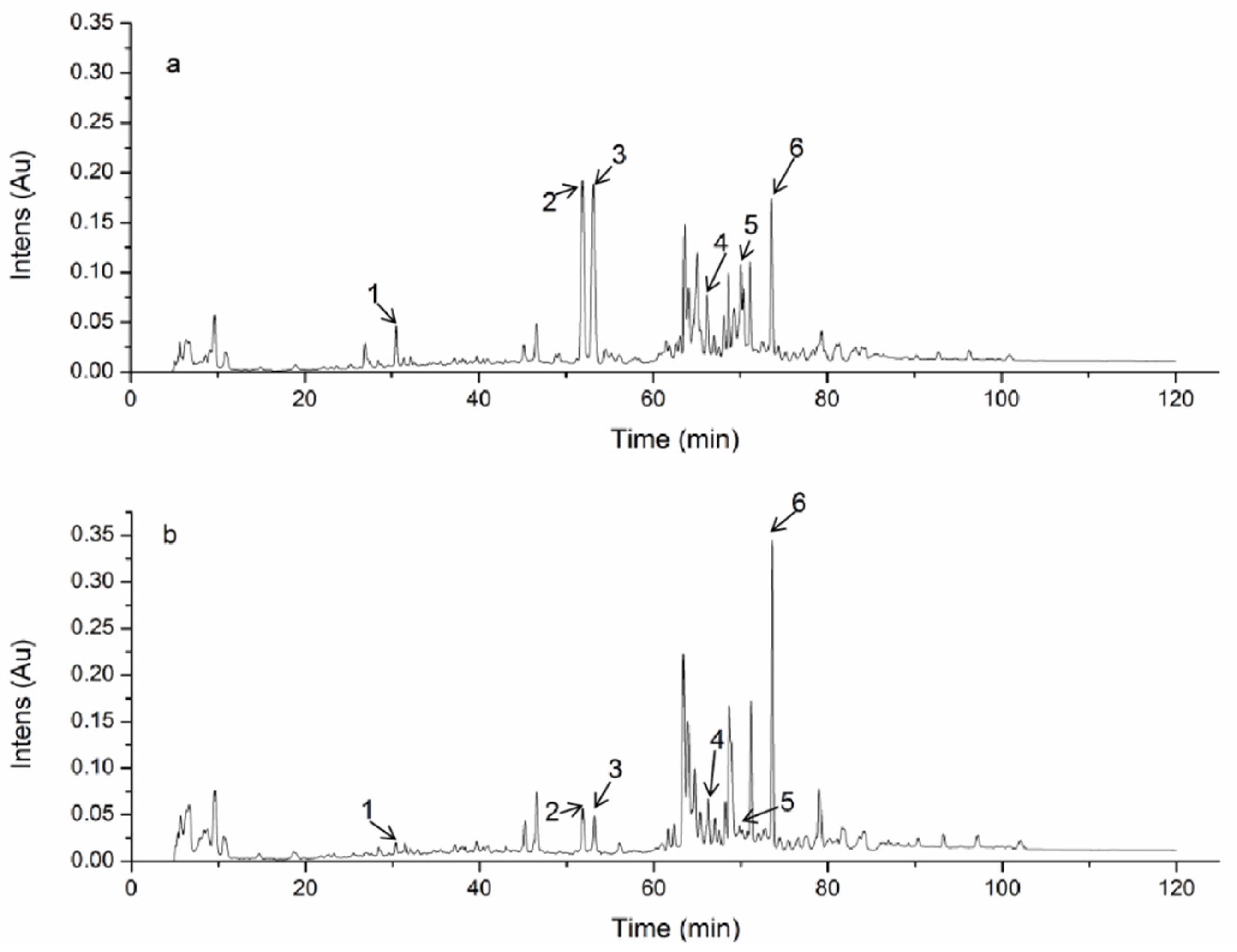
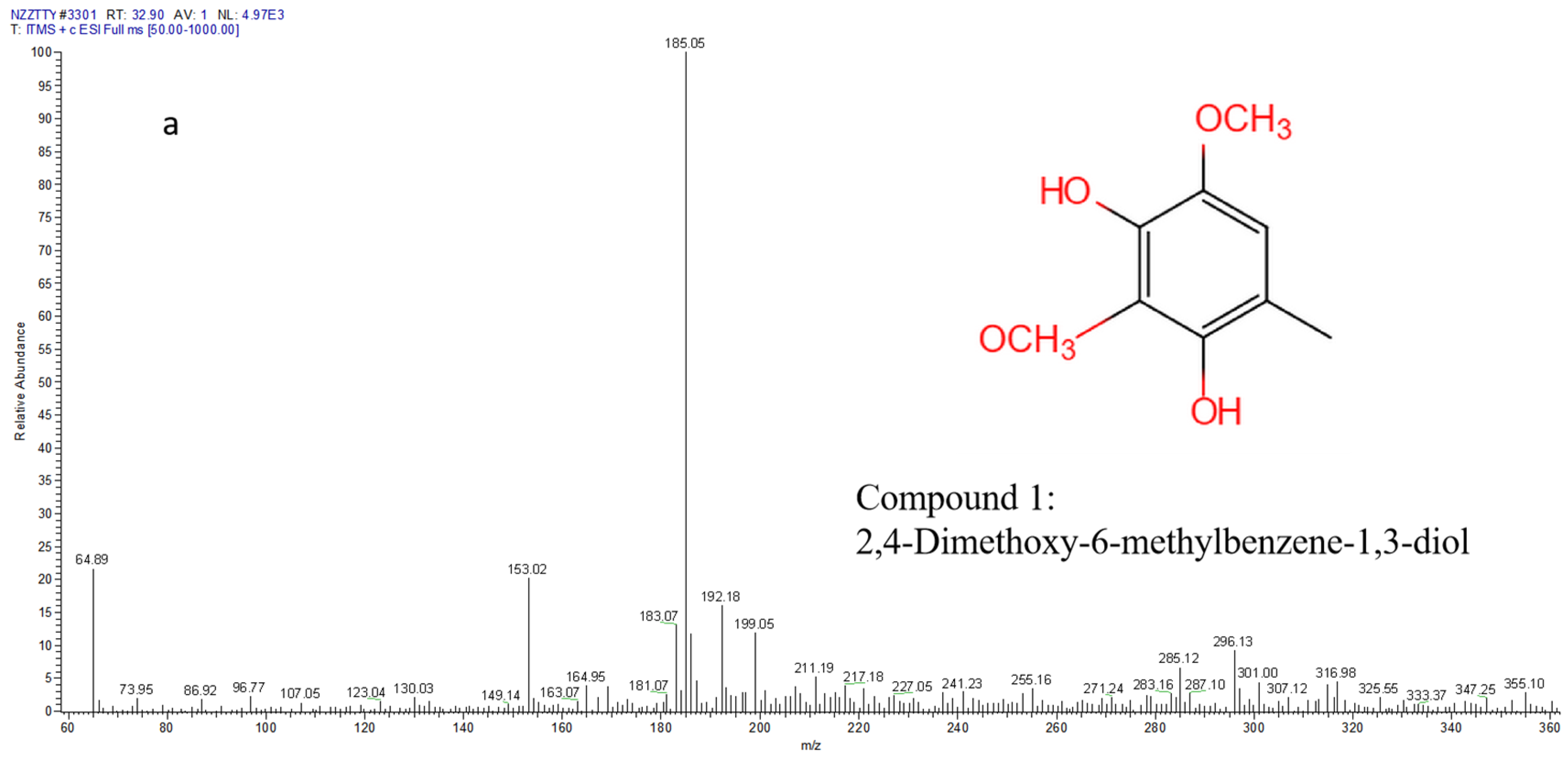







| Peak Number | Compound | Molecular Formula | TR (min) | [M + H]+ | [M − H]− | Molecular Weight | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2, 4-dimethoxy-6-methylbenzene-1,3-diol | C9H12O4 | 32.90 | 185 | 183 | 184 | [1] |
| 2 | Antcin K | C29H44O6 | 66.86 | 489 | 487 | 488 | [21] |
| 3 | Antcin K | C29H44O6 | 67.48 | 489 | 487 | 488 | [21] |
| 4 | Antcin C | C29H42O5 | 74.67 | 471 | 479 | 470 | [21] |
| 5 | Antcin H | C29H42O6 | 77.30 | 487 | 485 | 486 | [21] |
| 6 | Antcin A | C29H42O4 | 80.71 | 455 | 453 | 454 | [21] |
| Method | Temperature (°C) | Solvent | Time (min) | Yield c (%) |
|---|---|---|---|---|
| MAEM a | 50 | Water | 30 | 1.82 ± 0.04 |
| EHRE b | 90 | Ethanol | 120 | 1.02 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Wu, K.; Zhang, A.; Xie, Z.; Sun, P. Mechanochemical-Assisted Extraction and Pharmacological Study of Triterpenoids from Antrodia Camphorata. Appl. Sci. 2019, 9, 4281. https://doi.org/10.3390/app9204281
He R, Wu K, Zhang A, Xie Z, Sun P. Mechanochemical-Assisted Extraction and Pharmacological Study of Triterpenoids from Antrodia Camphorata. Applied Sciences. 2019; 9(20):4281. https://doi.org/10.3390/app9204281
Chicago/Turabian StyleHe, Rongjun, Kaixiang Wu, Anqiang Zhang, Zhangfu Xie, and Peilong Sun. 2019. "Mechanochemical-Assisted Extraction and Pharmacological Study of Triterpenoids from Antrodia Camphorata" Applied Sciences 9, no. 20: 4281. https://doi.org/10.3390/app9204281





