Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. General
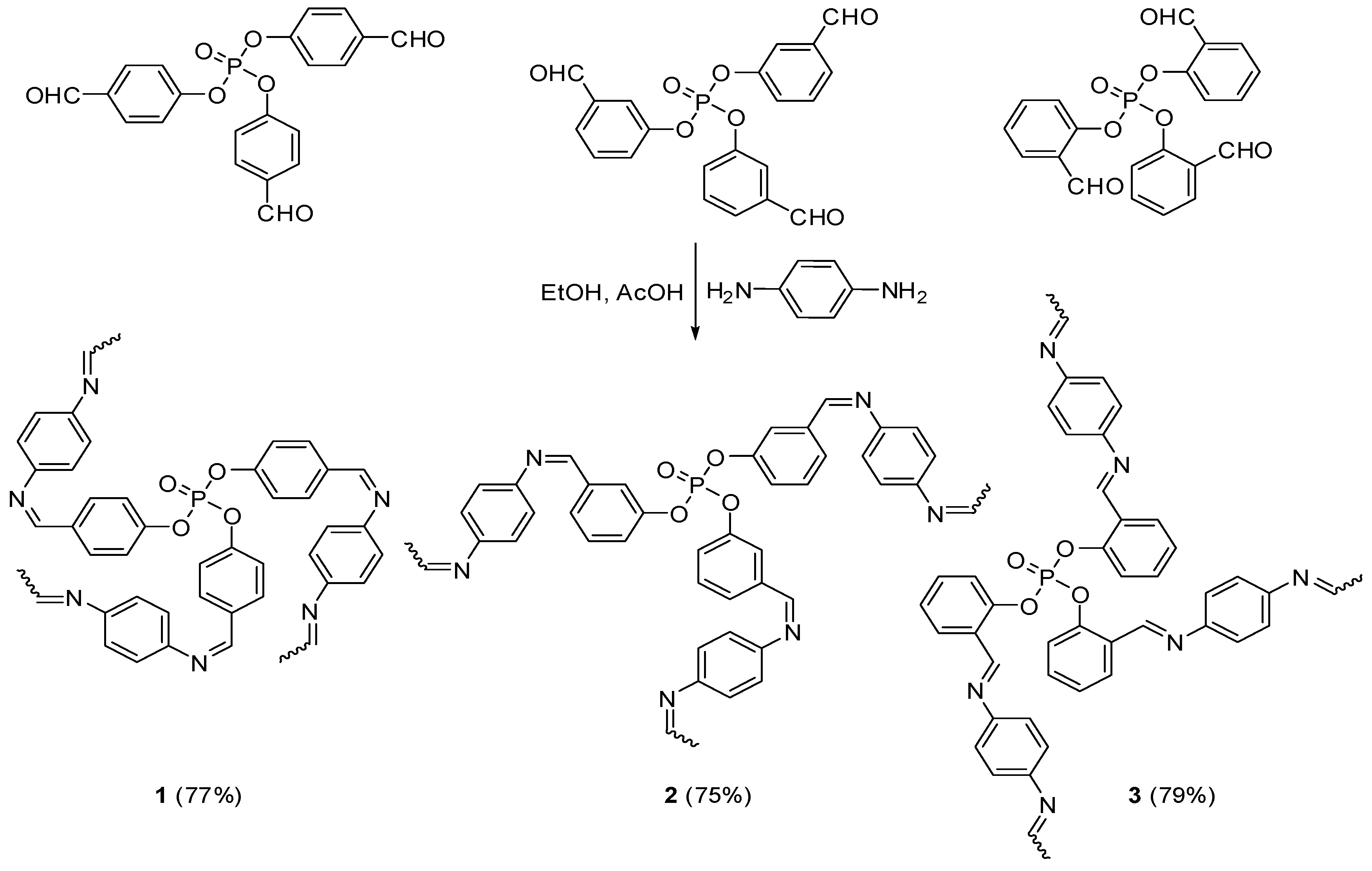
2.2. Synthesis of Polyphosphates 1–3
3. Results and Discussion
3.1. Structural Characterization of Polyphosphates 1–3
3.2. Morphologies of 1–3
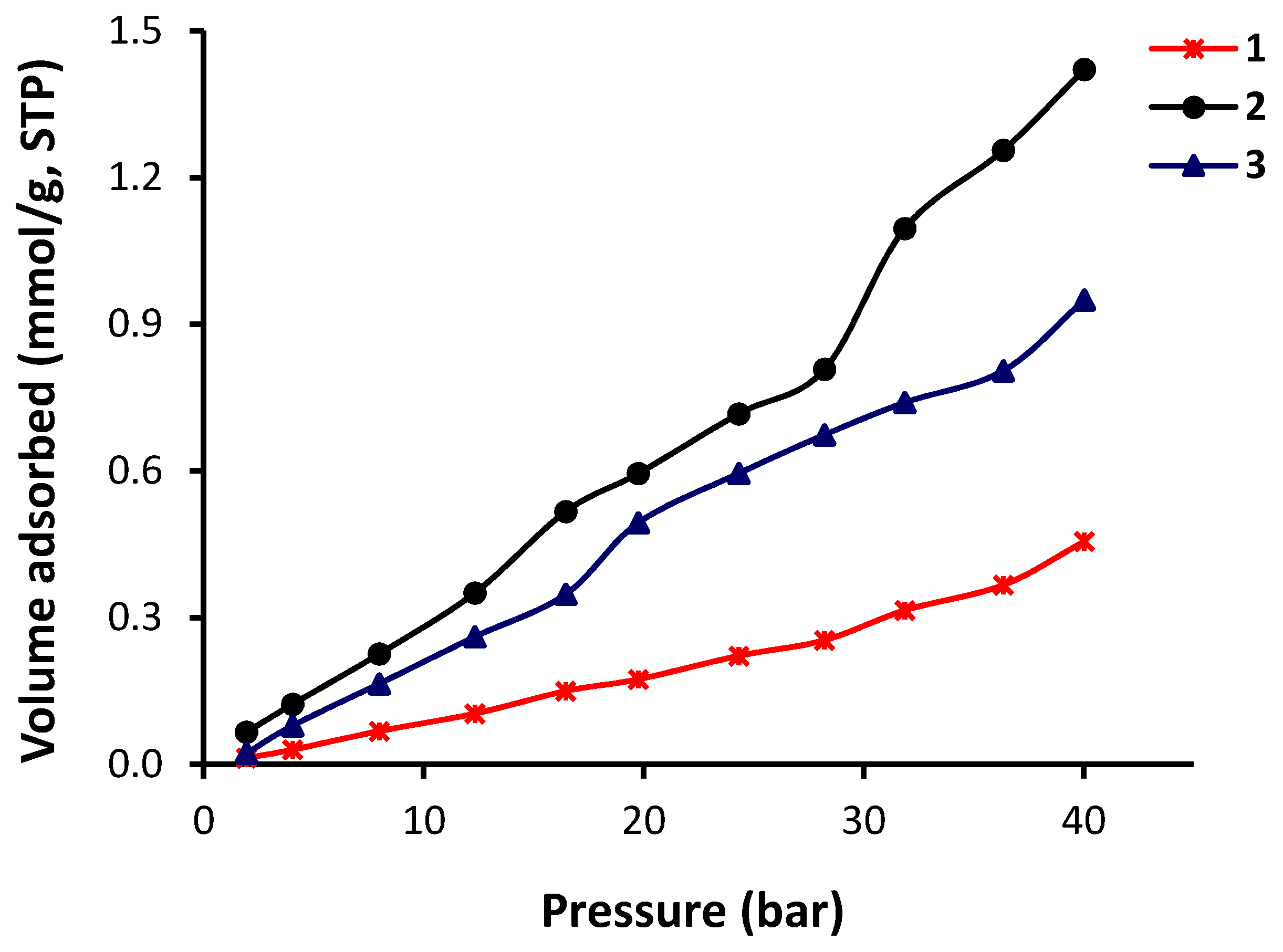
3.3. Porosity Measurements and Gas Storage Capacity of 1–3
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ben-Mansour, R.; Habib, M.A.; Bamidele, O.E.; Basha, M.; Qasem, N.A.A.; Peedikakkal, A.; Laoui, T.; Ali, M. Carbon capture by physical adsorption: Materials, experimental investigations and numerical modeling and simulations—A review. Appl. Energy 2016, 161, 225–255. [Google Scholar] [CrossRef]
- Yaumi, A.L.; Bakar, M.Z.A.; Hameed, B.H. Recent advances in functionalized composite solid materials for carbon dioxide capture. Energy 2017, 124, 461–480. [Google Scholar] [CrossRef]
- United States Environmental Agency. Global Greenhouse Gas Emissions Data. 2018. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 10 July 2019).
- Boamah, K.B.; Du, J.; Bediako, I.A.; Boamah, A.J.; Abdul-Rasheed, A.A.; Owusu, S.M. Carbon dioxide emission and economic growth of China–the role of international trade. Environ. Sci. Pollut. Res. 2017, 24, 13049. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.; Verolme, H.J.; Hunt, W.F., Jr. Overview of regulatory/policy/economic issues related to carbon dioxide. Environ. Int. 2003, 29, 303–310. [Google Scholar] [CrossRef]
- Sun, H.; Xin, Q.; Ma, Z.; Lan, S. Effects of plant diversity on carbon dioxide emissions and carbon removal in laboratory-scale constructed wetland. Environ. Sci. Pollut. Res. 2019, 26, 5076. [Google Scholar] [CrossRef] [PubMed]
- IPCC 2007. Climate Change 2007: Synthesis Report. In Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Core Writing Team; Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2008; p. 104. [Google Scholar]
- Sanz-Perez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Mukherjee, A.; Okolie, J.A.; Abdelrasoul, A.; Niu, C.; Dalai, A.K. Review of post-combustion carbon dioxide capture technologies using activated carbon. J. Environ. Sci. 2019, 83, 46–63. [Google Scholar] [CrossRef]
- Okesola, A.A.; Oyedeji, A.A.; Abdulhamid, A.F.; Olowo, J.; Ayodele, B.E.; Alabi, T.W. Direct air capture: A review of carbon dioxide capture from the air. Mater. Sci. Eng. 2018, 413, 12077. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khokarale, S.G.; Bui, T.Q.; Mikkola, J.-P.T. Ionic liquids: Potential materials for carbon dioxide capture and utilization. Front. Mater. 2019, 6, 42. [Google Scholar] [CrossRef]
- Aminua, M.D.; Nabavia, S.A.; Rochelleb, C.A.; Manovica, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Kelektsoglou, K. Carbon capture and storage: A review of mineral storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew Sust. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Goh, K.; Karahan, H.E.; Yang, E.; Bae, T.-H. Graphene-based membranes for CO2/CH4 separation: Key challenges and perspectives. Appl. Sci. 2019, 9, 2784. [Google Scholar] [CrossRef]
- Sabouni, R.; Kazemian, H.; Rohani, S. Carbon dioxide capturing technologies: A review focusing on metal organic framework materials (MOFs). Environ. Sci. Pollut. Res. 2014, 21, 5427. [Google Scholar] [CrossRef]
- Thomas, D.M.; Mechery, J.; Paulose, S.V. Carbon dioxide capture strategies from flue gas using microalgae: A review. Environ. Sci. Pollut. Res. 2016, 23, 16926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bacsik, Z.; Hedin, N.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.M. Carbon dioxide capture on amine-rich carbonaceous materials derived from glucose. ChemSusChem 2010, 3, 840–845. [Google Scholar] [CrossRef]
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. department of energy’s carbon sequestration program: A review. Int. J. Greenh. Gas Con. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Asadi-Sangachini, Z.; Galangash, M.M.; Younesi, H.; Nowrouzi, M. The feasibility of cost-effective manufacturing activated carbon derived from walnut shells for large-scale CO2 capture. Environ. Sci. Pollut. Res. 2019, 26, 26542–26552. [Google Scholar] [CrossRef]
- Gibson, J.A.A.; Mangano, E.; Shiko, E.; Greenaway, A.G.; Gromov, A.V.; Lozinska, M.M.; Friedrich, D.; Campbell, E.E.B.; Wright, P.A.; Brandani, S. Adsorption materials and processes for carbon capture from gas-fired power plants: AMPgas. Ind. Eng. Chem. Res. 2016, 551, 33840–33851. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. A review on solid adsorbents for carbon dioxide capture. J. Ind. Eng. Chem. 2015, 23, 1–11. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Su, F.; Chen, W.; Hwang, J.F.; Lee, H.-H. Adsorption of carbon dioxide from gas streams via mesoporous spherical-silica particles. J. Air Waste Manag. Assoc. 2010, 60, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef]
- Aquino, A.S.; Vieira, M.O.; Ferreira, A.S.D.; Cabrita, E.J.; Einloft, S.; de Souza, M.O. Hybrid ionic liquid–silica xerogels applied in CO2 capture. Appl. Sci. 2019, 9, 2614. [Google Scholar] [CrossRef]
- Staciwa, P.; Narkiewicz, U.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon spheres as CO2 sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Chiang, Y.-C.; Yeh, C.Y.; Weng, C.H. Carbon dioxide adsorption on porous and functionalized activated carbon fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Al-Ghurabi, E.H.; Ajbar, A.; Asif, M. Enhancement of CO2 removal efficacy of fluidized bed using particle mixing. Appl. Sci. 2018, 8, 1467. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, M.; Yuan, D. Carbon dioxide capture in amorphous porous organic polymers. J. Mater. Chem. A 2017, 5, 1334–1347. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous Carbon spheres from hydrothermal carbonization and KOH activation on cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Dziura, A.; Stachurska, K.; Jaroniec, M. Preparation and studies of adsorption properties of microporous carbon spheres. Eng. Prot. Environ. 2016, 19, 169–182. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of functionalization, catenation, and variation of the metal oxide and organic linking units on the low-pressure hydrogen adsorption properties of metal–organic frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide at high temperature-a review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen., B.; Reineke, T.M.; O’keeffe, M.; Yaghi, O.M. Modular chemistry: secondary building units as a basis for the design of highly porous and robust metal−organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Lu, W.; Yuan, D.; Sculley, J.; Zhao, D.; Krishna, R.; Zhou, H.-C. Sulfonate-grafted porous polymer networks for preferential CO2 adsorption at low pressure. J. Am. Chem. Soc. 2011, 133, 18126–18129. [Google Scholar] [CrossRef]
- Rabbani, M.G.; El-Kaderi, H.M. Template-free synthesis of a highly porous benzimidazole-linked polymer for CO2 capture and H2 storage. Chem. Mater. 2011, 23, 1650–1653. [Google Scholar] [CrossRef]
- Rabbani, M.G.; Reich, T.E.; Kassab, R.M.; Jackson, K.T.; El-Kaderi, H.M. High CO2 uptake and selectivity by triptycene-derived benzimidazole-linked polymers. Chem. Commun. 2012, 48, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Rashchi, F.; Finch, J.A. Polyphosphates: A review their chemistry and application with particular reference to mineral processing. Miner. Eng. 2000, 13, 1019–1035. [Google Scholar] [CrossRef]
- Corbridge, D.E.C. Phosphorus: Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: New York, NY, USA, 2013. [Google Scholar]
- Iliescu, S.; Zubizarreta, L.; Plesu, N.; Macarie, L.; Popa, A.; Ilia, G. Polymers containing phosphorus groups and polyethers: From synthesis to application. Chem. Cent. J. 2012, 6, 132. [Google Scholar] [CrossRef] [PubMed]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef]
- Ren, H.; Sun, J.; Wu, B.; Zhou, Q. Synthesis and properties of a phosphorus-containing flame retardant epoxy resin based on bis-phenoxy (3-hydroxy) phenyl phosphine oxide. Polym. Degrad. Stab. 2007, 92, 956–961. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.A.; Yousif, E.; Hameed, A.S.; Abdalla, M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers 2017, 9, 336. [Google Scholar] [CrossRef]
- Hadi, A.G.; Jawad, K.; Yousif, E.; El-Hiti, G.A.; Alotaibi, M.H.; Ahmed, D.S. Synthesis of telmisartan organotin(IV) complexes and their use as carbon dioxide capture Media. Molecules 2019, 24, 1631. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Ma, Q.-Y.; Yang, B.-X.; Li, J.-Q. Porous organic polymers derived from tetrahedral silicon-centered monomers and a stereocontorted spirobifluorene-based precursor: Synthesis, porosity and carbon dioxide sorption. RSC Adv. 2015, 5, 64163–64169. [Google Scholar] [CrossRef]
- Jin, Y.; Voss, B.A.; McCaffrey, R.; Baggett, C.T.; Noble, R.D.; Zhang, W. Microwave-assisted syntheses of highly CO2-selective organic cage frameworks (OCFs). Chem. Sci. 2012, 3, 874–877. [Google Scholar] [CrossRef]
- Katsoulidis, A.P.; Dyar, S.M.; Carmieli, R.; Malliakas, C.D.; Wasielewski, M.R.; Kanatzidis, M.G. Copolymerization of terephthalaldehyde with pyrrole, indole and carbazole gives microporous POFs functionalized with unpaired electrons. J. Mater. Chem. A 2013, 1, 10465–10473. [Google Scholar] [CrossRef]
- Yu, H.; Tian, M.; Shen, C.; Wang, Z. Facile preparation of porous polybenzimidazole networks and adsorption behavior of CO2 gas, organic and water vapors. Polym. Chem. 2013, 4, 961–968. [Google Scholar] [CrossRef]




| Polyphosphate | Color | Melting Point (°C) | Yield (%) |
|---|---|---|---|
| 1 | Light orange | 160–162 | 77 |
| 2 | Orange | 134–137 | 75 |
| 3 | Deep orange | 176–178 | 79 |
| Polyphosphate | FT-IR (Wavenumber; cm–1) a | 1H-NMR (Chemical Shift; ppm) b | |||
|---|---|---|---|---|---|
| P–O–C | P=O | C=C | CH=N | ||
| 1 | 1206 | 1162 | 1594 | 1600 | 6.84–7.78 (m, 24H, Ar), 9.03 (s, 3H, CH) |
| 2 | 1205 | 1135 | 1568 | 1620 | 6.77–7.82 (m, 24H, Ar), 9.03 (s, 3H, CH) |
| 3 | 1233 | 1185 | 1566 | 1616 | 6.80–7.68 (m, 24H, Ar), 9.30 (s, 3H, CH) |
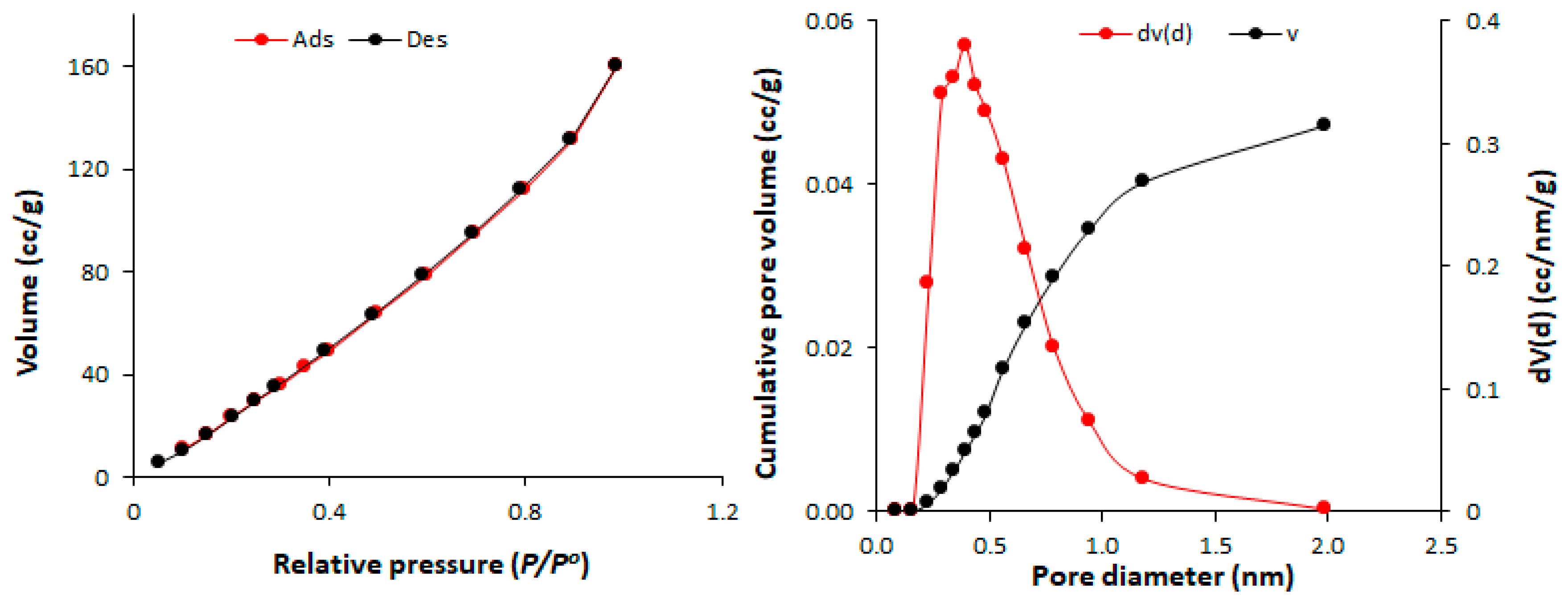
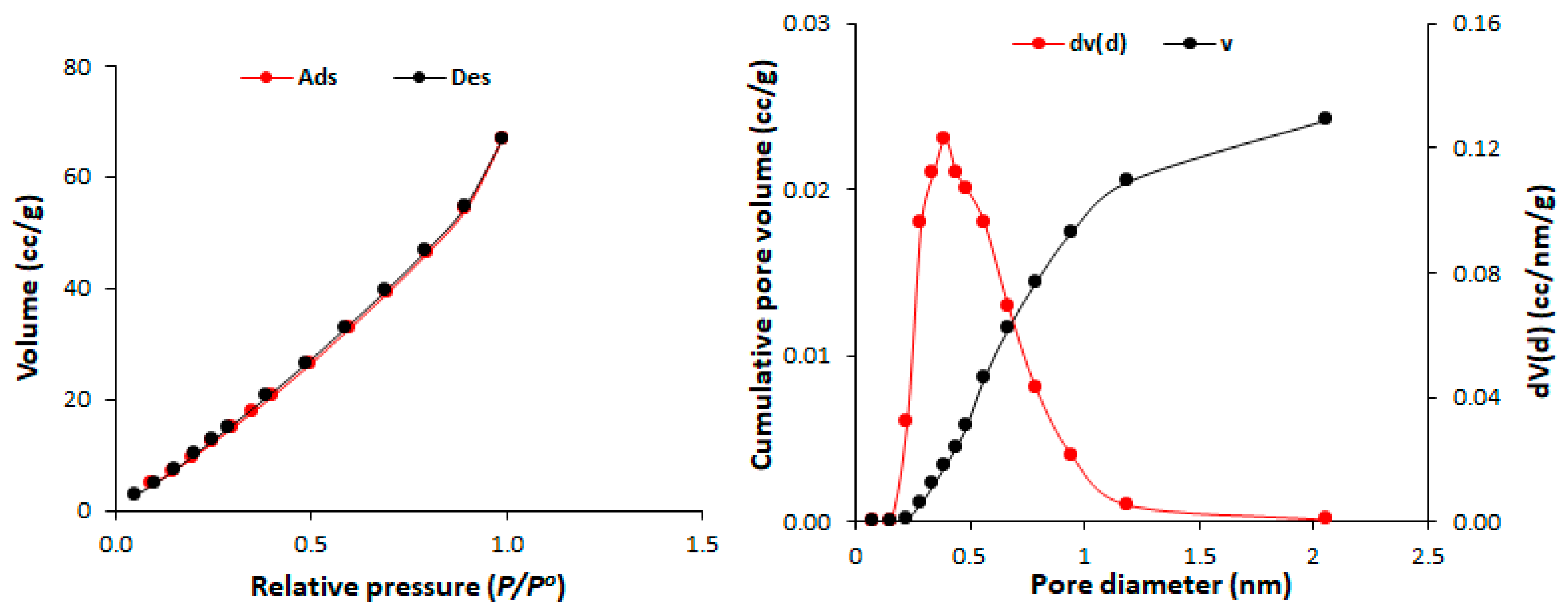
| Polyphosphate | SBET (m2/g) | Pore Volume (cm3/gm) | Average Pore Diameter (nm) |
|---|---|---|---|
| 1 | 82.7 | 0.11 | 2.43 |
| 2 | 213.5 | 0.32 | 1.96 |
| 3 | 86.1 | 0.13 | 2.43 |
| Polyphosphate | CO2 Uptake (mmol/g) | CO2 Uptake (wt %) |
|---|---|---|
| 1 | 0.46 | 2.04 |
| 2 | 1.42 | 6.00 |
| 3 | 0.95 | 4.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satar, H.A.; Ahmed, A.A.; Yousif, E.; Ahmed, D.S.; Alotibi, M.F.; El-Hiti, G.A. Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage. Appl. Sci. 2019, 9, 4314. https://doi.org/10.3390/app9204314
Satar HA, Ahmed AA, Yousif E, Ahmed DS, Alotibi MF, El-Hiti GA. Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage. Applied Sciences. 2019; 9(20):4314. https://doi.org/10.3390/app9204314
Chicago/Turabian StyleSatar, Hind A., Ahmed A. Ahmed, Emad Yousif, Dina S. Ahmed, Mohammed F. Alotibi, and Gamal A. El-Hiti. 2019. "Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage" Applied Sciences 9, no. 20: 4314. https://doi.org/10.3390/app9204314
APA StyleSatar, H. A., Ahmed, A. A., Yousif, E., Ahmed, D. S., Alotibi, M. F., & El-Hiti, G. A. (2019). Synthesis of Novel Heteroatom-Doped Porous-Organic Polymers as Environmentally Efficient Media for Carbon Dioxide Storage. Applied Sciences, 9(20), 4314. https://doi.org/10.3390/app9204314