Comparative Study of ZnO Thin Films Grown on Quartz Glass and Sapphire (001) Substrates by Means of Magnetron Sputtering and High-Temperature Annealing
Abstract
:1. Introduction
2. Experimental Section
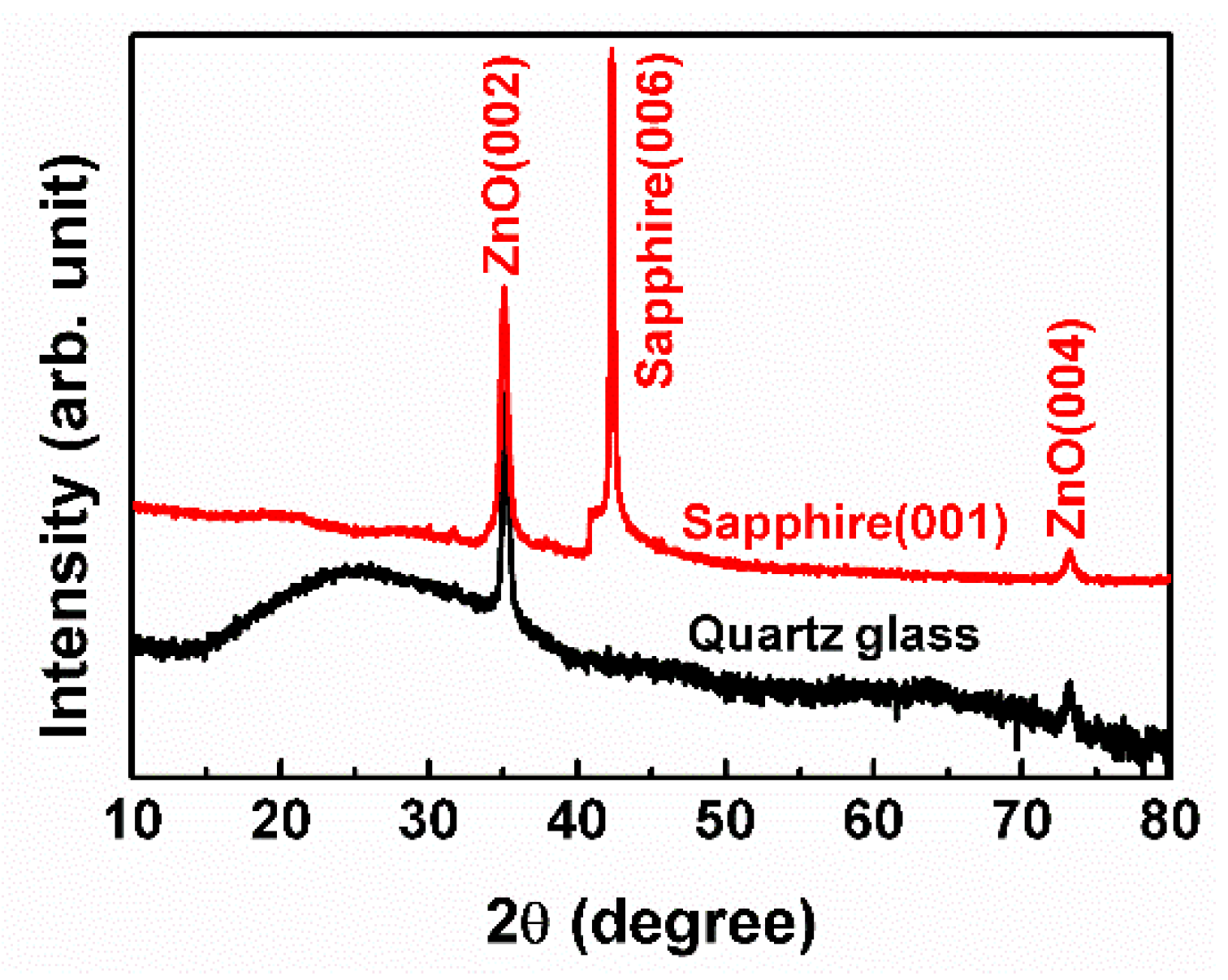
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Park, H.-H.; Zhang, X.; Lee, K.W.; Sohn, A.; Kim, D.-W.; Kim, J.; Song, J.-W.; Choi, Y.S.; Lee, H.K.; Jung, S.H.; et al. Selective photochemical synthesis of Ag nanoparticles on position-controlled ZnO nanorods for the enhancement of yellowgreen light emission. Nanoscale 2015, 7, 20717–20724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, F.; Xu, H.; Liu, W.; Yang, L.; Wang, Z.; Ma, J.; Kang, Z.; Liu, Y. Significant improvement of near-UV electroluminescence from ZnO quantum dot LEDs via coupling with carbon nanodot surface plasmons. Nanoscale 2017, 9, 14592–14601. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zheng, Y.; Li, X.; Huang, L.; Li, G. Lattice Structure and Bandgap Control of 2D GaN Grown on Graphene/Si Heterostructures. Small 2019, 15, 1802995. [Google Scholar] [CrossRef]
- Harriman, T.A.; Bi, Z.; Jia, Q.X.; Lucca, D.A. Frequency shifts of the E2 high Raman mode due to residual stress in epitaxial ZnO thin films. Appl. Phys. Lett. 2013, 103, 121904. [Google Scholar] [CrossRef]
- Alaie, Z.; Mohammad Nejad, S.; Yousefi, M.H. Recent advances in ultraviolet photodetectors. Mater. Sci. Semicond. Process. 2015, 29, 16–55. [Google Scholar] [CrossRef]
- Hung, N.L.; Kim, H.; Hong, S.-K.; Kim, D. Enhancement of CO gas sensing properties in ZnO thin films deposited on self-assembled Au nanodots. Sens. Actuators B Chem. 2010, 151, 127–132. [Google Scholar] [CrossRef]
- Karthik, T.V.K.; Martinez, L.; Agarwal, V. Porous silicon ZnO/SnO2 structures for CO2 detection. J. Alloys Compd. 2018, 731, 853–863. [Google Scholar] [CrossRef]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 2016, 11, 472–478. [Google Scholar] [CrossRef]
- Luo, Q.-P.; Wang, B.; Cao, Y. Single-crystalline porous ZnO nanosheet frameworks for efficient fully flexible dye-sensitized solar cells. J. Alloys Compd. 2017, 695, 3324–3330. [Google Scholar] [CrossRef]
- Chiu, H.-M.; Yang, T.-H.; Hsueh, Y.-C.; Perng, T.-P.; Wu, J.-M. Fabrication and characterization of well-dispersed plasmonic Pt nanoparticles on Ga-doped ZnO nanopagodas array with enhanced photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 156–166. [Google Scholar] [CrossRef]
- Correia, F.C.; Bundaleski, N.; Teodoro, O.M.N.D.; Correia, M.R.; Rebouta, L.; Mendes, A.; Tavares, C.J. XPS analysis of ZnO:Ga films deposited by magnetron sputtering: Substrate bias effect. Appl. Surf. Sci. 2018, 458, 1043–1049. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, Y.; Li, X.; Li, Y.; Zhao, H.; Huang, L.; Yang, Z.; Zhang, X.; Li, G. 2D AlN Layers Sandwiched Between Graphene and Si Substrates. Adv. Mater. 2019, 31, 1803448. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, L.; Liu, Y.; Liu, Y.; Jiang, P.; Liu, S.; Gao, M.; Tang, Z. Bioinspired Synthesis of Vertically Aligned ZnO Nanorod Arrays: Toward Greener Chemistry. Cryst. Growth Des. 2009, 9, 4793–4796. [Google Scholar] [CrossRef]
- Chu, C.-H.; Wu, H.-W.; Huang, J.-L. Effect of annealing temperature and atmosphere on aluminum-doped ZnO/Au/aluminum-doped ZnO thin film properties. Thin Solid Film. 2016, 605, 121–128. [Google Scholar] [CrossRef]
- Kumar, N.; Dubey, A.; Bahrami, B.; Venkatesan, S.; Qiao, Q.; Kumar, M. Origin of high carrier mobility and low residual stress in RF superimposed DC sputtered Al doped ZnO thin film for next generation flexible devices. Appl. Surf. Sci. 2018, 436, 477–485. [Google Scholar] [CrossRef]
- Tseng, S.-F. Investigation of post-annealing aluminum-doped zinc oxide (AZO) thin films by a graphene-based heater. Appl. Surf. Sci. 2018, 448, 163–167. [Google Scholar] [CrossRef]
- Wang, W.; Gu, H.; He, X.; Xuan, W.; Chen, J.; Wang, X.; Luo, J.K. Thermal annealing effect on ZnO surface acoustic wave-based ultraviolet light sensors on glass substrates. Appl. Phys. Lett. 2014, 104, 212107. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Ho, H.P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef]
- Nimbalkar, A.R.; Patil, M.G. Synthesis of highly selective and sensitive Cu-doped ZnO thin film sensor for detection of H 2 S gas. Mater. Sci. Semicond. Process. 2017, 71, 332–341. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, B.; Zhang, H.; Qiu, Y.; Lei, J.; Chang, Y.; Zhao, Y.; Ji, J.; Hu, L. Piezoelectric effect enhancing decay time of p-NiO/n-ZnO ultraviolet photodetector. Appl. Surf. Sci. 2016, 361, 157–161. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, G.; Wang, C.; Li, Y.; Dunphy, D.; Hasan, T.; Brinker, C.J.; Su, B.L. Bio-inspired Murray materials for mass transfer and activity. Nat. Commun. 2017, 8, 14921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Li, D.; Dai, J.; Su, H.; Wang, L.; Pu, Y.; Fang, W.; Jiang, F. Effect of the initial thin Ti buffer layers on the quality of ZnO thin films grown on Si(111) substrates by MOCVD. Superlattices Microstruct. 2006, 40, 56–63. [Google Scholar] [CrossRef]
- Chand, P.; Gaur, A.; Kumar, A.; Gaur, U.K. Structural, morphological and optical study of Li doped ZnO thin films on Si (100) substrate deposited by pulsed laser deposition. Ceram. Int. 2014, 40, 11915–11923. [Google Scholar] [CrossRef]
- Luo, C.-Q.; Ling, F.C.-C.; Rahman, M.A.; Phillips, M.; Ton-That, C.; Liao, C.; Shih, K.; Lin, J.; Tam, H.W.; Djurišić, A.B.; et al. Surface polarity control in ZnO films deposited by pulsed laser deposition. Appl. Surf. Sci. 2019, 483, 1129–1135. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Kong, X.; Gao, Z.; Han, L.; Li, X. Enhanced electrical properties of ZnO transparent conducting films prepared by electron beam annealing. Appl. Surf. Sci. 2018, 428, 191–198. [Google Scholar] [CrossRef]
- Su Kim, M.; Kim, S.; Leem, J.-Y. Laser-assisted sol-gel growth and characteristics of ZnO thin films. Appl. Phys. Lett. 2012, 100, 252108. [Google Scholar] [CrossRef]
- Shabannia, R.; Selman, A.M. Structural, optical, and electrical properties of ZnO nanofilms deposited over PS substrate. Micro Nano Lett. 2017, 12, 422–424. [Google Scholar] [CrossRef]
- Tian, J.-L.; Zhang, H.-Y.; Wang, G.-G.; Wang, X.-Z.; Sun, R.; Jin, L.; Han, J.-C. Influence of film thickness and annealing temperature on the structural and optical properties of ZnO thin films on Si (100) substrates grown by atomic layer deposition. Superlattices Microstruct. 2015, 83, 719–729. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Tran Nguyen, N.H.; Nguyen, T.H.; Liu, Y.R.; Aminzare, M.; Pham, A.T.; Cho, S.; Wong, D.P.; Chen, K.H.; Seetawan, T.; Pham, N.K.; et al. Thermoelectric Properties of Indium and Gallium Dually Doped ZnO Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 33916–33923. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, C.; Xu, B.; Wang, P.; Liu, J. Effects of the annealing temperature on the structure and up-conversion photoluminescence of ZnO film. J. Mater. Sci. Technol. 2018, 34, 2392–2397. [Google Scholar] [CrossRef]
- Boryło, P.; Matus, K.; Lukaszkowicz, K.; Kubacki, J.; Balin, K.; Basiaga, M.; Szindler, M.; Mikuła, J. The influence of atomic layer deposition process temperature on ZnO thin film structure. Appl. Surf. Sci. 2019, 474, 177–186. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics PerkinElmer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Alfaro Cruz, M.R.; Ceballos-Sanchez, O.; Luévano-Hipólito, E.; Torres-Martínez, L.M. ZnO thin films deposited by RF magnetron sputtering: Effects of the annealing and atmosphere conditions on the photocatalytic hydrogen production. Int. J. Hydrog. Energy 2018, 43, 10301–10310. [Google Scholar] [CrossRef]
- Zaier, A.; Meftah, A.; Jaber, A.Y.; Abdelaziz, A.A.; Aida, M.S. Annealing effects on the structural, electrical and optical properties of ZnO thin films prepared by thermal evaporation technique. J. King Saud Univ.-Sci. 2015, 27, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Ooi, P.K.; Ng, S.S.; Abdullah, M.J.; Hassan, Z. Structural and optical properties of In-doped ZnO thin films under wet annealing. Mater. Lett. 2014, 116, 396–398. [Google Scholar] [CrossRef]
- Irimpan, L.; Ambika, D.; Kumar, V.; Nampoori, V.P.N.; Radhakrishnan, P. Effect of annealing on the spectral and nonlinear optical characteristics of thin films of nano-ZnO. J. Appl. Phys. 2008, 104, 033118. [Google Scholar] [CrossRef]
- Knutsen, K.E.; Galeckas, A.; Zubiaga, A.; Tuomisto, F.; Farlow, G.C.; Svensson, B.G.; Kuznetsov, A.Y. Zinc vacancy and oxygen interstitial in ZnO revealed by sequential annealing and electron irradiation. Phys. Rev. B 2012, 86, 121203. [Google Scholar] [CrossRef]
- Wang, X.J.; Vlasenko, L.S.; Pearton, S.J.; Chen, W.M.; Buyanova, I.A. Oxygen and zinc vacancies in as-grown ZnO single crystals. J. Phys. D Appl. Phys. 2009, 42, 175411. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef] [Green Version]
- Ahn, C.H.; Kim, Y.Y.; Kim, D.C.; Mohanta, S.K.; Cho, H.K. A comparative analysis of deep level emission in ZnO layers deposited by various methods. J. Appl. Phys. 2009, 105, 013502. [Google Scholar] [CrossRef]
- Chandrinou, C.; Boukos, N.; Stogios, C.; Travlos, A. PL study of oxygen defect formation in ZnO nanorods. Microelectron. J. 2009, 40, 296–298. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Hsu, Y.F.; Ding, L.; Ge, W.K.; Zhong, Y.C.; Wong, K.S.; Chan, W.K.; Tam, H.L.; et al. Defect emissions in ZnO nanostructures. Nanotechnology 2007, 18, 095702. [Google Scholar] [CrossRef]
- Sarsfield, B.A.; Davidovich, M.; Desikan, S.; Fakes, M.; Futernik, S.; Hilden, J.L.; Tan, J.S.; Yin, S.; Young, G.; Vakkalagadda, B.; et al. Powder X-ray Diffraction Detection of Crystalline Phases in Amorphous Pharmceuticals; The International Centre for Diffraction Data: Newtown Square, PA, USA, 2006. [Google Scholar]
- Wermelinger, T.; Mornaghini, F.C.F.; Hinderling, C.; Spolenak, R. Correlation between the defect structure and the residual stress distribution in ZnO visualized by TEM and Raman microscopy. Mater. Lett. 2010, 64, 28–30. [Google Scholar] [CrossRef]
- Jannotti, P.; Subhash, G.; Zheng, J.; Halls, V. Measurement of microscale residual stresses in multi-phase ceramic composites using Raman spectroscopy. Acta Mater. 2017, 129, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Si, B.; Chen, S.; Jiao, B.; Yan, X. A nondestructive raman spectra stress 2D analysis for the pressure sensor sensitive silicon membrane. Eng. Fail. Anal. 2019, 105, 1252–1261. [Google Scholar] [CrossRef]
- Kunj, S.; Sreenivas, K. Residual stress and defect content in magnetron sputtered ZnO films grown on unheated glass substrates. Curr. Appl. Phys. 2016, 16, 748–756. [Google Scholar] [CrossRef]
- Kharroubi, B.; Baghdad, R.; Abdiche, A.; Bousmaha, M.; Bousquet, M.; Zeinert, A.; Marssi, M.E.; Zellama, K.; Hamzaoui, S. Mn doping effect on the structural properties of ZnO-nanostructured films deposited by the ultrasonic spray pyrolysis method. Phys. Scr. 2012, 86, 015805. [Google Scholar] [CrossRef]







| Substrate | 2θ/° | c(002)/nm | FWHM/° | D/nm | δ/(×10−3 nm) | σ/GPa |
|---|---|---|---|---|---|---|
| Quartz glass | 35.151 | 0.5132 | 0.271 | 30.8 | 1.05 | 3.312 |
| Sapphire (001) | 35.020 | 0.5137 | 0.299 | 27.9 | 1.28 | 3.088 |
| Substrate | Annealing | Peak | Position (eV) | FWHM (eV) | Area (%) |
|---|---|---|---|---|---|
| Quartz glass | 600 °C | O1 | 529.96 | 1.23 | 36.32 |
| O2 | 530.86 | 0.85 | 4.40 | ||
| O3 | 531.46 | 1.73 | 59.28 | ||
| Sapphire | 600 °C | O1 | 529.94 | 1.24 | 37.06 |
| O2 | 531.31 | 1.51 | 33.16 | ||
| O3 | 531.97 | 1.81 | 29.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, F.; Guan, Z.; He, P.; Liu, Z.; Hu, L.; Chen, M.; Zhang, C.; He, X.; Fu, Y. Comparative Study of ZnO Thin Films Grown on Quartz Glass and Sapphire (001) Substrates by Means of Magnetron Sputtering and High-Temperature Annealing. Appl. Sci. 2019, 9, 4509. https://doi.org/10.3390/app9214509
Yang W, Wang F, Guan Z, He P, Liu Z, Hu L, Chen M, Zhang C, He X, Fu Y. Comparative Study of ZnO Thin Films Grown on Quartz Glass and Sapphire (001) Substrates by Means of Magnetron Sputtering and High-Temperature Annealing. Applied Sciences. 2019; 9(21):4509. https://doi.org/10.3390/app9214509
Chicago/Turabian StyleYang, Weijia, Fengming Wang, Zeyi Guan, Pengyu He, Zhihao Liu, Linshun Hu, Mei Chen, Chi Zhang, Xin He, and Yuechun Fu. 2019. "Comparative Study of ZnO Thin Films Grown on Quartz Glass and Sapphire (001) Substrates by Means of Magnetron Sputtering and High-Temperature Annealing" Applied Sciences 9, no. 21: 4509. https://doi.org/10.3390/app9214509





