3-Sulphinyl-5-Amino-1H-1,2,4-Triazoles as Inhibitors of Copper Corrosion
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Evaluation of Corrosion Inhibition Efficiency
2.2. Potentiodynamic Polarisation Measurements
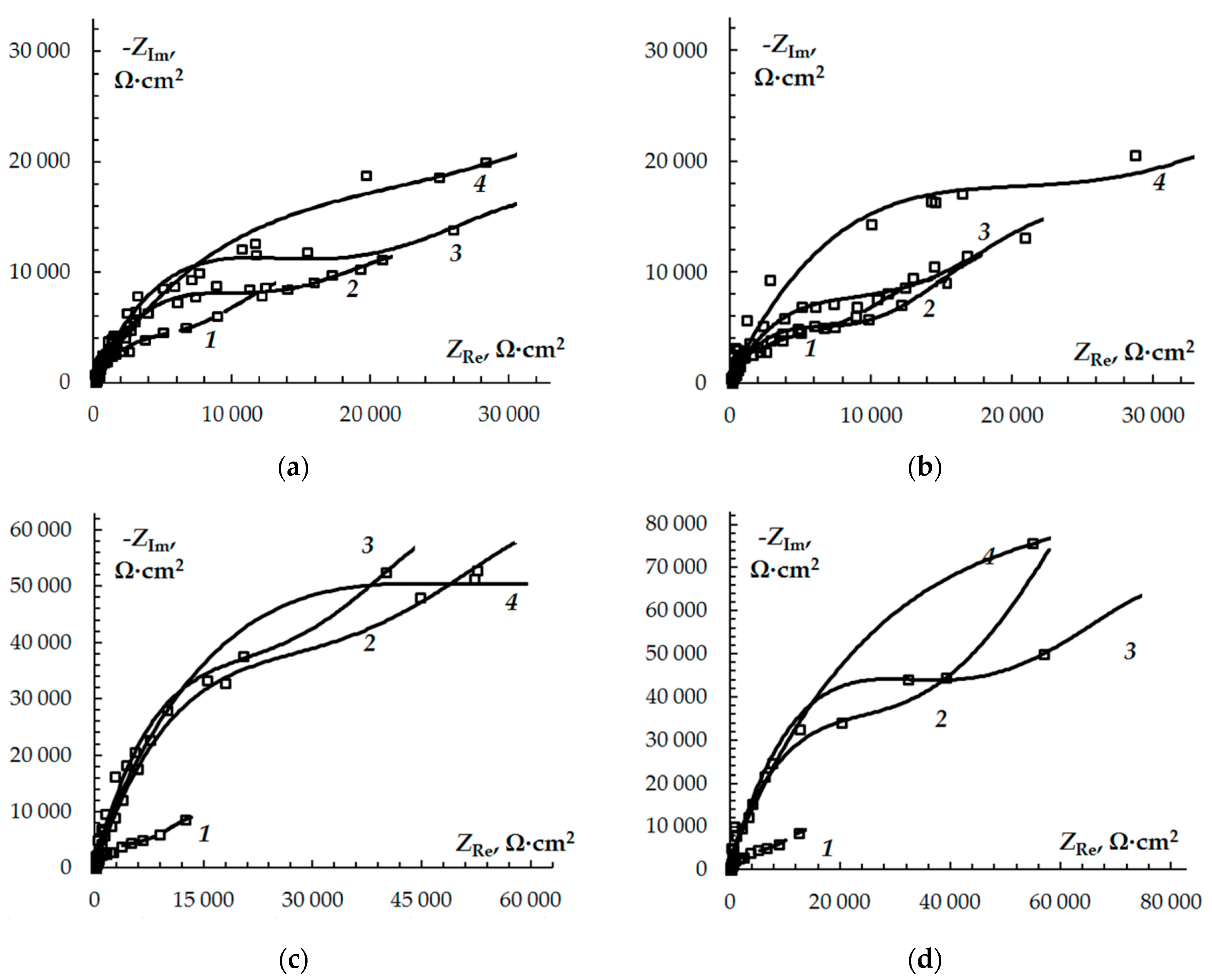
2.3. Electrochemical Impedance Spectroscopy (EIS)
2.4. Accelerated Corrosion Test
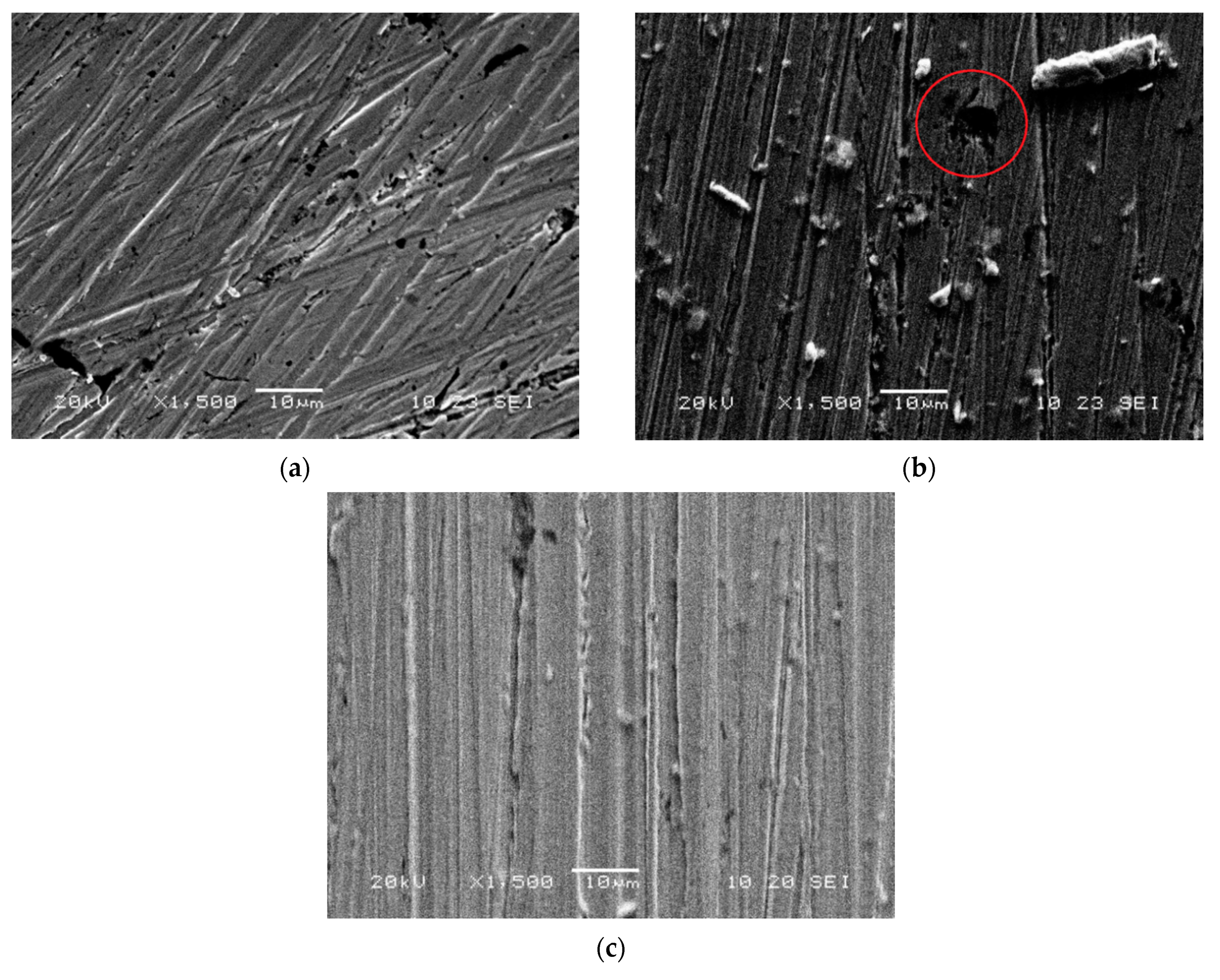
2.5. Scanning Electron Microscopy (SEM)
2.6. Quantum-Chemical Simulation
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sherif, E.S.M. Effects of 2-amino-5-(ethylthio)-1,3,4-thiadiazole on copper corrosion as a corrosion inhibitor in 3% NaCl solutions. Appl. Surf. Sci. 2006, 252, 8615–8623. [Google Scholar] [CrossRef]
- Sherif, E.M.; Park, S.M. 2-Amino-5-ethyl-1,3, 4-thiadiazole as a corrosion inhibitor for copper in 3.0% NaCl solutions. Corros. Sci. 2006, 48, 4065–4079. [Google Scholar] [CrossRef]
- Sherif, E.M.; Erasmus, R.M.; Comins, J.D. Corrosion of copper in aerated synthetic sea water solutions and its inhibition by 3-amino-1,2,4-triazole. J. Colloid Interface Sci. 2007, 309, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Sherif, E.M.; Shamy, A.M.E.; Ramla, M.M.; El Nazhawy, A.O. 5-(Phenyl)-4H-1, 2, 4-triazole-3-thiol as a corrosion inhibitor for copper in 3.5% NaCl solutions. Mater. Chem. Phys. 2007, 102, 231–239. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Al-Kharafi, F.M.; Ateya, B.G. Intergranular corrosion of copper in the presence of benzotriazole. Scr. Mater. 2006, 54, 1673–1677. [Google Scholar] [CrossRef]
- Elmorsi, M.A.; Hassanein, A.M. Corrosion inhibition of copper by heterocyclic compounds. Corros. Sci. 1999, 41, 2337–2352. [Google Scholar] [CrossRef]
- Mihit, M.; Salghi, R.; Issami, S.E.; Bazzi, L.; Hammouti, B.; Addi, E.A.; Kertit, S. A study of tetrazoles derivatives as corrosion inhibitors of copper in nitric acid. Pigment Resin Technol. 2006, 35, 151–157. [Google Scholar] [CrossRef]
- Szőcs, E.; Vastag, G.; Shaban, A.; Kálmán, E. Electrochemical behaviour of an inhibitor film formed on copper surface. Corros. Sci. 2005, 47, 893–908. [Google Scholar] [CrossRef]
- Tromans, D.; Sun, R.H. Anodic polarisation behavior of copper in aqueous chloride/benzotriazole solutions. J. Electrochem. Soc. 1991, 138, 3235–3244. [Google Scholar] [CrossRef]
- Lalitha, A.; Ramesh, S.; Rajeswari, S. Surface protection of copper in acid medium by azoles and surfactants. Electrochim. Acta 2005, 51, 47–55. [Google Scholar] [CrossRef]
- Otmačić, H.; Stupnišek-Lisac, E. Copper corrosion inhibitors in near neutral media. Electrochim. Acta 2003, 48, 985–991. [Google Scholar] [CrossRef]
- Yu, P.; Liao, D.M.; Luo, Y.B.; Chen, Z.G. Studies of benzotriazole and tolytriazole as inhibitors for copper corrosion in deionized water. Corrosion 2003, 59, 314–318. [Google Scholar] [CrossRef]
- Qafsaoui, W.; Blanc, C.; Pebere, N.; Takenouti, H.; Srhiri, A.; Mankowski, G. Quantitative characterization of protective films grown on copper in the presence of different triazole derivative inhibitors. Electrochim. Acta 2002, 47, 4339–4346. [Google Scholar] [CrossRef]
- Lewis, G. The corrosion inhibition of copper by benzimidazole. Corros. Sci. 1982, 22, 579–584. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Thomas, J.G.N.; Mercer, A.D. Organic Inhibitors of Corrosion of Metals; Springer Science & Business Media: New York, NY, USA, 1996; ISBN 978-1-4899-1958-8. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Zhou, G.D. Synergistic effect of 2-mercapto benzimidazole and KI on copper corrosion inhibition in aerated sulfuric acid solution. J. Appl. Electrochem. 2003, 33, 361–366. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Zhou, G.D. Inhibition of copper corrosion in aerated hydrochloric acid solution by heterocyclic compounds containing a mercapto group. Corros. Sci. 2004, 46, 3031–3040. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Gao, L.X.; Zhou, G.D. Inhibition of copper corrosion by bis-(1-benzotriazolymethylene)-(2, 5-thiadiazoly)-disulfide in chloride media. Appl. Surf. Sci. 2004, 225, 287–293. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper Corrosion Inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Blajiev, O.; Hubin, A. Inhibition of copper corrosion in chloride solutions by amino-mercapto-thiadiazole and methyl-mercapto-thiadiazole: An impedance spectroscopy and a quantum chemical investigation. Electrochim. Acta 2004, 49, 2761–2770. [Google Scholar] [CrossRef]
- Issa, I.M.; Issa, R.M.; Temerk, Y.M.; Ghoneim, M.M. Die Reaktionen der Benzoyl- und Salicoylhydrazone des Vanillins, Furfurols und Zimtaldehyds mit zweiwertigen Ionen der Übergangsmetalle. Monatsh. Chem. 1973, 104, 963–972. [Google Scholar] [CrossRef]
- Issa, R.M.; Temerk, Y.M.; Mahmoud, M.R.; Khattab, M.A. Die Chelate von Co (II), Ni (II), Cu (II), Zn (II) und Cd (II) mit Malonsäurehydrazid und dessen Arylidenderivaten. Monatsh. Chem. 1976, 107, 485–493. [Google Scholar] [CrossRef]
- Tan, Y.S.; Srinivasan, M.P.; Pehkonen, S.O.; Chooi, S.Y. Effects of ring substituents on the protective properties of self-assembled benzenethiols on copper. Corros. Sci. 2006, 48, 840–862. [Google Scholar] [CrossRef]
- Singh, M.M.; Rastogi, R.B.; Upadhyay, B.N.; Yadav, M. Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions. Mater. Chem. Phys. 2003, 80, 283–293. [Google Scholar] [CrossRef]
- El-Maksoud, S.A.A. Some phthalazin derivatives as non toxic corrosion inhibitors for copper in sulphuric acid. Electrochim. Acta 2004, 49, 4205–4212. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Agafonkina, M.O.; Shikhaliev, H.S.; Andreeva, N.P.; Potapov, A.Y. Adsorption and passivation of copper by triazoles in neutral aqueous solution. Int. J. Corros. Scale Inhib. 2014, 3, 137–148. [Google Scholar] [CrossRef]
- Sasse, K.; Niedrig, H. Kohlensäure-Derivate von 3-Alkylthio-5-amino-und 3-Alkylsulfonyl-5-amino-1,2,4-triazolen. Angew. Chem. 1981, 93, 835–836. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel slopes and corrosion rates obtained in the pre-Tafel region of polarisation curves. Corros. Sci. 2005, 47, 3178–3186. [Google Scholar] [CrossRef]
- Shih, H.; Mansfeld, F. Software for quantitative analysis of polarisation curves. Comput. Model. Corros. ASTM Int. 1992, 174–183. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Chirkunov, A.A.; Kuznetsov, Y.I.; Shikhaliev, K.S.; Agafonkina, M.O.; Andreeva, N.P.; Kazansky, L.P.; Potapov, A.Y. Adsorption of 5-alkyl-3-amino-1, 2, 4-triazoles from aqueous solutions and protection of copper from atmospheric corrosion. Corros. Sci. 2018, 144, 230–236. [Google Scholar] [CrossRef]
- Kuznetsov, Y.I.; Andreeva, N.P. The designing of nanometer-sized passive films at metal surfaces using organic inhibitors. Russ. J. Electrochem. 2012, 48, 442–449. [Google Scholar] [CrossRef]
- Chira, A.; Bucur, B.; Radu, G.L. Electrodeposited Organic Layers Formed from Aryl Diazonium Salts for Inhibition of Copper Corrosion. Materials 2017, 10, 235. [Google Scholar] [CrossRef] [PubMed]




| Symbol | Name | Formula |
|---|---|---|
| A | 3-sulphinylpropyl-5-amino-1H-1,2,4-triazole | 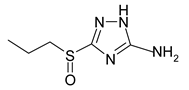 |
| B | 3-sulphinylbutyl-5-amino-1H-1,2,4-triazole | 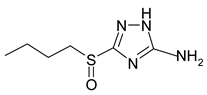 |
| C | 3-sulphinylpentyl-5-amino-1H-1,2,4-triazole |  |
| D | 3-phenethylsulphinyl-5-amino-1H-1,2,4-triazole |  |
| Inhibitor | Cinh, mM | Ecorr, V | Rp, kΩ∙cm2 | icorr μA∙cm−2 | Zi, % |
|---|---|---|---|---|---|
| - | - | 0.172 | 41.9 ± 4.6 | 1.2 ± 0.3 | - |
| A | 0.01 | 0.198 | 20.8 ± 4.8 | 0.7 ± 0.2 | 41.7 |
| 0.10 | 0.140 | 25.9 ± 1.7 | 0.6 ± 0.2 | 50.0 | |
| 1.00 | 0.128 | 107.2 ± 17.7 | 0.30 ± 0.10 | 75.0 | |
| B | 0.01 | 0.162 | 34.8 ± 5.3 | 0.9 ± 0.3 | 25.0 |
| 0.10 | 0.226 | 62.6 ± 8.4 | 0.31 ± 0.10 | 74.2 | |
| 1.00 | 0.295 | 115.8 ± 18.3 | 0.20 ± 0.10 | 83.3 | |
| C | 0.01 | 0.174 | 42.4 ± 7.3 | 0.89 ± 0.11 | 25.8 |
| 0.10 | 0.179 | 140 ± 19 | 0.24 ± 0.08 | 80.0 | |
| 1.00 | 0.160 | 172 ± 13 | 0.15 ± 0.02 | 57.5 | |
| D | 0.01 | 0.159 | 223 ± 33 | 0.28 ± 0.08 | 76.6 |
| 0.10 | 0.136 | 208 ± 23 | 0.10 ± 0.02 | 91.7 | |
| 1.00 | 0.210 | 195 ± 17 | 0.14 ± 0.04 | 88.3 |
| Inhibitor | Cinh, mM | RΩ, Ω∙cm2 | CPE | RpEIS, kΩ∙cm2 | BW, kΩ∙cm2∙s−0,5 | Degree of protection, ηinh,% | |
|---|---|---|---|---|---|---|---|
| Y0, Ω−1∙cm−2∙sn | n | ||||||
| - | 0.00 | 214 | 6.39 × 10−6 | 0.86 | 10.06 | 12.43 | − |
| A | 0.01 | 195 | 5.86 × 10−6 | 0.86 | 24.07 | 11.80 | 58.2 |
| 0.10 | 200 | 3.71 × 10−6 | 0.91 | 27.14 | 14.84 | 62.9 | |
| 1.00 | 187 | 2.80 × 10−6 | 1.00 | 41.23 | 0.085 | 75.6 | |
| B | 0.01 | 179 | 1.36 × 10−6 | 0.92 | 12.88 | 19.49 | 21.8 |
| 0.10 | 184 | 2.90 × 10−6 | 0.83 | 16.60 | 14.69 | 39.3 | |
| 1.00 | 179 | 1.92 × 10−6 | 0.93 | 43.65 | 41.62 | 76.9 | |
| C | 0.01 | 143 | 4.10 × 10−6 | 0.88 | 65.48 | 43.70 | 84.6 |
| 0.10 | 150 | 1.55 × 10−6 | 0.98 | 83.79 | 82.75 | 88.0 | |
| 1.00 | 155 | 3.40 × 10−6 | 0.87 | 101.02 | 35.20 | 90.0 | |
| D | 0.01 | 145 | 2.29 × 10−6 | 0.98 | 63.24 | 32.44 | 84.2 |
| 0.10 | 145 | 1.27 × 10−6 | 1.00 | 76.70 | 59.95 | 86.9 | |
| 1.00 | 140 | 1.07 × 10−6 | 1.00 | 115.01 | 99.46 | 91.3 | |
| Inhibitor | Cinh, mM | k, g/m2/day−1 | γ | Zk, % | τcorr, h |
|---|---|---|---|---|---|
| - | - | 44 | - | - | 1-2 |
| A | 1.0 | 17.3 | 1.3 | 22.9 | 26 |
| 5.0 | 13.7 | 1.6 | 38.9 | 43 | |
| 10.0 | 10.3 | 2.2 | 54.1 | 43 | |
| B | 1.0 | 18.4 | 1.2 | 17.8 | 47 |
| 5.0 | 5.9 | 3.8 | 73.9 | 63 | |
| 10.0 | 4.3 | 5.2 | 80.9 | 63 | |
| C | 1.0 | 22.4 | 1.0 | 0.0 | 68 |
| 5.0 | 4.7 | 4.8 | 79.0 | 86 | |
| 10.0 | 2.3 | 9.8 | 89.8 | 117 | |
| D | 1.0 | 0,9 | 10,9 | 90,8 | 70 |
| 5.0 | 0,7 | 13,1 | 92,4 | 95 | |
| 10.0 | 1,5 | 6,4 | 84,3 | 100 |
| Polarisation Mode | Element | |
|---|---|---|
| Cu | O | |
| No polarisation | 100.0 | 0.0 |
| After polarisation in borate buffer + 10 mM NaCl | 47.2 | 52.8 |
| After polarisation in borate buffer + 10 mM NaCl + 1.00 mM of 3-phenethylsulphinyl-5-amino-1H-1,2,4-triazole | 93.1 | 6.9 |
| Molecule | HOMO | LUMO | HLG | IP | EA | χ | η | σ |
|---|---|---|---|---|---|---|---|---|
| A | −6.55 | −0.68 | 5.87 | 6.55 | 0.68 | 3.62 | 2.94 | 0.34 |
| B | −6.57 | −0.72 | 5.85 | 6.57 | 0.72 | 3.64 | 2.92 | 0.34 |
| C | −6.57 | −0.71 | 5.86 | 6.57 | 0.71 | 3.64 | 2.93 | 0.34 |
| D | −6.58 | −0.86 | 5.72 | 6.58 | 0.86 | 3.72 | 2.86 | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevtsov, D.; Kozaderov, O.; Shikhaliev, K.; Komarova, E.; Kruzhilin, A.; Potapov, A.; Prabhakar, C.; Zartsyn, I. 3-Sulphinyl-5-Amino-1H-1,2,4-Triazoles as Inhibitors of Copper Corrosion. Appl. Sci. 2019, 9, 4882. https://doi.org/10.3390/app9224882
Shevtsov D, Kozaderov O, Shikhaliev K, Komarova E, Kruzhilin A, Potapov A, Prabhakar C, Zartsyn I. 3-Sulphinyl-5-Amino-1H-1,2,4-Triazoles as Inhibitors of Copper Corrosion. Applied Sciences. 2019; 9(22):4882. https://doi.org/10.3390/app9224882
Chicago/Turabian StyleShevtsov, Dmitry, Oleg Kozaderov, Khidmet Shikhaliev, Ekaterina Komarova, Alexei Kruzhilin, Andrei Potapov, Chetti Prabhakar, and Ilya Zartsyn. 2019. "3-Sulphinyl-5-Amino-1H-1,2,4-Triazoles as Inhibitors of Copper Corrosion" Applied Sciences 9, no. 22: 4882. https://doi.org/10.3390/app9224882
APA StyleShevtsov, D., Kozaderov, O., Shikhaliev, K., Komarova, E., Kruzhilin, A., Potapov, A., Prabhakar, C., & Zartsyn, I. (2019). 3-Sulphinyl-5-Amino-1H-1,2,4-Triazoles as Inhibitors of Copper Corrosion. Applied Sciences, 9(22), 4882. https://doi.org/10.3390/app9224882





