Shape Memory Polyurethane and its Composites for Various Applications
Abstract
:1. Introduction
2. Shape Memory Polyurethane in Different Applications
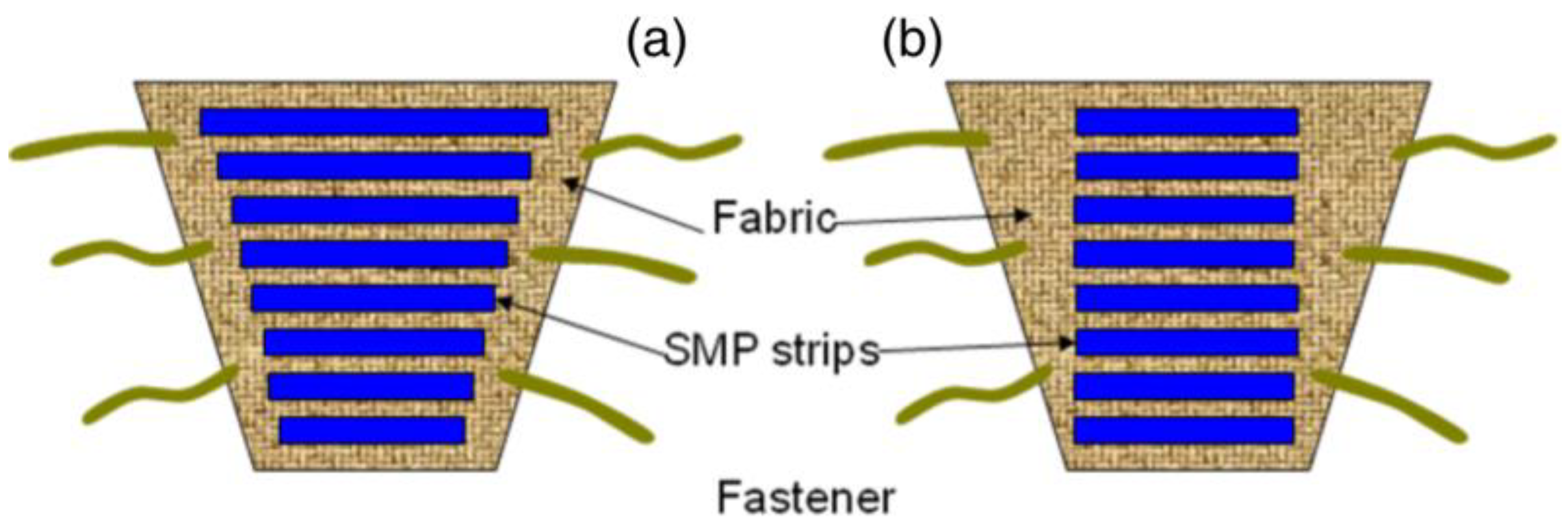
2.1. Electromagnetic Interference Shielding
2.2. Pressure Bandage Application
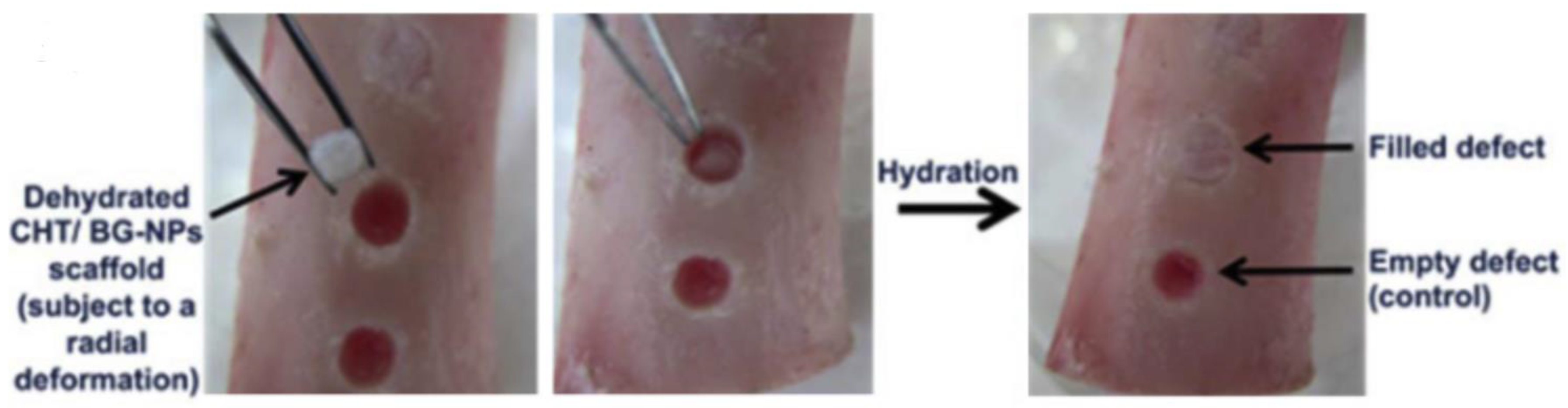
2.3. Bone Tissue Engineering
2.4. Self-Healing
2.5. Cardiovascular Implants
3. Author’s Perspective on the Shape Memory Polyurethane Composites
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543–1558. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, W.; Zhao, W.; Peng, C.; Ren, J.; Yu, Q.; Hu, Y.; Zhang, X. One-way and two-way shape memory effects of a high-strain cis-1,4-polybutadiene–polyethylene copolymer based dynamic network via self-complementary quadruple hydrogen bonding. Polym. Chem. 2019, 10, 718–726. [Google Scholar] [CrossRef]
- Ware, T.; Hearon, K.; Lonnecker, A.; Wooley, K.L.; Maitland, D.J.; Voit, W. Triple-Shape Memory Polymers Based on Self-Complementary Hydrogen Bonding. Macromolecules 2012, 45, 1062–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xian, J.; Geng, J.; Wang, Y.; Xia, L. Quadruple-shape-memory effect of TPI/LDPE/HDPE composites. Polym. Adv. Technol. 2018, 29, 982–988. [Google Scholar] [CrossRef]
- Bothe, M.; Pretsch, T. Bidirectional actuation of a thermoplastic polyurethane elastomer. J. Mater. Chem. A 2013, 1, 14491–14497. [Google Scholar] [CrossRef]
- Goo, N.S.; Paik, I.H.; Yoon, K.J. The durability of a conducting shape memory polyurethane actuator. Smart Mater. Struct. 2007, 16, N23–N26. [Google Scholar] [CrossRef]
- Tobushi, H.; Hayashi, S.; Kojima, S. Mechanical Properties of Shape Memory Polymer of Polyurethane Series: Basic Characteristics of Stress-Strain-Temperature Relationship. Jsme Int. J. Ser. 1 Solid Mech. Strength Mater. 1992, 35, 296–302. [Google Scholar]
- Tobushi, H.; Hara, H.; Yamada, E.; Hayashi, S. Thermomechanical properties in a thin film of shape memory polymer of polyurethane series. Smart Mater. Struct. 1996, 5, 483–491. [Google Scholar] [CrossRef]
- Meng, Q.; Hu, J. A review of shape memory polymer composites and blends. Compos. Part. A Appl. Sci. Manuf. 2009, 40, 1661–1672. [Google Scholar] [CrossRef]
- Lendlein, A.; Gould, O.E.C. Reprogrammable recovery and actuation behaviour of shape-memory polymers. Nat. Rev. Mater. 2019, 4, 116–133. [Google Scholar] [CrossRef]
- Thakur, S.; Hu, J. Polyurethane: A Shape Memory Polymer (SMP). In Aspects of Polyurethanes; Faris, Y., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [Green Version]
- McCaig, C.D.; Song, B.; Rajnicek, A.M. Electrical dimensions in cell science. J. Cell Sci. 2009, 122, 4267–4276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oschman, J.L. Chapter 16-The Electromagnetic Environment. In Energy Medicine (Second Edition); Oschman, J.L., Ed.; Churchill Livingstone: Edinburgh, UK, 2016; pp. 269–295. [Google Scholar]
- Taki, M.; Watanabe, S. Biological and health effects of exposure to electromagnetic field from mobile communications systems. Iatss Res. 2001, 25, 40–50. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Huang, X.; Liao, X.; Shi, B. Absorption and Reflection Contributions to the High Performance of Electromagnetic Waves Shielding Materials Fabricated by Compositing Leather Matrix with Metal Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 14036–14044. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Yin, X.; Zhang, Y.; Yuan, X.; Li, Q.; Ye, F.; Cheng, L.; Zhang, L. Electromagnetic Wave Absorption Properties of Reduced Graphene Oxide Modified by Maghemite Colloidal Nanoparticle Clusters. J. Phys. Chem. C 2013, 117, 19701–19711. [Google Scholar] [CrossRef]
- Mishra, R. Specific functional properties of 3D woven glass nanocomposites. J. Compos. Mater. 2014, 48, 1745–1754. [Google Scholar] [CrossRef]
- Jin, X.; Ni, Q.-Q.; Natsuki, T. Composites of multi-walled carbon nanotubes and shape memory polyurethane for electromagnetic interference shielding. J. Compos. Mater. 2011, 45, 2547–2554. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Menon, A.V.; Madras, G.; Bose, S. Shape memory polyurethane nanocomposites with porous architectures for enhanced microwave shielding. Chem. Eng. J. 2018, 352, 590–600. [Google Scholar] [CrossRef]
- Lamberti, P.; Kuzhir, P.; Tucci, V. A robust approach to the design of an electromagnetic shield based on pyrolitic carbon. Aip Adv. 2016, 6, 075301. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Xia, H.; Qiu, Y.; Xu, Z.; Ni, Q.-Q. Shape memory driving thickness-adjustable G@SMPU sponge with ultrahigh carbon loading ratio for excellent microwave shielding performance. Mater. Lett. 2019, 236, 116–119. [Google Scholar] [CrossRef]
- Suarato, G.; Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Koch, M.; Krieger, A.; Brachvogel, B.; Kreft, S. Differential Proteomic Analysis Distinguishes Tissue Repair Biomarker Signatures in Wound Exudates Obtained from Normal Healing and Chronic Wounds. J. Proteome Res. 2010, 9, 4758–4766. [Google Scholar] [CrossRef] [PubMed]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013, 9. [Google Scholar] [CrossRef]
- De La Brassinne, M.; Thirion, L.; Horvat, L.-I. A novel method of comparing the healing properties of two hydrogels in chronic leg ulcers. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 131–135. [Google Scholar] [CrossRef]
- O’Meara, S.; Cullum, N.; Nelson, E.A.; Dumville, J.C. Compression for venous leg ulcers. Cochrane Database Syst. Rev. 2012, 11, CD000265. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers Cns 2014, 11, 26. [Google Scholar] [CrossRef]
- Hettrick, H. The science of compression therapy for chronic venous insufficiency edema. J. Am. Coll. Certif. Wound Spec. 2009, 1, 20–24. [Google Scholar] [CrossRef]
- Ahmad, M.; Luo, J.; Miraftab, M. Feasibility study of polyurethane shape-memory polymer actuators for pressure bandage application. Sci. Technol. Adv. Mater. 2012, 13, 015006. [Google Scholar] [CrossRef]
- Sáenz-Pérez, M.; Bashir, T.; Laza, J.M.; García-Barrasa, J.; Vilas, J.L.; Skrifvars, M.; León, L.M. Novel shape-memory polyurethane fibers for textile applications. Text. Res. J. 2019, 89, 1027–1037. [Google Scholar] [CrossRef]
- Jahid, M.A.; Hu, J.; Wong, K.; Wu, Y.; Zhu, Y.; Sheng Luo, H.H.; Zhongmin, D. Fabric Coated with Shape Memory Polyurethane and Its Properties. Polymers 2018, 10, 681. [Google Scholar] [CrossRef]
- Liu, Y.; Chung, A.; Hu, J.; Lv, J. Shape memory behavior of SMPU knitted fabric. J. Zhejiang Univ.-Sci. A 2007, 8, 830–834. [Google Scholar] [CrossRef]
- Narayana, H.; Hu, J.; Kumar, B.; Shang, S.; Ying, M.; Young, R.J. Designing of advanced smart medical stocking using stress-memory polymeric filaments for pressure control and massaging. Mater. Sci. Eng. C 2018, 91, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Wnek, G.E.; Carr, M.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Nanofiber Fibrinogen Structures. Nano Lett. 2003, 3, 213–216. [Google Scholar] [CrossRef]
- Guerado, E.; Caso, E. Challenges of bone tissue engineering in orthopaedic patients. World J. Orthop. 2017, 8, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Mulchandani, N.; Gupta, A.; Katiyar, V. Polylactic Acid Based Hydrogels and Its Renewable Characters: Tissue Engineering Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–24. [Google Scholar]
- Cortizo, M.S.; Belluzo, M.S. Biodegradable Polymers for Bone Tissue Engineering. In Industrial Applications of Renewable Biomass Products: Past, Present and Future; Goyanes, S.N., D’Accorso, N.B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 47–74. [Google Scholar]
- Kashirina, A.; Yao, Y.; Liu, Y.; Leng, J. Biopolymers as bone substitutes: A review. Biomater. Sci. 2019, 7, 3961–3983. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Zhao, X.; Xie, R.; Qin, T.; Ji, F. Mechanically Robust Shape Memory Polyurethane Nanocomposites for Minimally Invasive Bone Repair. ACS Appl. Bio Mater. 2019, 2, 1056–1065. [Google Scholar] [CrossRef]
- Correia, C.O.; Mano, J.F. Chitosan scaffolds with a shape memory effect induced by hydration. J. Mater. Chem. B 2014, 2, 3315–3323. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.O.; Leite, Á.J.; Mano, J.F. Chitosan/bioactive glass nanoparticles scaffolds with shape memory properties. Carbohydr. Polym. 2015, 123, 39–45. [Google Scholar] [CrossRef]
- Leite, Á.J.; Caridade, S.G.; Mano, J.F. Synthesis and characterization of bioactive biodegradable chitosan composite spheres with shape memory capability. J. Non-Cryst. Solids 2016, 432, 158–166. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Jeng, U.S.; Hsu, S.-h. Biodegradable Water-Based Polyurethane Shape Memory Elastomers for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 1397–1406. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, B.; Cao, M.; Sun, J.; Wu, H.; Zhao, P.; Xing, J.; Yang, Y.; Zhang, X.; Ji, M.; et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 2016, 86, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Et Biophys. Acta (Bba)-Gen. Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Villa, M.M.; Wang, L.; Huang, J.; Rowe, D.W.; Wei, M. Bone tissue engineering with a collagen–hydroxyapatite scaffold and culture expanded bone marrow stromal cells. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2015, 103, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef]
- Zhang, P.; Li, G. Advances in healing-on-demand polymers and polymer composites. Progress Polym. Sci. 2016, 57, 32–63. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.C.; Bartelt, J.A.; Braun, P.V. Transparent Self-Healing Polymers Based on Encapsulated Plasticizers in a Thermoplastic Matrix. Adv. Funct. Mater. 2011, 21, 4705–4711. [Google Scholar] [CrossRef]
- Nakahata, M.; Takashima, Y.; Yamaguchi, H.; Harada, A. Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2011, 2, 511. [Google Scholar] [CrossRef]
- Takashima, Y.; Harada, A. Self-Healing Polymers. In Encyclopedia of Polymeric Nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 2209–2214. [Google Scholar]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682–9695. [Google Scholar] [CrossRef] [PubMed]
- Aïssa, B.; Therriault, D.; Haddad, E.; Jamroz, W. Self-Healing Materials Systems: Overview of Major Approaches and Recent Developed Technologies. Adv. Mater. Sci. Eng. 2012, 2012, 17. [Google Scholar] [CrossRef]
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49, 175–220. [Google Scholar] [CrossRef]
- Fan, W.; Li, W.; Zhang, Y.; Wang, W.; Zhang, X.; Song, L.; Liu, X. Cooperative self-healing performance of shape memory polyurethane and Alodine-containing microcapsules. RSC Adv. 2017, 7, 46778–46787. [Google Scholar] [CrossRef] [Green Version]
- Yan, P.; Zhao, W.; Fu, X.; Liu, Z.; Kong, W.; Zhou, C.; Lei, J. Multifunctional polyurethane-vitrimers completely based on transcarbamoylation of carbamates: Thermally-induced dual-shape memory effect and self-welding. RSC Adv. 2017, 7, 26858–26866. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D. Shape memory-assisted self-healing polyurethane inspired by a suture technique. J. Mater. Sci. 2018, 53, 10582–10592. [Google Scholar] [CrossRef]
- Wen, H.; Chen, S.; Ge, Z.; Zhuo, H.; Ling, J.; Liu, Q. Development of humidity-responsive self-healing zwitterionic polyurethanes for renewable shape memory applications. RSC Adv. 2017, 7, 31525–31534. [Google Scholar] [CrossRef] [Green Version]
- González-García, Y.; Mol, J.M.C.; Muselle, T.; De Graeve, I.; Van Assche, G.; Scheltjens, G.; Van Mele, B.; Terryn, H. A combined mechanical, microscopic and local electrochemical evaluation of self-healing properties of shape-memory polyurethane coatings. Electrochim. Acta 2011, 56, 9619–9626. [Google Scholar] [CrossRef]
- Ghosh, T.; Karak, N. Tough interpenetrating polymer network of silicone containing polyurethane and polystyrene with self-healing, shape memory and self-cleaning attributes. Rsc Adv. 2018, 8, 17044–17055. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.F.; Rong, M.Z.; Zhang, M.Q.; Chen, X.D. Repeated Intrinsic Self-Healing of Wider Cracks in Polymer via Dynamic Reversible Covalent Bonding Molecularly Combined with a Two-Way Shape Memory Effect. Acs Appl. Mater. Interfaces 2018, 10, 38538–38546. [Google Scholar] [CrossRef] [Green Version]
- Ban, J.; Zhu, L.; Chen, S.; Wang, Y. The Effect of 4-Octyldecyloxybenzoic Acid on Liquid-Crystalline Polyurethane Composites with Triple-Shape Memory and Self-Healing Properties. Materials 2016, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mo, F.; Yang, Y.; Stadler, F.J.; Chen, S.; Yang, H.; Ge, Z. Development of zwitterionic polyurethanes with multi-shape memory effects and self-healing properties. J. Mater. Chem. A 2015, 3, 2924–2933. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Li, Y.; Sun, J.; Pan, X.; Yu, Q.; Zhou, N.; Zhang, Z.; Zhu, X. Shape-memory and self-healing polyurethanes based on cyclic poly (ε-caprolactone). Polym. Chem. 2016, 7, 6789–6797. [Google Scholar] [CrossRef]
- Deng, X.-Y.; Xie, H.; Du, L.; Fan, C.-J.; Cheng, C.-Y.; Yang, K.-K.; Wang, Y.-Z. Polyurethane networks based on disulfide bonds: From tunable multi-shape memory effects to simultaneous self-healing. Sci. China Mater. 2019, 62, 437. [Google Scholar] [CrossRef]
- Du, W.; Jin, Y.; Lai, S.; Shi, L.; Fan, W.; Pan, J. Near-infrared light triggered shape memory and self-healable polyurethane/functionalized graphene oxide composites containing diselenide bonds. Polymer 2018, 158, 120–129. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D. Self-healing thermoplastic polyurethane (TPU)/polycaprolactone (PCL) /multi-wall carbon nanotubes (MWCNTs) blend as shape-memory composites. Compos. Sci. Technol. 2018, 168, 255–262. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-Like Malleable Materials from Permanent Organic Networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.e.; Rong, M.Z.; Zhang, M.Q.; Zhang, Z.P.; Yuan, Y.C. Self-Healing of Polymers via Synchronous Covalent Bond Fission/Radical Recombination. Chem. Mater. 2011, 23, 5076–5081. [Google Scholar] [CrossRef]
- Blackman, L.D.; Gunatillake, P.A.; Cass, P.; Locock, K.E.S. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem. Soc. Rev. 2019, 48, 757–770. [Google Scholar] [CrossRef]
- Zheng, L.; Sundaram, H.S.; Wei, Z.; Li, C.; Yuan, Z. Applications of zwitterionic polymers. React. Funct. Polym. 2017, 118, 51–61. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Synthesis and Solution Properties of Zwitterionic Polymers. Chem. Rev. 2002, 102, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Boccafoschi, F.; Fusaro, L.; Cannas, M. 15-Immobilization of peptides on cardiovascular stent. In Functionalised Cardiovascular Stents; Wall, J.G., Podbielska, H., Wawrzyńska, M., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2018; pp. 305–318. [Google Scholar]
- Weems, A.C.; Boyle, A.J.; Maitland, D.J. Two-year performance study of porous, thermoset, shape memory polyurethanes intended for vascular medical devices. Smart Mater. Struct. 2017, 26, 035054. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.C.; Wacker, K.T.; Carrow, J.K.; Boyle, A.J.; Maitland, D.J. Shape memory polyurethanes with oxidation-induced degradation: In vivo and in vitro correlations for endovascular material applications. Acta Biomaterialia 2017, 59, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Wong, C.S.; Wang, X. Medical Textiles as Vascular Implants and Their Success to Mimic Natural Arteries. J. Funct. Biomater. 2015, 6, 500–525. [Google Scholar] [CrossRef] [Green Version]
- Bussooa, A.; Neale, S.; Mercer, J.R. Future of Smart Cardiovascular Implants. Sensors 2018, 18, 2008. [Google Scholar] [CrossRef]
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-expanding nitinol stents: Material and design considerations. Eur. Radiol. 2004, 14, 292–301. [Google Scholar] [CrossRef]
- Cui, C. 8-Biocompatibility and fabrication of in situ bioceramic coating/titanium alloy biocomposites. In Metals for Biomedical Devices; Niinomi, M., Ed.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2010; pp. 202–232. [Google Scholar]
- Zheng, Y.; Dong, R.; Shen, J.; Guo, S. Tunable Shape Memory Performances via Multilayer Assembly of Thermoplastic Polyurethane and Polycaprolactone. ACS Appl. Mater. Interfaces 2016, 8, 1371–1380. [Google Scholar] [CrossRef]
- Wache, H.M.; Tartakowska, D.J. Development of a polymer stent with shape memory effect as a drug delivery system. J. Mater. Sci. Mater. Med. 2003, 14, 109–112. [Google Scholar] [CrossRef]
- Ahmad Zubir, S.; Mat Saad, N.; Harun, F.W.; Ali, E.S.; Ahmad, S. Incorporation of palm oil polyol in shape memory polyurethane: Implication for development of cardiovascular stent. Polym. Adv. Technol. 2018, 29, 2926–2935. [Google Scholar] [CrossRef]
- Gu, S.-Y.; Chang, K.; Jin, S.-P. A dual-induced self-expandable stent based on biodegradable shape memory polyurethane nanocomposites (PCLAU/Fe3O4) triggered around body temperature. J. Appl. Polym. Sci. 2018, 135, 45686. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Wang, Y. Fluorinated waterborne shape memory polyurethane urea for potential medical implant application. J. Appl. Polym. Sci. 2013, 127, 710–716. [Google Scholar] [CrossRef]
- Baer, G.M.; Wilson, T.S.; Small, W.t.; Hartman, J.; Benett, W.J.; Matthews, D.L.; Maitland, D.J. Thermomechanical properties, collapse pressure, and expansion of shape memory polymer neurovascular stent prototypes. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 90, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.; Hasirci, N. Polyurethanes in Biomedical Applications. In Biomaterials; Springer: Boston, MA, USA, 2004. [Google Scholar]
- Ajili, S.H.; Ebrahimi, N.G.; Soleimani, M. Polyurethane/polycaprolactane blend with shape memory effect as a proposed material for cardiovascular implants. Acta Biomater. 2009, 5, 1519–1530. [Google Scholar] [CrossRef]
- Kim, T.; Lee, Y.-G. Shape transformable bifurcated stents. Sci. Rep. 2018, 8, 13911. [Google Scholar] [CrossRef]
- Kuribayashi, K.; Tsuchiya, K.; You, Z.; Tomus, D.; Umemoto, M.; Ito, T.; Sasaki, M. Self-deployable origami stent grafts as a biomedical application of Ni-rich TiNi shape memory alloy foil. Mater. Sci. Eng. A 2006, 419, 131–137. [Google Scholar] [CrossRef]
- Shyu, T.C.; Damasceno, P.F.; Dodd, P.M.; Lamoureux, A.; Xu, L.; Shlian, M.; Shtein, M.; Glotzer, S.C.; Kotov, N.A. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat. Mater. 2015, 14, 785. [Google Scholar] [CrossRef]
- Neville, R.M.; Scarpa, F.; Pirrera, A. Shape morphing Kirigami mechanical metamaterials. Sci. Rep. 2016, 6, 31067. [Google Scholar] [CrossRef] [Green Version]
- Chalissery, D.; Pretsch, T.; Staub, S.; Andrä, H. Additive Manufacturing of Information Carriers Based on Shape Memory Polyester Urethane. Polymers 2019, 11, 1005. [Google Scholar] [CrossRef]
- Raasch, J.; Ivey, M.; Aldrich, D.; Nobes, D.S.; Ayranci, C. Characterization of polyurethane shape memory polymer processed by material extrusion additive manufacturing. Addit. Manuf. 2015, 8, 132–141. [Google Scholar] [CrossRef]
- Pretsch, T.; Ecker, M.; Schildhauer, M.; Maskos, M. Switchable information carriers based on shape memory polymer. J. Mater. Chem. 2012, 22, 7757–7766. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Leng, J. Programmable and Shape-Memorizing Information Carriers. Acs Appl. Mater. Interfaces 2017, 9, 44792–44798. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Leow, W.R.; Wang, T.; Wang, J.; Yu, J.; He, K.; Qi, D.; Wan, C.; Chen, X. 3D Printed Photoresponsive Devices Based on Shape Memory Composites. Adv. Mater. 2017, 29, 1701627. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Xu, M.; Ye, G.; Guo, R.; Cai, L.; Ren, Z. Mechanical, Thermal, and Shape Memory Properties of Three-Dimensional Printing Biomass Composites. Polymers 2018, 10, 1234. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Wei, Y.; Li, Y. 3D printing of shape memory polymer for functional part fabrication. Int. J. Adv. Manuf. Technol. 2016, 84, 2079–2095. [Google Scholar] [CrossRef]
- Villacres, J. Additive manufacturing of shape memory polymers: Effects of print orientation and infill percentage on mechanical properties. Rapid Prototyp. J. 2018, 24, 744–751. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.-Y.; Zheng, M.; Moorlag, C.; Yang, J.; Wang, Z.L. 3D printing of thermoreversible polyurethanes with targeted shape memory and precise in situ self-healing properties. J. Mater. Chem. A 2019, 7, 6972–6984. [Google Scholar] [CrossRef]
- Gupta, A.; Kim, B.S. Shape Memory Polyurethane Biocomposites Based on Toughened Polycaprolactone Promoted by Nano-Chitosan. Nanomaterials 2019, 9, 225. [Google Scholar] [CrossRef]
















| Material | Mechanical Properties | Transition Temperatures | Self-Healing Properties | Shape Memory | Ref | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deformation Parameters | UTS | E (%) | Toughness | Impact Resistance | Thermal Stability | Tg | Tm | Parameters | Self-Healing Efficiency | Recovery Ratio | Fixing Ratio | ||
| PUVs | stretch under 0.1 MPa load | 7.3 MPa | 833.4 | — | — | 300 °C | -- | 40 °C | 80 °C, heating rate 30 °C min−1 | -- | 100% | 96% | [60] |
| PCL diol | scratched using razor blade | -- | -- | — | — | — | — | 50 ~ 60 °C | 80 °C, 1 h (oven) | 97% | 94.6% | 95% | [61] |
| SHZPU | scratch by glass slide | — | — | — | — | 200 °C | 23.5 °C | — | heating at 80 °C | 97% | 88.2% | ~100% | [62] |
| SMPU | scratched with a razor blade | — | — | — | — | -- | -- | 70 °C | heating at 80 °C for 24 h | — | -- | -- | [63] |
| IPNs | heated at 70 °C | 12.6 MPa | 1608% | 92.34 MJ m−3 | 26.8 kJ m−1 | 245 °C | 44 to 44.1 °C | 29.2 °C | 62 s at 450 W microwave 6–8 min under sunlight | – | 100% | 98% | [64] |
| PU/SBS/MWCNTs | stretched to a strain of 500% at 60 °C for 5 min | — | — | — | — | — | — | 44.1 °C | 100 °C for 24 h | 81.4% | –– | –– | [65] |
| SMPU-OOBAm | elongated at 100 °C | — | — | — | — | — | — | 77 °C | 40 min at 80 °C and 100 °C | 90% at 100 °C for approximately 20 min | 90% | 98% | [66] |
| ZSMPU | 100% strain heated to 60 °C | — | 200 | — | — | — | 26.6 to 48.7 °C | — | moisture-rich conditions (30 °C and 80% RH and drying at 50 °C for 2 h | –– | 97.50% | 98.05% | [67] |
| c-PCL-2OH | surgical blade and put in an oven | 16 MPa | 135 | — | — | 48 h at 60 °C | — | 55 °C | 130°C for 4 h, followed by being kept at 60 °C for 48 h | –– | 60% | ~99.5% | [68] |
| PEUR-SSx-Ns | scraped by a fresh razor blade | 15.5 MPa | 1864 | — | — | — | 25 to 75 °C | 23.2 °C | heated to 55 °C about 12 h | 94% at 55 °C for 12 h | 97.4% | 99.9% | [69] |
| PIB-FGOs | surgical blade | 9.1 MPa | 125.8 | — | — | — | 53.3–62.2 °C | 17.9–19.8 °C | exposed to near infrared light lamp wavelength of 808 nm at a distance of ~20 cm for 10 min | 40–60% | 87.22–95.06% | 83.75–91.78% | [70] |
| SMPU-TDI | stretched into a length as long as possible at a sufficiently high temperature (70 °C) for 1 min with a hair dryer | — | — | — | — | — | — | — | heated at 75 °C for 2 h | –– | 79.6% | –– | [59] |
| TPU/PCL/MWCNTs | under the external forces | — | — | — | — | — | ~60 °C | ~60 °C | — | 96% | 63.9% | 96.8% | [71] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Maharjan, A.; Kim, B.S. Shape Memory Polyurethane and its Composites for Various Applications. Appl. Sci. 2019, 9, 4694. https://doi.org/10.3390/app9214694
Gupta A, Maharjan A, Kim BS. Shape Memory Polyurethane and its Composites for Various Applications. Applied Sciences. 2019; 9(21):4694. https://doi.org/10.3390/app9214694
Chicago/Turabian StyleGupta, Arvind, Anoth Maharjan, and Beom Soo Kim. 2019. "Shape Memory Polyurethane and its Composites for Various Applications" Applied Sciences 9, no. 21: 4694. https://doi.org/10.3390/app9214694
APA StyleGupta, A., Maharjan, A., & Kim, B. S. (2019). Shape Memory Polyurethane and its Composites for Various Applications. Applied Sciences, 9(21), 4694. https://doi.org/10.3390/app9214694







