The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Waste to Be Used in This Study
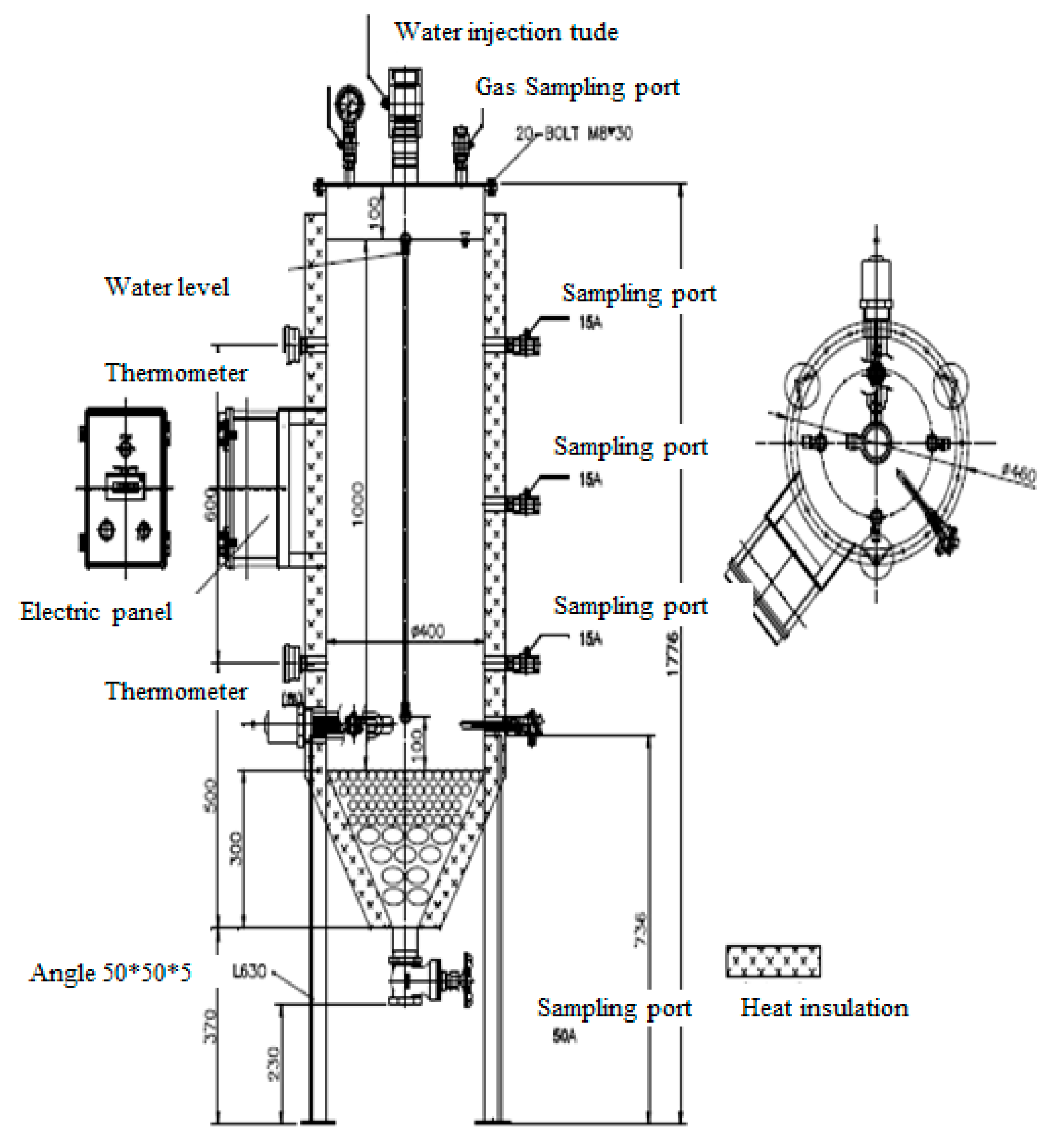
2.2. Design of the Lysimeter Reactor and Filling
2.3. Three Component and Chemical Composition
2.4. Gas and Leachate Analysis Method
3. Results
3.1. Results of the Analysis of the Three Components
3.2. Results of Elemental Analysis
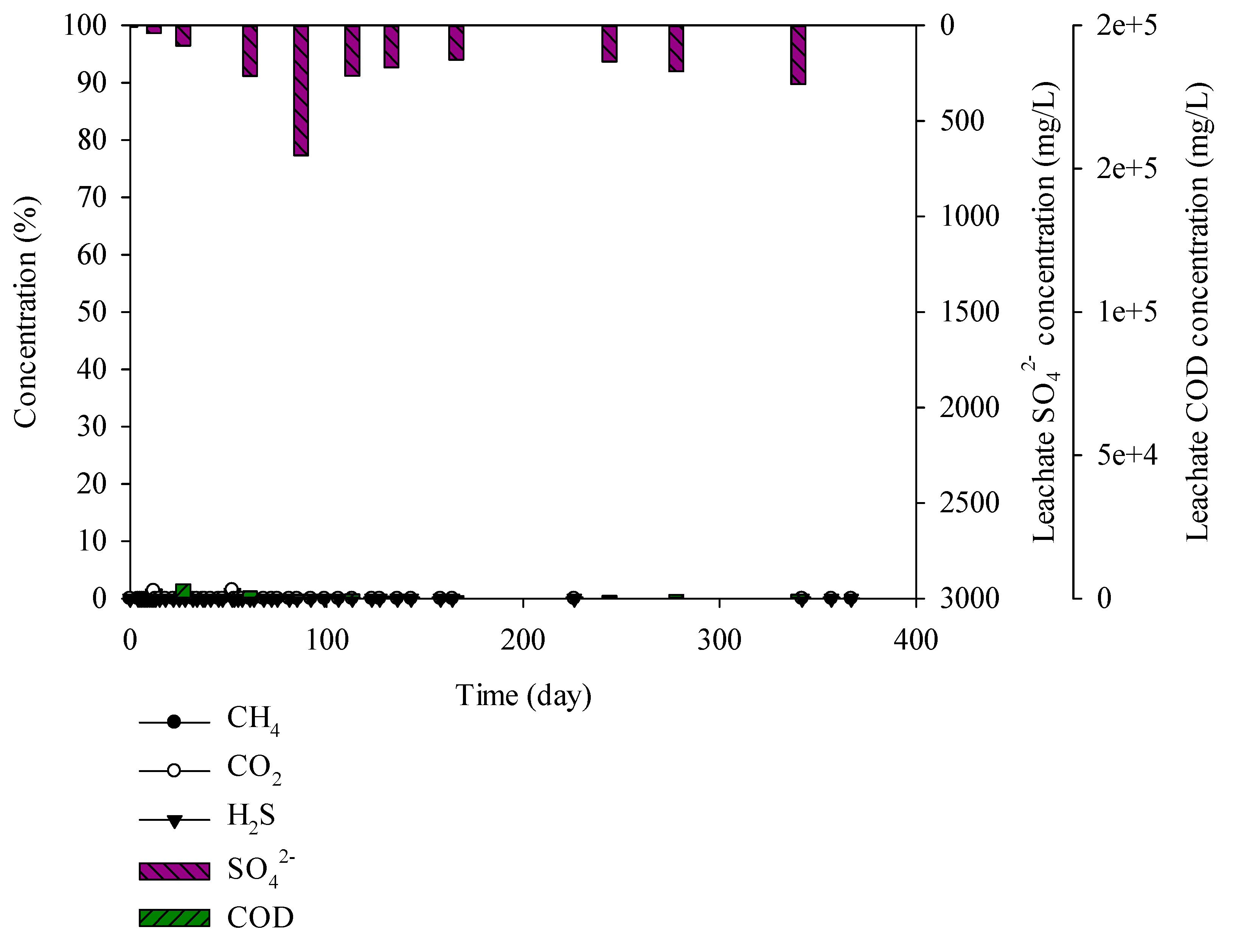
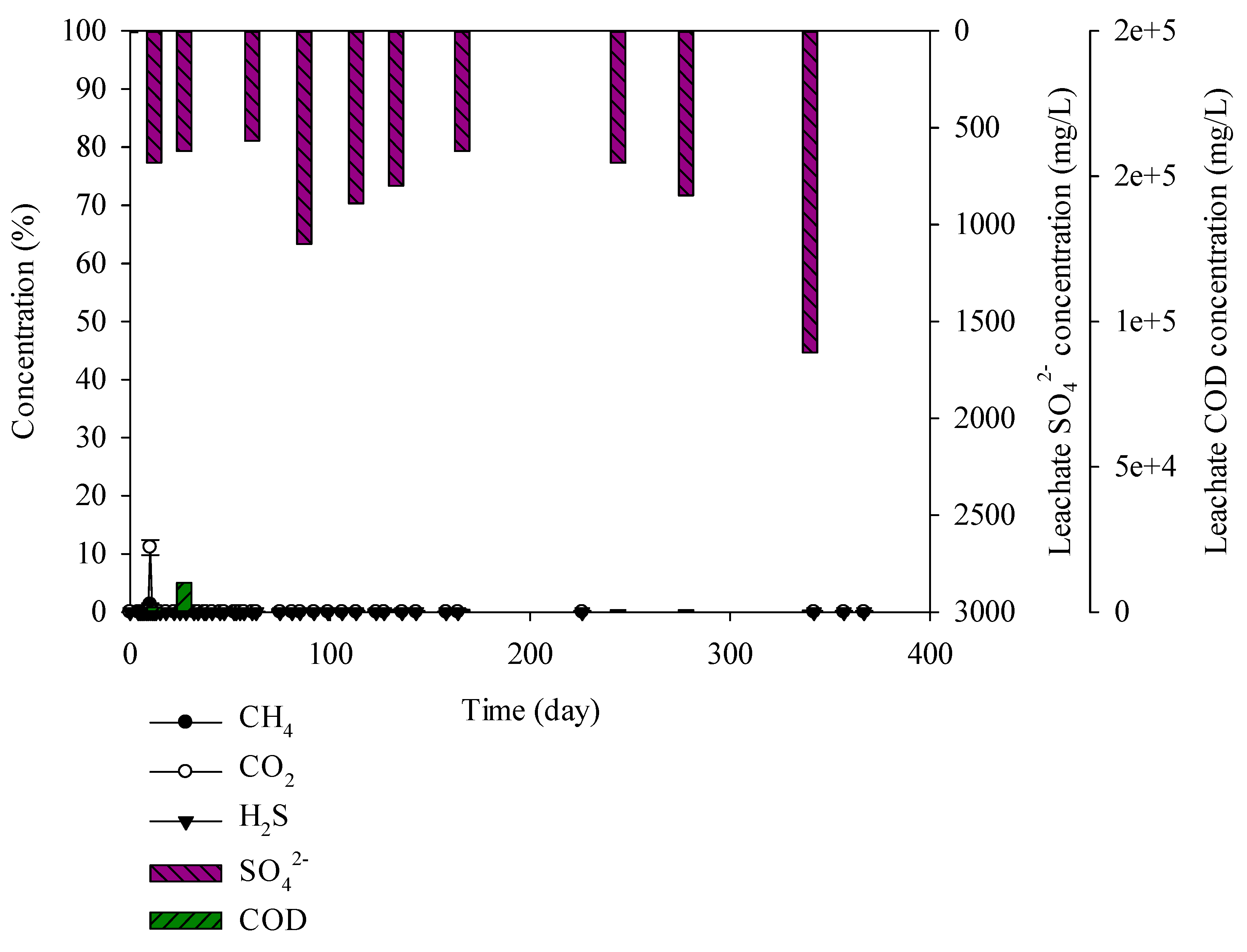
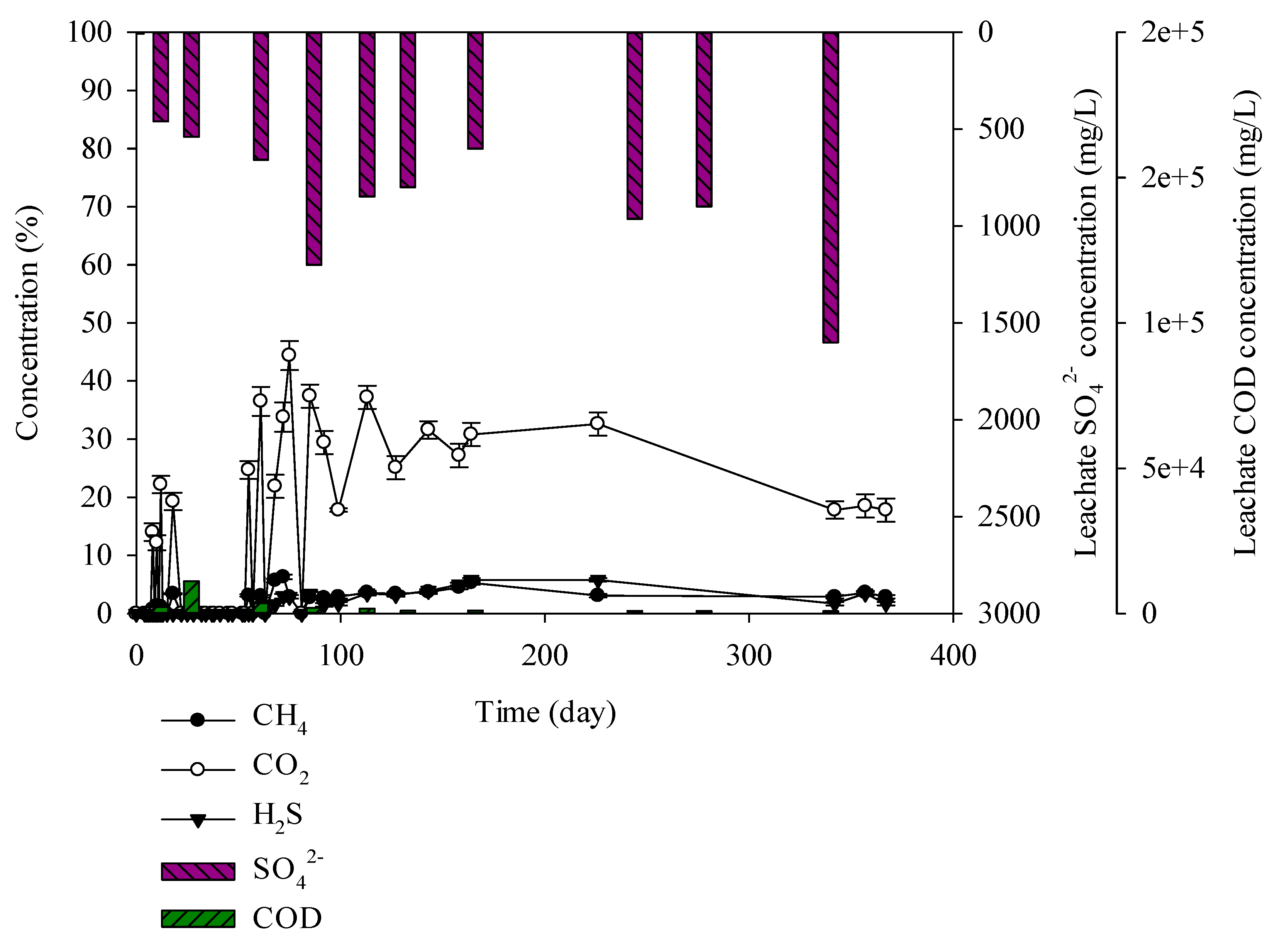
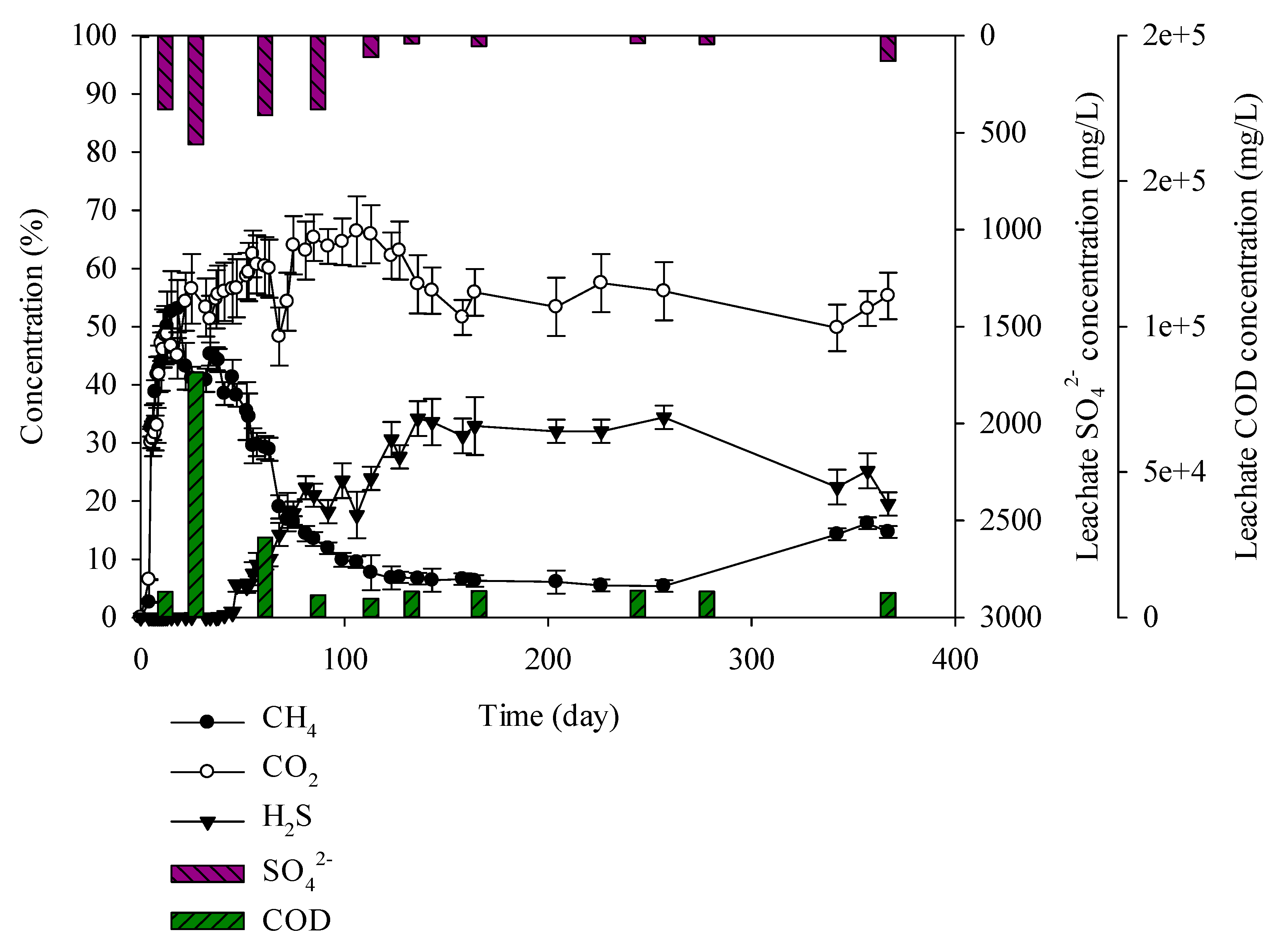
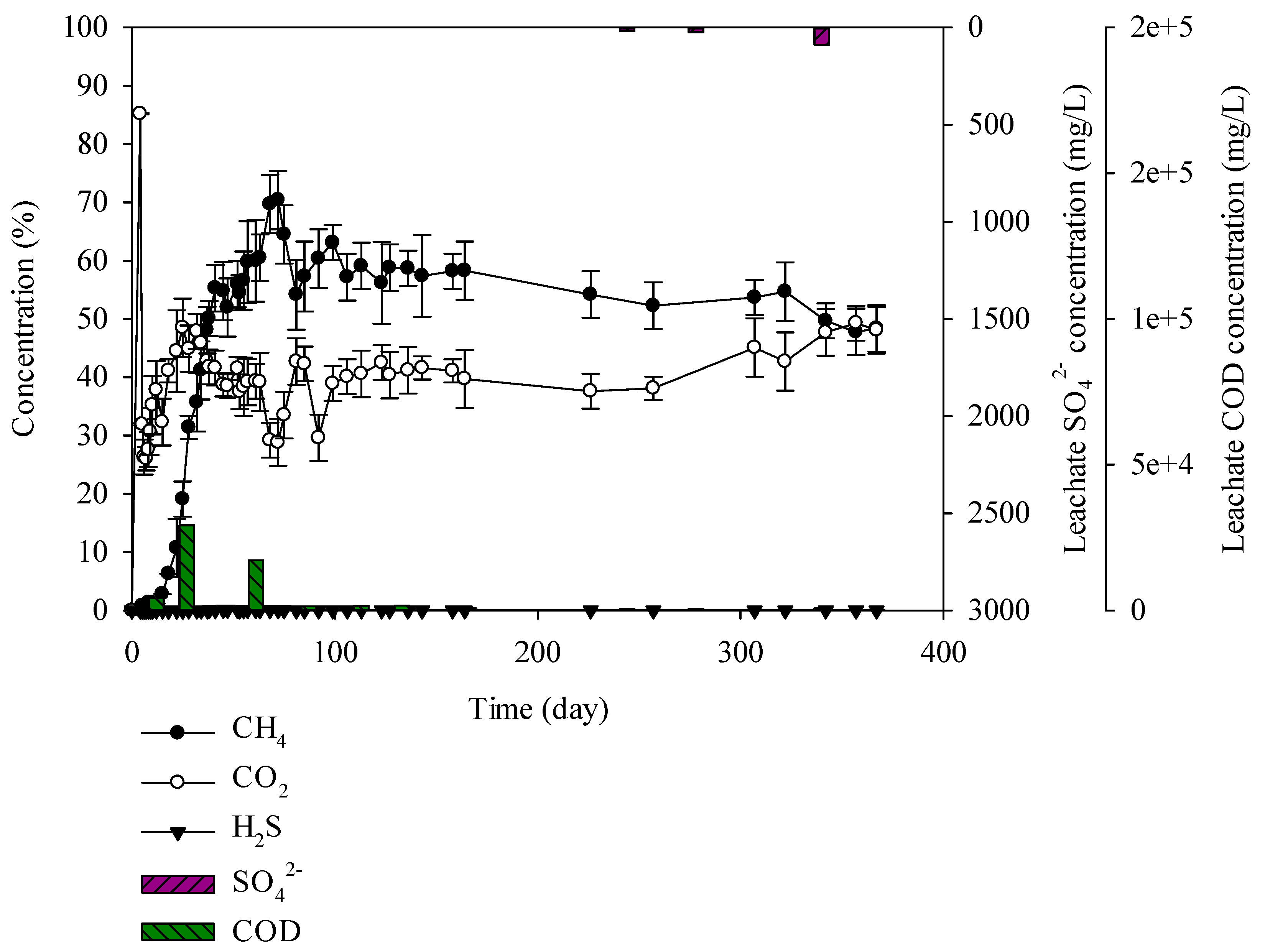
3.3. Characteristics of Gases and Leachate Generated from the Lysimeter Reactor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Department for Environment Food and Rural Affairs (DEFRA). Waste Prevention Programme for England: Overview of Evidence—A Rationale for Waste Prevention in England; Defra: London, UK, 2013.
- Yu, R.; Shui, Z. Influence of agglomeration of a recycled cement additive on the hydration and microstructure development of cement based materials. Constr. Build. Mater. 2013, 49, 841–851. [Google Scholar] [CrossRef]
- Lu, W.; Tam, V.W.Y. Construction waste management policies and their effectiveness in Hong Kong: A longitudinal review. Renew. Sustain. Energy Rev. 2013, 23, 214–223. [Google Scholar] [CrossRef]
- Oyedele, L.O.; Ajayi, S.O.; Kadiri, K.O. Use of recycled products in UK construction industry: An empirical investigation into critical impediments and strategies for improvement. Res. Conserv. Recycl. 2014, 93, 23–31. [Google Scholar] [CrossRef]
- Solis Guzman, J.; Marrero, M.; Montes Delgado, M.V.; Ramirez de Arellano, A. A Spanish model for quantification and management of construction waste. Waste Manag. 2009, 29, 2542–2548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sung, J.H.; Jeon, C.S.; Lee, S.H.; Kim, H.S. A study on the properties of Recycled aggregate concrete and its production facilities. Appl. Sci. 2019, 9, 1935. [Google Scholar] [CrossRef]
- Sudokwon Landfill Management Corporation. Sudokwon Landfill Statistical Yearbook; Sudokwon Landfill Management Corporation: Seo-gu, Korea, 2019; p. 17. [Google Scholar]
- Plaza, C.; Xu, Q.; Townsend, T.; Bitton, G.; Booth, M. Evaluation of alternative landfill cover soils for attenuating hydrogen sulfide from construction and demolition (C&D) debris landfills. J. Environ. Manag. 2007, 84, 314–322. [Google Scholar]
- Rabus, R.; Hansen, T.A.; Widdel, F. Dissimilatory Sulfate-and Sulfur-Reducing Prokaryotes. Prokaryotes Prokaryot. Physiol. Biochem. 2013, 309–404. [Google Scholar]
- Townsend, T.G.; Jang, Y.; Thurn, L.G. Simulation of construction and demolition waste leachate. J. Environ. Eng. 1999, 125, 1071–1081. [Google Scholar] [CrossRef]
- Einsiedl, F.; Maloszewski, P.; Stichler, W. Multiple isotope approach to the determination of the natural attenuation potential of a high-alpine karst system. J. Hydrol. 2009, 365, 113–121. [Google Scholar] [CrossRef]
- Shin, H.S.; Oh, S.E.; Bae, B.U. Competition between SRB and MPB according to temperature change in the anaerobic treatment of tannery wastes containing high sulfate. Environ. Technol. 1996, 17, 361–370. [Google Scholar] [CrossRef]
- Munusamy, P.; Ravichandran, M.; Natarajan, S.D.; Varadhaaraju, C. Biolofical aspects of anaerobic digestion and its kinetics: An overview. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1090–1097. [Google Scholar] [CrossRef]
- Fang, H.H. Microbial distribution in UASB granules and its resulting effects. Water Sci. Technol. 2000, 42, 201–208. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, J.; Jo, J.H. Municipal solid waste landfill performance with different biogas collection practices: Biogas and leachate generations. J. Clean. Prod. 2019, 222, 446–454. [Google Scholar] [CrossRef]
- Fadel, M.E.; Zeid, E.B.; Chahine, W.; Alayli, B. Temporal variation of leachate quality from pre-sorted and baled municipal solid waste with high organic and moisture content. Waste Manag. 2002, 22, 269–282. [Google Scholar] [CrossRef]
- Ko, J.H.; Yang, F.; Xu, Q. The impact of compaction and leachate recirculation on waste degradation in simulated landfills. Bioresour. Technol. 2016, 211, 72–79. [Google Scholar] [CrossRef]
- Lu, X.; Ni, J.; Zhen, G.; Kubota, K.; Li, Y.Y. Response of morphology and microbial community structure of granules to influent COD/SO42– ratios in an upflow anaerobic sludge blanket (UASB) reactor treating starch wastewater. Bioresour. Technol. 2018, 256, 456–465. [Google Scholar] [CrossRef]
- Sun, M.; Sun, W.; Barlaz, M.A. A batch assay to measure microbial hydrogen sulfide production from sulfur-containing solid wastes. Sci. Total Environ. 2016, 551, 23–31. [Google Scholar] [CrossRef]
- Simonton, S.; Spears, M. Human health effects from exposure to low-level concentrations of hydrogen sulfide. J. Occup. Health Saf. 2007, 76, 102–104. [Google Scholar]
- Sudokwon Landfill Management Corporation. Effect of Landfill Evaporation on Landfill Gas Generation; Sudokwon Landfill Management Corporation: Seo-gu, Korea, 2013. [Google Scholar]
- Xu, Q.; Tian, Y.; Wang, S.; Ko, J.H. A comparative study of leachate quality and biogas generation in simulated anaerobic and hybrid bioreactors. Waste Manag. 2015, 41, 94–100. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef]
- Alabi, A.O.; Bakare, A.A. Genetic damage induced by electronic waste leachates and contaminated underground water in two prokaryotic systems. Toxicol. Mech. Methods 2017, 27, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Tetteh, E.K.; Ansah Amano, K.O.; Asante-Sackey, D.; Armah, E.K. Biochemical Methane Potential (BMP) of Miscanthus Fuscus for Anaerobic Digestion. Int. J. Sci. Res. Publ. 2017, 7, 434–439. [Google Scholar]
- Im, S.; Petersen, S.O.; Lee, D.; Kim, D.H. Effects of storage temperature on CH4 emissions from cattle manure and subsequent biogas production potential. Waste Manag. 2019, 101, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sudokwon Landfill Management Corporation. Sudokwon Landfill Statistical Yearbook; Sudokwon Landfill Management Corporation: Seo-gu, Korea, 2012; p. 10. [Google Scholar]
- Zaman, N.Q.; Sukor, N.S.A.; Rahman, S.A.A.; Yaacof, N. The influence of moisture content on the production of odor from food waste using path analysis. Environ. Sci. Pollut. Res. 2019, 26, 13658–13663. [Google Scholar] [CrossRef] [PubMed]
- Townsend, T.G.; Jang, Y.; Lee, S. Characterization of Recovered Screened Material from C&D Recycling Facilities in Florida; Center for Solid and Hazardous waste Management Report No. 1998 98-13; University of Florida: Gainesville, FL, USA, 1998. [Google Scholar]
- Yong, C.J.; Townsend, T. Sulfate leaching from recovered construction and demolition debris fines. Adv. Environ. Res. 2001, 5, 203–217. [Google Scholar]
- Lopez, A.; Lobo, A. Emissions of C&D refuse in landfills: A European case. Waste Manag. 2014, 34, 1446–1454. [Google Scholar]
- Asakura, H. Sulfate and organic matter concentration in relation to hydrogen sulfide generation at inert solid waste landfill site—Limit value for gypsum. Waste Manag. 2015, 43, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Lay, J.J.; Li, Y.Y.; Noike, T. Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res. 1997, 31, 1518–1524. [Google Scholar] [CrossRef]
- Yang, K.; Xu, Q.; Townsend, T.G.; Chadik, P.; Bitton, G.; Booth, M. Hydrogen Sulfide Generation in Simulated Construction and Demolition Debris Landfills: Impact of Waste Composition. J. Air Waste Manag. Assoc. 2006, 56, 1130–1138. [Google Scholar] [CrossRef]
- Aerts, S.; Canniere, P.D.; Moors, H. Presence of Sulphate Reducing Bacteria Near a Boom Clay-Steel Interface; External Report; The Belgian Nuclear Research Centre: Boeretang, Belgium, 2009; pp. 251–262. [Google Scholar]
- Chen, Y.; Wen, Y.; Zhou, J.; Tang, Z.; Li, L.; Zhou, Q.; Vymazal, J. Effects of cattail biomass on sulfate removal and carbon sources competition in subsurface-flow constructed wetlands treating secondary effluent. Water Res. 2014, 59, 1–10. [Google Scholar] [CrossRef]
- Fairweather, R.; Barlaz, M. Hydrogen Sulfide Production During Decomposition of Landfill Inputs. J. Environ. Eng. 1998, 124, 353–361. [Google Scholar] [CrossRef]
- Swati, M.; Kurgan, J.; Nagendran, R. Bioreactor landfill lysimeter studies on indian urban refuse. In Proceedings of the Tenth International Waste Management and Landfill Symposium, Cagliari, Italy, 3–7 October 2005; pp. 229–237. [Google Scholar]
- Yang, K.J. Hydrogen Sulfide Generation in Simulated Landfill Columns. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2000. [Google Scholar]
- Lay, J.J.; Li, Y.Y.; Noike, T. The influence of pH and ammonia concentration on the methane production in high-solids digestion processes. Water Environ. Res. 1998, 70, 1075–1082. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhu, N. Progress in inhibition mechanisms and process control of intermediates and by-products in sewage sludge anaerobic digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]



| Waste Type | Composition Ratio (a) |
|---|---|
| C&D debris waste (b) | Brick construction waste 12%, Earth and sand construction waste 38%, Combustibles (c) 50% |
| C&D debris waste + sewage sludge | C&D debris waste 72%, Sewage sludge 28% |
| C&D debris waste + solidified sludge | C&D debris waste 79%, Solidified sludge 21% |
| Domestic waste | Food 13%, Paper 52%, Plastic 28%,Wood 1%, Textile 5% |
| Mixed waste | C&D debris waste 55%, Domestic waste 24%, Sewage sludge 21% |
| Brick construction waste | Waste concrete, construction aggregates, and bricks 100% |
| Earth and sand construction waste | Earth and sand construction waste 100% |
| Content | Method |
|---|---|
| CODCr | Potassium dichromate method (Closed Reflux Method) |
| SO42− | Absorbance method |
| Waste Type | Solidified Substances (%) | Three Components | ||
|---|---|---|---|---|
| Moisture (%) | Combustibles (%) | Ash (%) | ||
| C&D debris waste | 91.05 | 8.95 | 43.65 | 47.4 |
| C&D debris waste + sewage sludge | 69.57 | 30.43 | 35.52 | 34.05 |
| C&D debris waste + solidified sludge | 76.15 | 23.85 | 37.25 | 38.90 |
| Domestic waste | 73.14 | 26.86 | 64.25 | 8.89 |
| Mixed waste | 71.52 | 28.48 | 37.25 | 34.27 |
| Brick construction waste | 99.99 | 0.01 | 3.59 | 96.41 |
| Earth and sand construction waste | 84.12 | 15.88 | 13.25 | 70.87 |
| Waste Type | C | H | O | N | S | |
|---|---|---|---|---|---|---|
| C&D debris waste | Brick construction waste | 3.24 | 0.45 | 36.01 | 0.20 | 0.00 |
| Earth and sand construction waste | 7.23 | 1.42 | 42.42 | 0.67 | 5.15 | |
| Combustibles | 47.27 | 5.53 | 38.97 | 2.88 | 0.25 | |
| C&D debris waste | 26.77 | 3.36 | 39.93 | 1.72 | 2.08 | |
| Sludge | Sewage sludge | 32.25 | 4.67 | 23.32 | 5.15 | 0.41 |
| Solidified sludge | 23.93 | 2.27 | 13.59 | 2.35 | 1.41 | |
| Domestic waste | 57.17 | 8.42 | 21.78 | 0.41 | 0.00 | |
| C&D debris waste + sewage sludge | 28.31 | 3.73 | 35.28 | 2.68 | 1.62 | |
| C&D debris waste + solidified sludge | 26.17 | 3.13 | 34.40 | 1.85 | 1.94 | |
| Mixed waste | 35.22 | 4.85 | 32.09 | 2.12 | 1.23 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.; Jung, S.; Chang, S. The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter. Appl. Sci. 2019, 9, 4677. https://doi.org/10.3390/app9214677
Chung W, Jung S, Chang S. The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter. Applied Sciences. 2019; 9(21):4677. https://doi.org/10.3390/app9214677
Chicago/Turabian StyleChung, Woojin, Sukyoung Jung, and Soonwoong Chang. 2019. "The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter" Applied Sciences 9, no. 21: 4677. https://doi.org/10.3390/app9214677
APA StyleChung, W., Jung, S., & Chang, S. (2019). The Influence of Waste Composition on Landfill Gas Generation in a Pilot-Scale Lysimeter. Applied Sciences, 9(21), 4677. https://doi.org/10.3390/app9214677





