Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
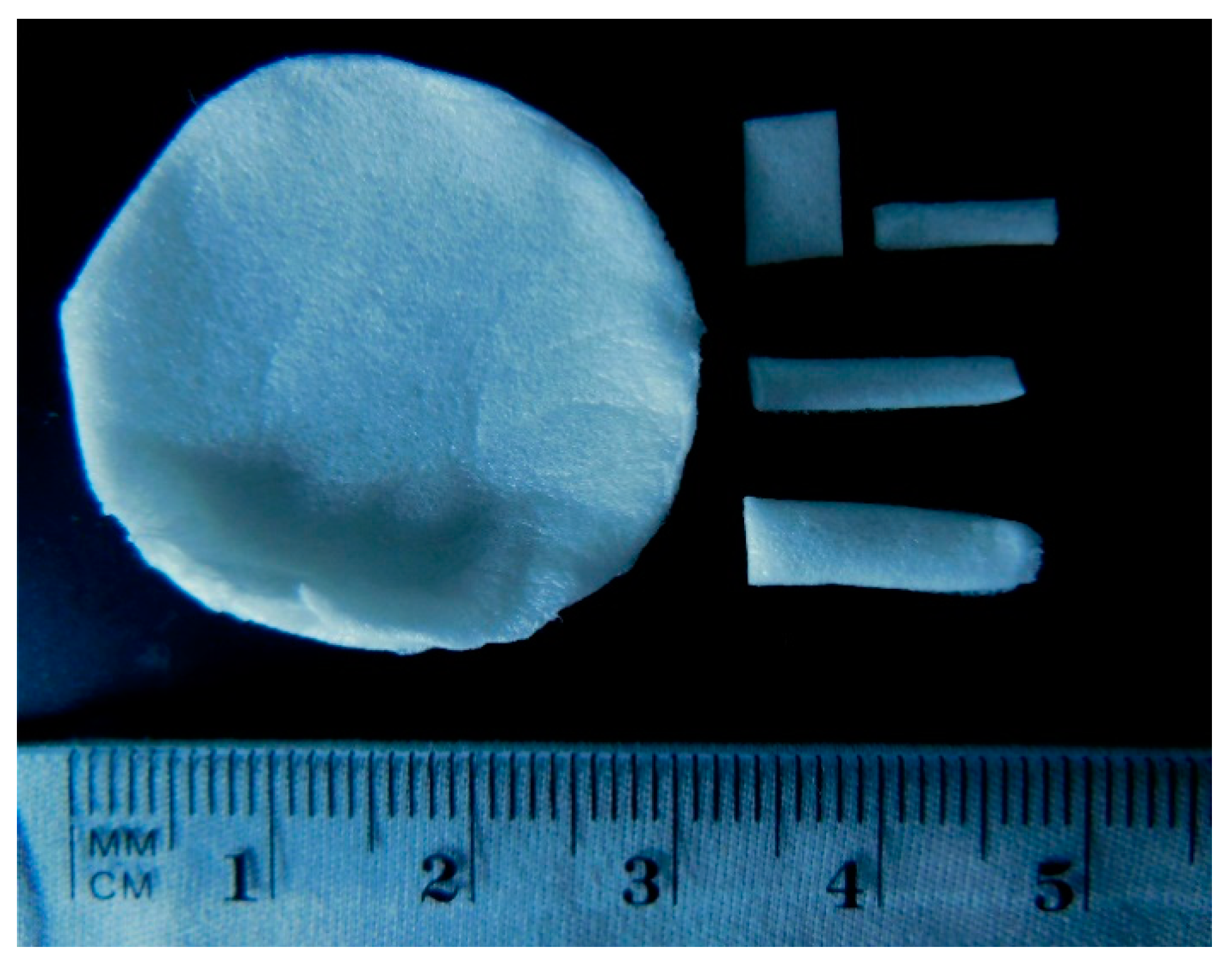
2.1. Matrix Preparation
2.2. Physical Properties of Matrix
2.2.1. Surface Morphology Observation
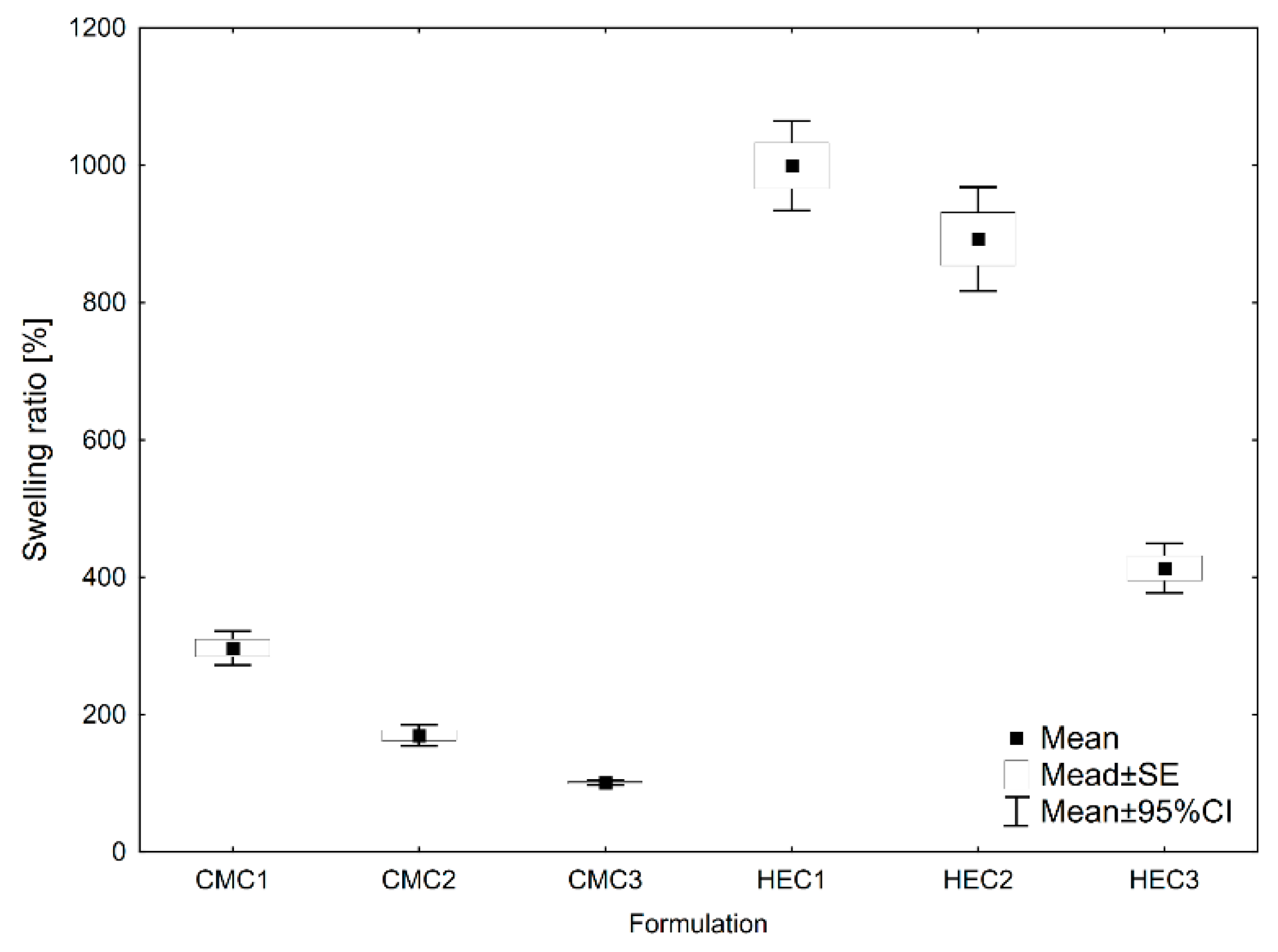
2.2.2. Swelling Ratio and Degradation Weight Loss Analysis
2.2.3. Mechanical Properties Testing
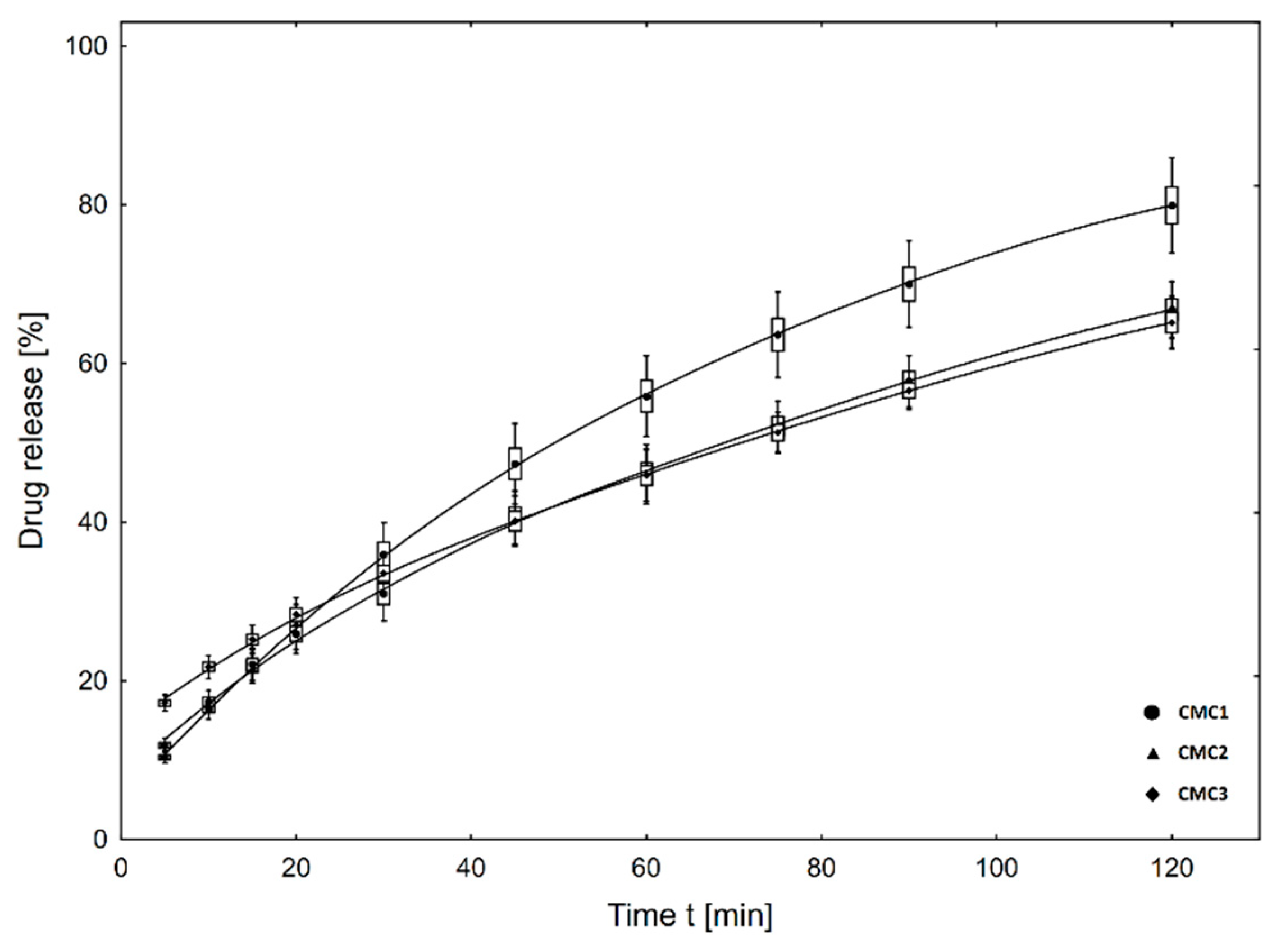
2.3. In Vitro Metronidazole Release Studies
Drug Release Kinetics
2.4. Preparation of Extract for Cell Viability Assay
Cytotoxicity Assay of MTZ-Loaded Matrices Toward Fibroblast and Osteoblast Cell Cultures
2.5. Metronidazole Disk Diffusion Test
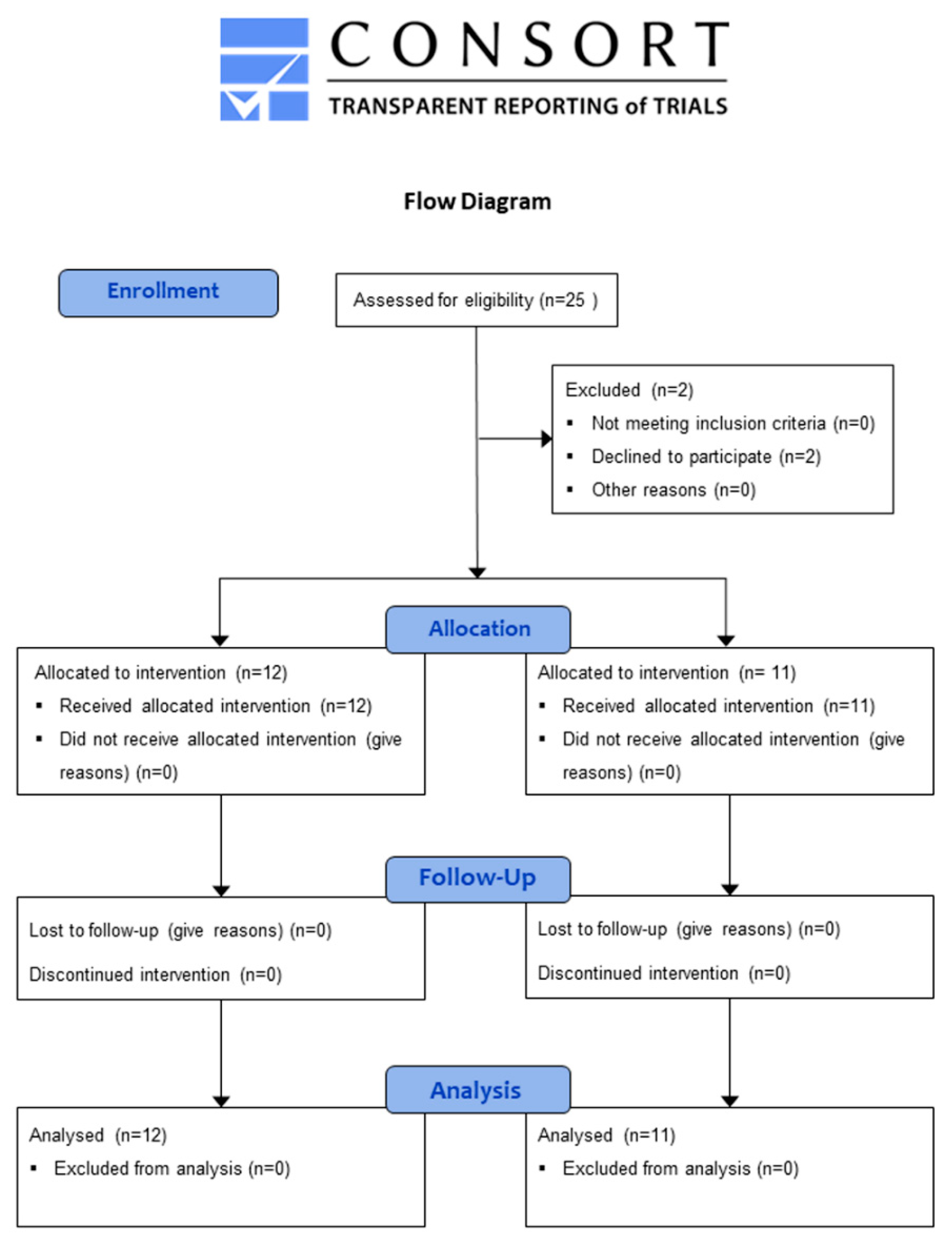
2.6. Clinical Pilot Study
2.7. Statistical Analyses
3. Results
3.1. Physical Properties of Matrices
3.1.1. Morphological Observation of Surface
3.1.2. Swelling Ratio and Degradation Weight-Loss Analysis
3.1.3. Mechanical Testing
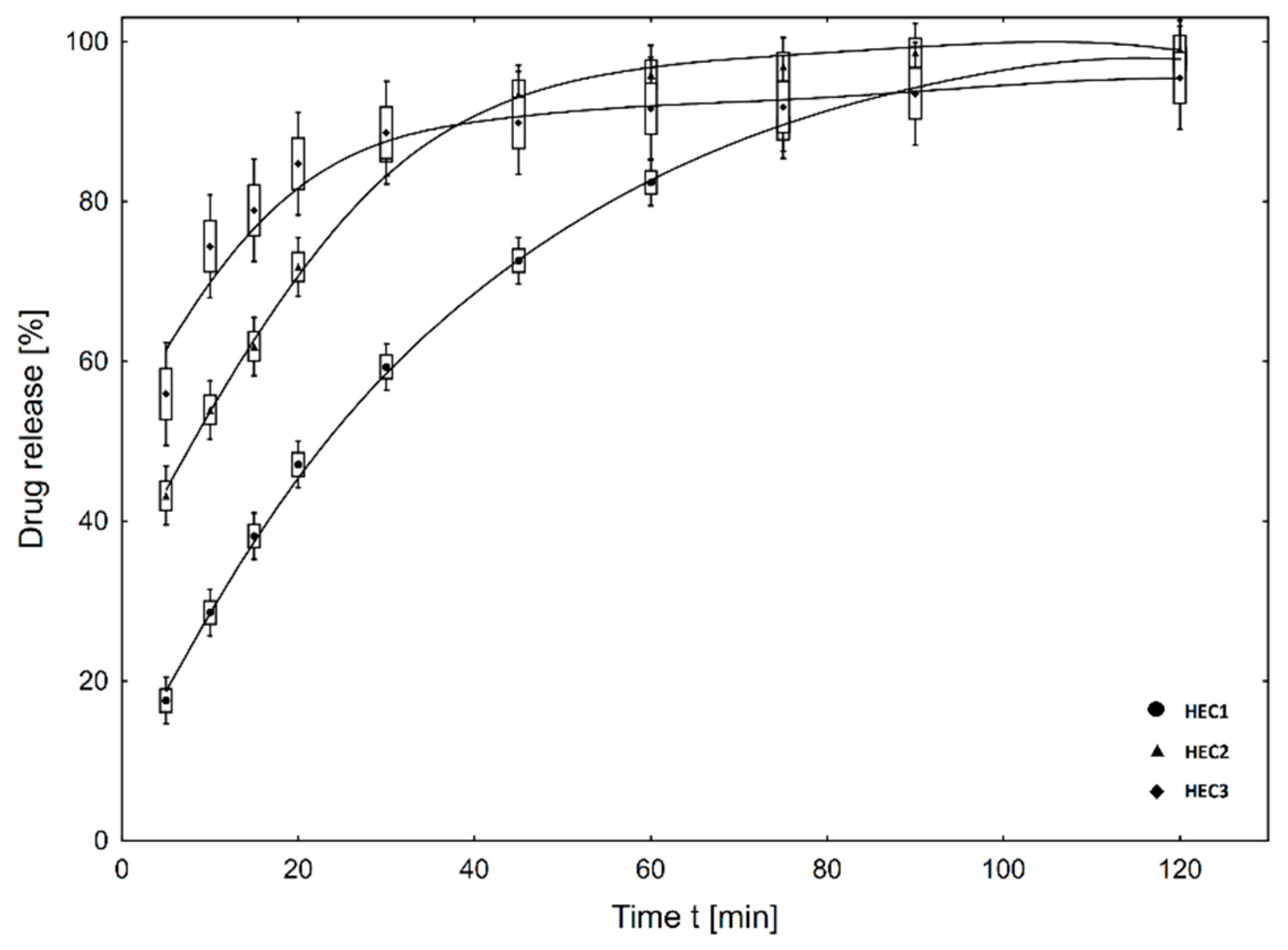
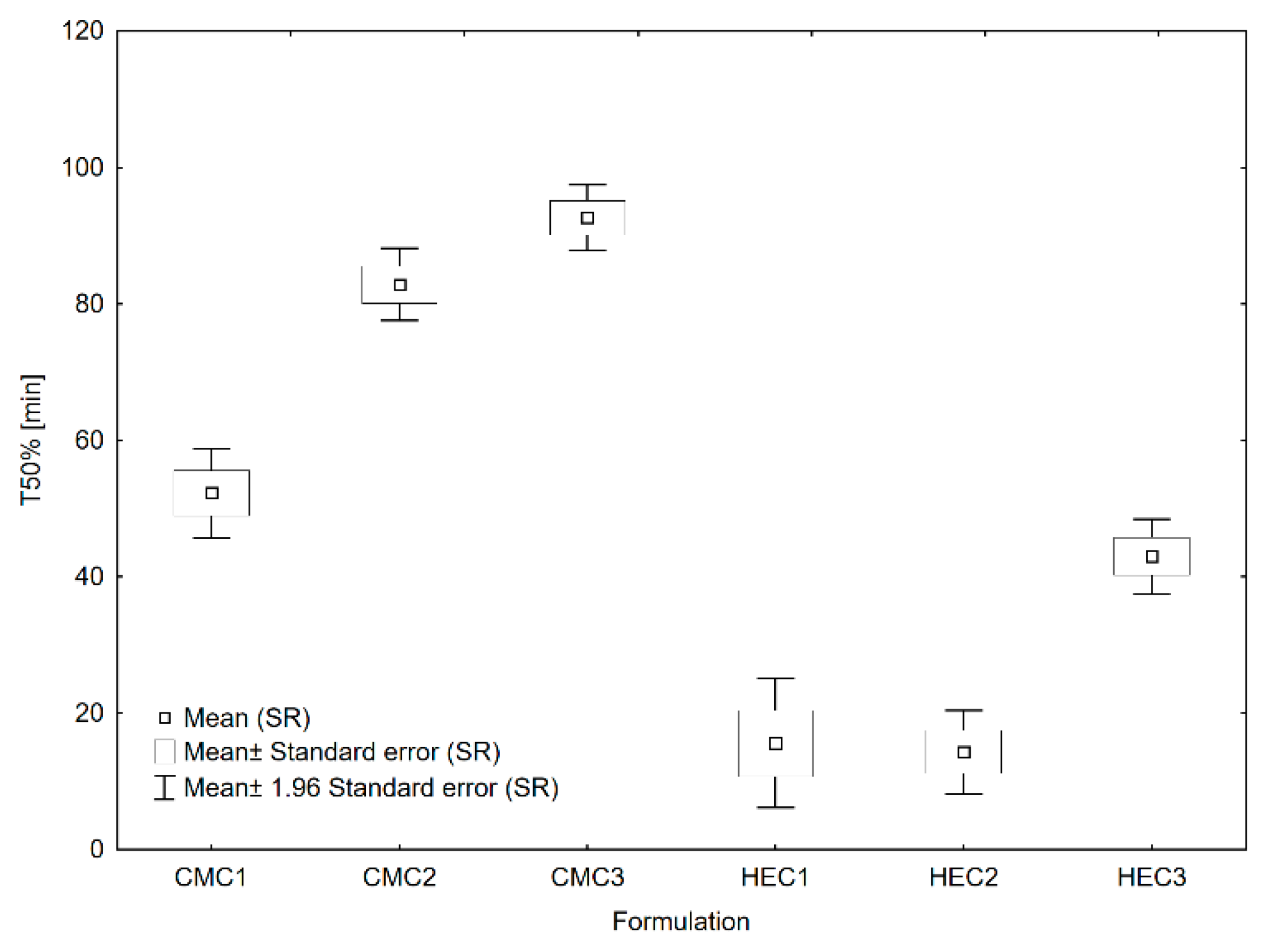
3.2. In Vitro Drug Release
3.3. Evaluation of Cytotoxic Properties of Matrices with Regard to Fibroblast and Osteoblast Reference Cell Cultures
3.4. Metronidazole Disk Diffusion Test
3.5. Clinical Pilot Study
4. Discussion
Limitations of Pilot Study
5. Conclusions
- Metronidazole-loaded matrices based on gelatin and CMC or HEC synthesized by whipping and lyophilization methods were soft, porous, and swellable in water.
- Both matrices based on hydroxyethyl cellulose (CMC2) and hydroxyethyl cellulose (HEC2) containing metronidazole showed effective antimicrobial activity in vitro.
- No adverse effects were displayed by the metronidazole-containing matrix based on HEC applied clinically, and its single application lead to positive clinical outcomes.
- Intra-pocket application of metronidazole in the designed matrix is a worthwhile supplementation of the classical periodontal inflammation treatment and a feasible alternative to the use of antibiotics in periodontal diseases.
- Based on the elaborated pilot study, it can be concluded that the performance of the main clinical study is feasible and may bring high added value to local treatment of periodontal diseases.
Author Contributions
Funding
Conflicts of Interest
References
- Dusane, J.; Vrushali, M.; Borse, P.; Thakare, P.; Kshirsagar, S. Recent Trends in Treatment of Periodontitis. Pharm. Biol. Eval. 2016, 3, 19–31. [Google Scholar]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontol. 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. On the inability of root debridement and periodontal surgery to eliminate actinobacillus actinomycetemcomitans from periodontal pockets. J. Clin. Periodontol. 1990, 17, 351–355. [Google Scholar] [CrossRef]
- Horliana, A.; Chambrone, L.; Foz, A.; Artese, H.P.C.; Rabelo, M.; Pannuti, C.M.; Romito, G.A. Dissemination of periodontal pathogens in the bloodstream after periodontal procedures: A systematic review. PLoS ONE 2014, 9, 21–23. [Google Scholar] [CrossRef]
- Forner, L.; Larsen, T.; Kilian, M.; Holmstrup, P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 2006, 33, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Mayorga-Fayad, I.; Torres, M.F.; Castillo, D.M.; Aya, M.R.; Barón, A.; Hurtado, P.A. Periodontopathic microorganisms in peripheric blood after scaling and root planing. J. Clin. Periodontol. 2007, 34, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.M.; Sánchez-Beltrán, M.C.; Castellanos, J.E.; Sanz, I.; Mayorga-Fayad, I.; Sanz, M.; Lafaurie, G.I. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J. Clin. Periodontol. 2011, 38, 418–427. [Google Scholar] [CrossRef]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of actinobacillus actinomycetemcomitans and bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef]
- Soares, G.M.; Mendes, J.A.; Silva, M.P.; Faveri, M.; Teles, R.; Socransky, S.S.; Wang, X.; Figueiredo, L.C.; Feres, M. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: A secondary analysis of microbiological results from a randomized clinical trial. J. Clin. Periodontol. 2014, 41, 1149–1158. [Google Scholar] [CrossRef]
- Addy, M.; Martin, M.V. Systemic antimicrobials in the treatment of chronic periodontal diseases: A dilemma. Oral. Dis. 2003, 9, 38–44. [Google Scholar] [CrossRef]
- Sanz, I.; Alonso, B.; Carasol, M.; Herrera, D.; Sanz, M. Nonsurgical treatment of periodontitis. J. Evid. Based Dent. Pract. 2012, 12, 76–86. [Google Scholar] [CrossRef]
- Matesanz-Pérez, P.; García-Gargallo, M.; Figuero, E.; Bascones-Martínez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef]
- Haris, M.; Panickal, D.M. Role of metronidazole as a local drug delivery in the treatment of periodontitis: A review. Int. J. Oral. Heal. Med. Res. 2017, 3, 141–145. [Google Scholar]
- Nair, S.C.; Anoop, K.R. Intraperiodontal pocket: An ideal route for local antimicrobial drug delivery. J. Adv. Pharm. Technol. Res. 2012, 3, 9–15. [Google Scholar]
- Djagny, V.B.; Wang, Z.; Xu, S. Gelatin: A valuable protein for food and pharmaceutical industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Wong, M.; Tabata, Y.; Mikos, A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J. Control. Release 2005, 109, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Tylingo, R.; Gorczyca, G.; Mania, S.; Szweda, P.; Milewski, S. Preparation and characterization of porous scaffolds from chitosan-collagen-gelatin composite. React. Funct. Polym. 2016, 103, 131–140. [Google Scholar] [CrossRef]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Selvaraj, D.; Viswanadha, V.; Elango, S. Wound dressings-a review. BioMedicine 2015, 5, 24–28. [Google Scholar]
- Pachuau, L. Recent developments in novel drug delivery systems for wound healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Khorasani, S.N.; Saadatkish, N.; Khoshakhlagh, K. Characterization of gelatin/cellulose acetate nanofibrous scaffolds: Prediction and optimization by response surface methodology and artificial neural networks. Polym. Sci. Ser. A 2016, 58, 399–408. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous scaffolds with biomimetic composition for skin regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef]
- Polish Pharmacopeia; Office for Registration of Medicinal Products, Medical Devices and Biocidal Products: Warsaw, Poland, 2014.
- Eskandarinia, A.; Kefayat, A.; Rafienia, M.; Agheb, M.; Navid, S.; Ebrahimpour, K. Cornstarch-based wound dressing incorporated with hyaluronic acid and propolis: In vitro and in vivo studies. Carbohydr. Polym. 2019, 216, 25–35. [Google Scholar] [CrossRef]
- Waghmare, V.S.; Wadke, P.R.; Dyawanapelly, S.; Deshpande, A.; Jain, R.; Dandekar, P. Starch based nanofibrous scaffolds for wound healing applications. Bioact. Mater. 2018, 3, 255–266. [Google Scholar] [CrossRef]
- Dias, A.M.; da Silva, F.G.; de Figueiredo Monteiro, A.P.; Pinzón-García, A.D.; Sinisterra, R.D.; Cortés, M.E. Polycaprolactone nanofibers loaded oxytetracycline hydrochloride and zinc oxide for treatment of periodontal disease. Mater. Sci. Eng. C 2019, 103, 109798. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45, S162–S170. [Google Scholar] [CrossRef]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.P.A. Klinische Bewertungsverfahren zur Objektivierung der Mundhygiene. Dtsch. Zahnarztl. 1977, 32, 44–47. [Google Scholar]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Saxer UPMühlemann, H.R. Motivation und Aufklärung. Schweiz. Mschr. Zahnheilk. 1975, 85, 905–1002. [Google Scholar]
- Domínguez, L.; Cepeda, J.; Sánchez, L.; Márquez, R.; Aranda Romo, S. Evaluation of the clinical effect of a probiotic mouthwash in the treatment of generalized marginal chronic gingivitis. Randomized pilot study. J. Oral. Res. 2018, 7, 134–140. [Google Scholar] [CrossRef] [Green Version]
- García-Gargallo, M.; Zurlohe, M.; Montero, E.; Alonso, B.; Serrano, J.; Sanz, M.; Herrera, D. Evaluation of new chlorhexidine- and cetylpyridinium chloride-based mouthrinse formulations adjunctive to scaling and root planing: Pilot study. Int. J. Dent. Hyg. 2017, 15, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontol. 2000. 2018, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Mapstone, N.P.; Lynch, D.A.; Lewis, F.A.; Axon, A.T.; Tompkins, D.S.; Dixon, M.F.; Quirke, P. Identification of Helicobacter pylori DNA in the mouths and stomachs of patients with gastritis using PCR. J. Clin. Pathol. 1993, 46, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Marini, M.F.; Ciusa, M.L.; Isola, D.; Cotti, M.; Baldoni, M.; Piras, V.; Pisano, E.; Montaldo, C. Usefulness of real time PCR for the differentiation and quantification of 652 and JP2 Actinobacillus actinomycetemcomitans genotypes in dental plaque and saliva. BMC Infect. Dis. 2006, 6, 98. [Google Scholar] [CrossRef]
- Radwan-Oczko, M.; Jaworski, A.; Duś, I.; Plonek, T.; Szulc, M.; Kustrzycki, W. Porphyromonas gingivalis in periodontal pockets and heart valves. Virulence 2014, 5, 575–580. [Google Scholar] [CrossRef]
- Kanimozhi, K.; Khaleel Basha, S.; Sugantha Kumari, V. Processing and characterization of chitosan/PVA and methylcellulose porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2016, 61, 484–491. [Google Scholar] [CrossRef]
- Labib, G.S.; Aldawsari, H.M.; Badr-Eldin, S.M. Metronidazole and pentoxifylline films for the local treatment of chronic periodontal pockets: Preparation, in vitro evaluation and clinical assessment. Expert Opin. Drug Deliv. 2014, 11, 1981. [Google Scholar] [CrossRef]
- Morales, J.O.; McConville, J.T. Manufacture and characterization of mucoadhesive buccal films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Hydroxypropylmethyl cellulose-based sponges loaded self-microemulsifying curcumin: Preparation, characterization, and in vivo oral absorption studies. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Thakur, G.; Singh, A.; Singh, I. Chitosan-montmorillonite polymer composites: Formulation and evaluation of sustained release tablets of aceclofenac. Sci. Pharm. 2016, 84, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.C.; Kumar, K.S.; Ramalingam, M. Design and release characteristics of sustained release tablet containing metformin HCl. Rev. Bras. Ciencias. Farm. J. Pharm. Sci. 2008, 44, 477–483. [Google Scholar] [CrossRef]
- Rodrigues, I.F.; Machion, L.; Casati, M.Z.; Nociti, F.H.; Toledo, S., Jr.; Sallum, A.W.; Sallum, E.A. Clinical evaluation of the use of locally delivered chlorhexidine in periodontal maintenance therapy. J. Periodontol. 2007, 78, 624–628. [Google Scholar] [CrossRef]
- Grover, V.; Kapoor, A.; Malhotra, R.; Battu, V.S.; Bhatia, A.; Sachdeva, S. To assess the effectiveness of a chlorhexidine chip treatment of chronic periodontitis: A clinical and radiographic study. J. Indian. Soc. Periodontol. 2011, 15, 139–146. [Google Scholar] [CrossRef]
- Chaturvedi, T.P.; Srivastava, R.; Srivastava, A.K.; Gupta, V.; Verma, P.K. Evaluation of metronidazole nanofibers in patients with chronic periodontitis: A clinical study. Int. J. Pharm. Investig. 2012, 2, 213–217. [Google Scholar]


| Batch Code | GE | CMC | HEC | GLY | MTZ |
|---|---|---|---|---|---|
| (mg per matrix) | |||||
| CMC1 | 72.0 | 43.2 | - | 180.0 | 19.8 |
| CMC2 | 144.0 | 32.4 | - | 180.0 | 19.8 |
| CMC3 | 216.0 | 21.6 | - | 180.0 | 19.8 |
| HEC1 | 72.0 | - | 43.2 | 180.0 | 19.8 |
| HEC2 | 144.0 | - | 32.4 | 180.0 | 19.8 |
| HEC3 | 216.0 | - | 21.6 | 180.0 | 19.8 |
| Batch Code | Drug Content in Matrices ± SE (%) n = 6 | Mass of Matrices ± SE (g) n = 20 | Thickness of Matrices ± SE (mm) n = 6 |
|---|---|---|---|
| CMC1 | 96.307 ± 1.104 | 0.3495 ± 0.0017 | 2.02 ± 0.02 |
| CMC2 | 92.410 ± 0.939 | 0.3885 ± 0.0013 | 2.98 ± 0.03 |
| CMC3 | 104.445 ± 1.304 | 0.4147 ± 0.0006 | 3.05 ± 0.05 |
| HEC1 | 99.993 ± 0.699 | 0.3159 ± 0.0015 | 1.01 ± 0.02 |
| HEC2 | 91.669 ± 1.022 | 0.3785 ± 0.0030 | 2.06 ± 0.03 |
| HEC3 | 90.600 ± 0.780 | 0.4181 ± 0.0013 | 2.10 ± 0.03 |
| Batch Code | Weight Loss of Matrices ± SE (%) n = 6 |
|---|---|
| CMC1 | 11.58 ± 1.83 |
| CMC2 | 20.89 ± 2.55 |
| CMC3 | 27.83 ± 2.76 |
| HEC1 | 36.62 ± 1.12 |
| HEC2 | 45.12 ± 1.40 |
| HEC3 | 77.00 ± 2.80 |
| Batch Code | Rupture Force (g) | Elongation at Break (%) |
|---|---|---|
| CMC1 | 251.26 ± 18.68 | 679.4 ± 42.83 |
| CMC2 | 1345.19 ± 72.10 | 1027.9 ± 53.82 |
| CMC3 | 2023.21 ± 48.33 | 918.7 ± 66.56 |
| HEC1 | 469.59 ± 24.49 | 750.5 ± 44.12 |
| HEC2 | 1007.95 ± 41.78 | 679.0 ± 54.13 |
| HEC3 | 1647.21 ± 88.61 | 632.6 ± 15.26 |
| Batch Code | First-Order Kinetic Model | Zero-Order Kinetic Model | Higuchi Model | Korsmeyer–Peppas Model |
|---|---|---|---|---|
| CMC1 | ||||
| CMC2 | ||||
| CMC3 | ||||
| HEC1 | ||||
| HEC2 | ||||
| HEC3 |
| Batch Code | ANOVA: F = 98.9, df = 30, p = 1.04 × 10−17 (Significance Level α = 0.05) | |||||
|---|---|---|---|---|---|---|
| CMC1 | CMC2 | CMC3 | HEC1 | HEC2 | HEC3 | |
| CMC1 | 3.22 × 10−7 | 1.27 × 10−9 | 9.30 × 10−9 | 4.55 × 10−9 | 5.59 × 10−2 | |
| CMC2 | 3.22 × 10−7 | 4.45 × 10−2 | 5.39 × 10−15 | 3.26 × 10−15 | 1.67 × 10−9 | |
| CMC3 | 1.27 × 10−9 | 4.45 × 10−2 | 1.42 × 10−16 | 9.03 × 10−17 | 1.12 × 10−11 | |
| HEC1 | 9.30 × 10−9 | 5.39 × 10−15 | 1.42 × 10−16 | 7.85 × 10−1 | 2.07 × 10−6 | |
| HEC2 | 4.55 × 10−9 | 3.26 × 10−15 | 9.03 × 10−17 | 7.85 × 10−1 | 9.58 × 10−7 | |
| HEC3 | 5.59 × 10−2 | 1.67 × 10−9 | 1.12 × 10−11 | 2.07 × 10−6 | 9.58 × 10−7 | |
| Test 1 | Test 2 | Test 3 | |
|---|---|---|---|
| Mean API | 54 | 32 | 26 |
| mean BOP | 25 | 20 | 16 |
| mean PBI | 3.14 | 1.71 | 1.21 |
| Friedman ANOVA and Kendall Coeff of Concordance ANOVA Chi Sqr (n = 84, df = 4) = 231.032 p = 0.0000, Coeff of Concordance = 0.69057, Aver Rank r = 0.68684 | ||||
|---|---|---|---|---|
| Variable | Average Rank | Sum of Ranks | Mean | Std Dev |
| Pocket depth | 3.089286 | 259.5000 | 4.88095 | 2.39681 |
| No. | 4.767857 | 400.5000 | 42.50000 | 24.39262 |
| Pretest | 3.089286 | 259.5000 | 4.88095 | 2.39681 |
| After 1 week | 2.244048 | 188.5000 | 4.33333 | 2.16952 |
| After 4 weeks | 1.809524 | 152.0000 | 3.98810 | 1.94809 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kida, D.; Karolewicz, B.; Junka, A.; Sender-Janeczek, A.; Duś, I.; Marciniak, D.; Szulc, M. Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study. Appl. Sci. 2019, 9, 4545. https://doi.org/10.3390/app9214545
Kida D, Karolewicz B, Junka A, Sender-Janeczek A, Duś I, Marciniak D, Szulc M. Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study. Applied Sciences. 2019; 9(21):4545. https://doi.org/10.3390/app9214545
Chicago/Turabian StyleKida, Dorota, Bożena Karolewicz, Adam Junka, Aleksandra Sender-Janeczek, Irena Duś, Dominik Marciniak, and Małgorzata Szulc. 2019. "Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study" Applied Sciences 9, no. 21: 4545. https://doi.org/10.3390/app9214545
APA StyleKida, D., Karolewicz, B., Junka, A., Sender-Janeczek, A., Duś, I., Marciniak, D., & Szulc, M. (2019). Metronidazole-Loaded Porous Matrices for Local Periodontitis Treatment: In Vitro Evaluation and In Vivo Pilot Study. Applied Sciences, 9(21), 4545. https://doi.org/10.3390/app9214545







