Effects of Precursor Concentration in Solvent and Nanomaterials Room Temperature Aging on the Growth Morphology and Surface Characteristics of Ni–NiO Nanocatalysts Produced by Dendrites Combustion during SCS
Abstract
1. Introduction
1.1. Metallic Nickel
1.2. Nickel Oxide
1.3. Solution Combustion Synthesis
2. Room Temperature Aging Effects
3. Materials and Methods
3.1. Materials
3.2. Solution Combustion Synthesis of Ni-Based Catalysts
3.3. Characterization of the Solution Combustion Synthesized Catalysts
3.4. Hydrogenation of Maleic Acid
4. Results and Discussion
4.1. Transmission Electron Microscopy (TEM)
4.2. Infa-Red (IR)- Camera
4.3. X-ray Photoelectron Spectroscopy (XPS)
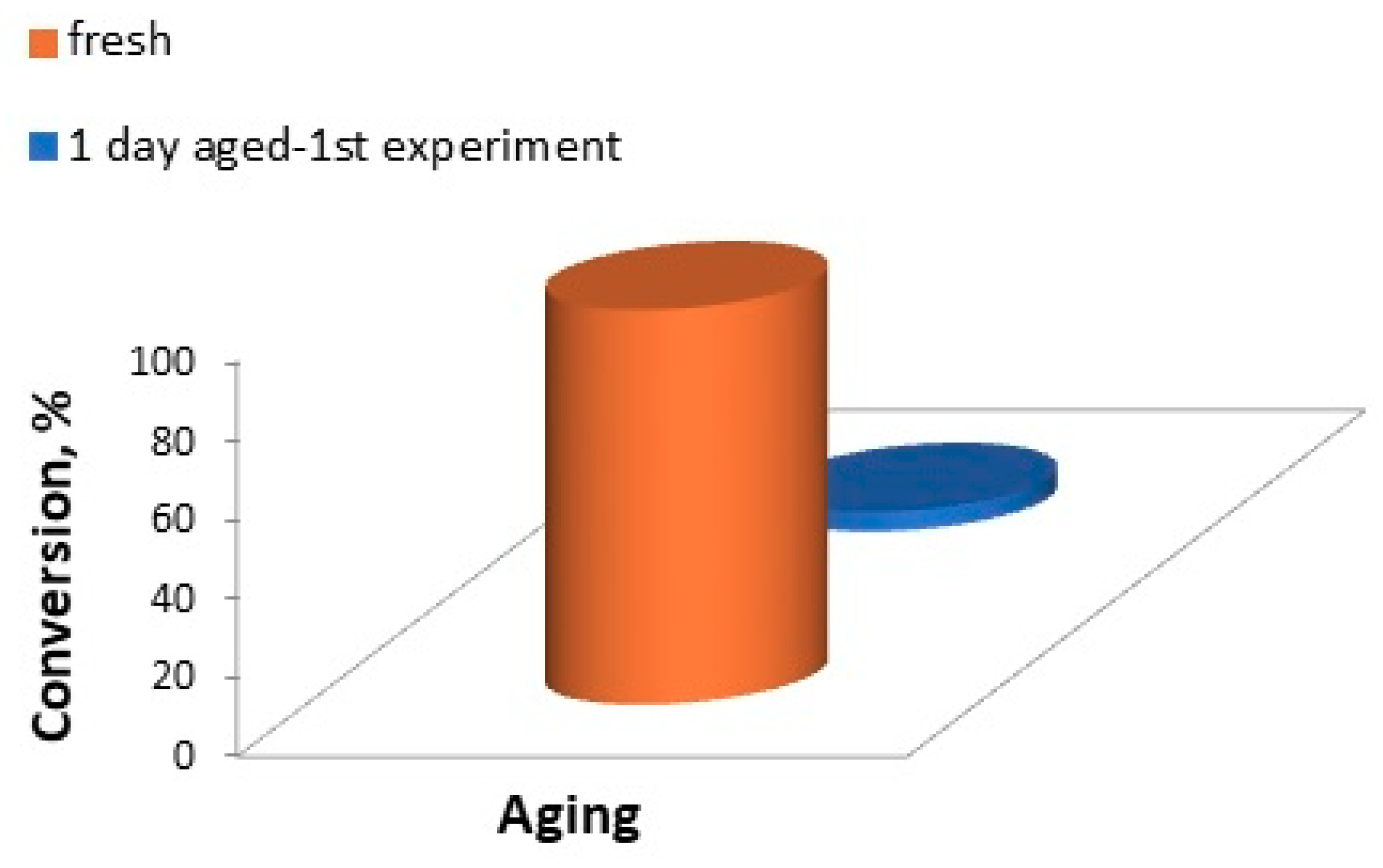
4.4. Catalytic Hydrogenation of Maleic Acid
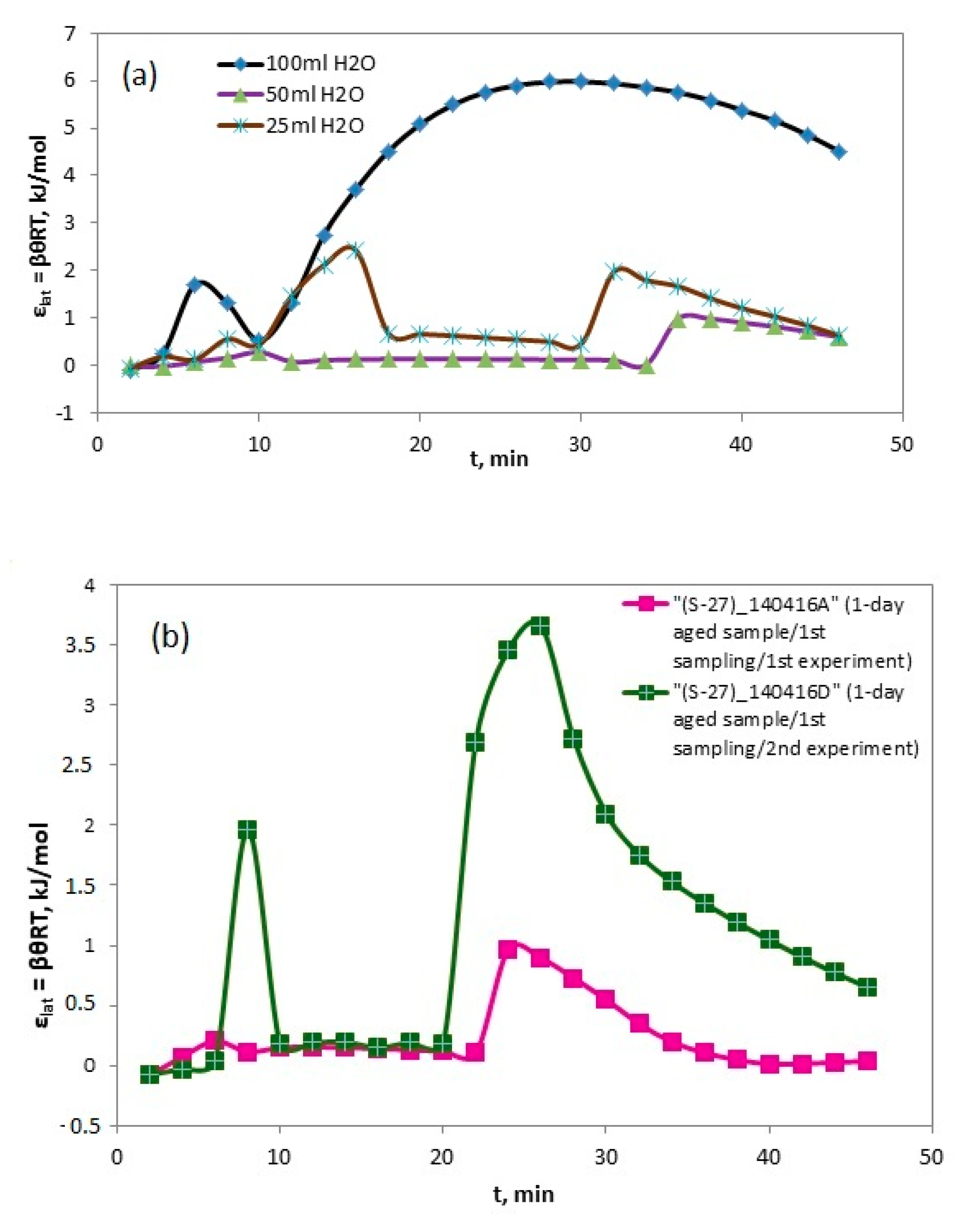
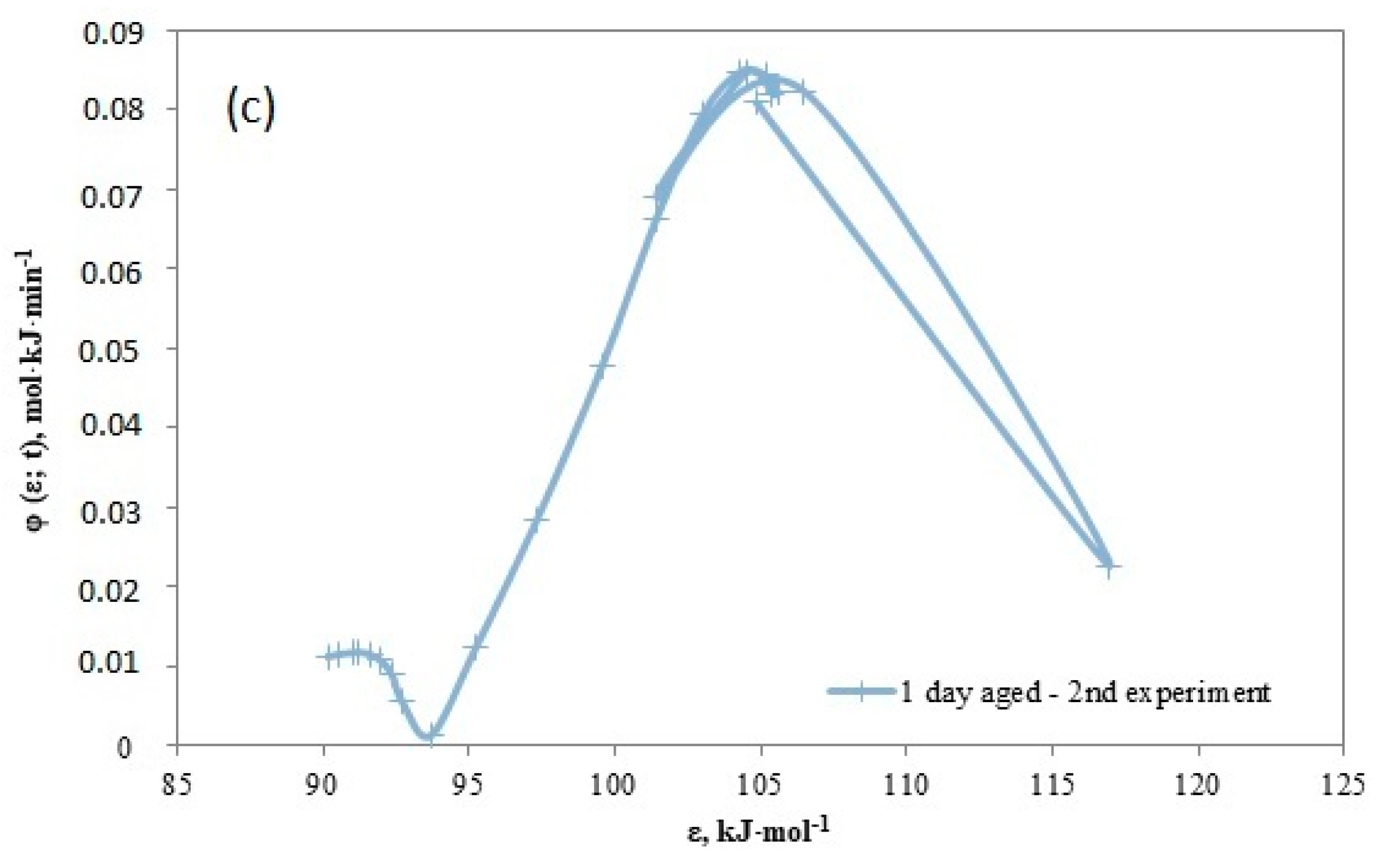
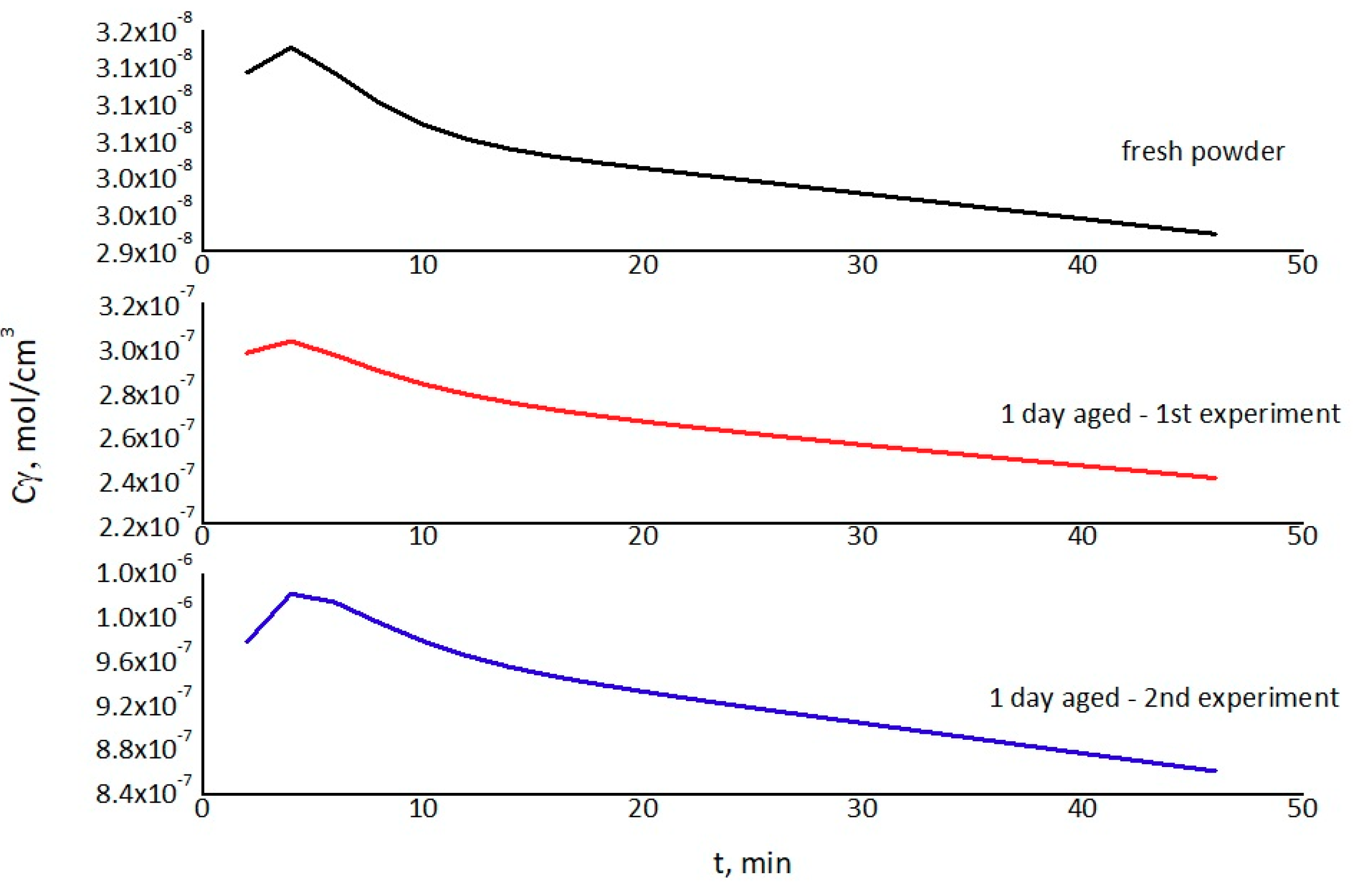
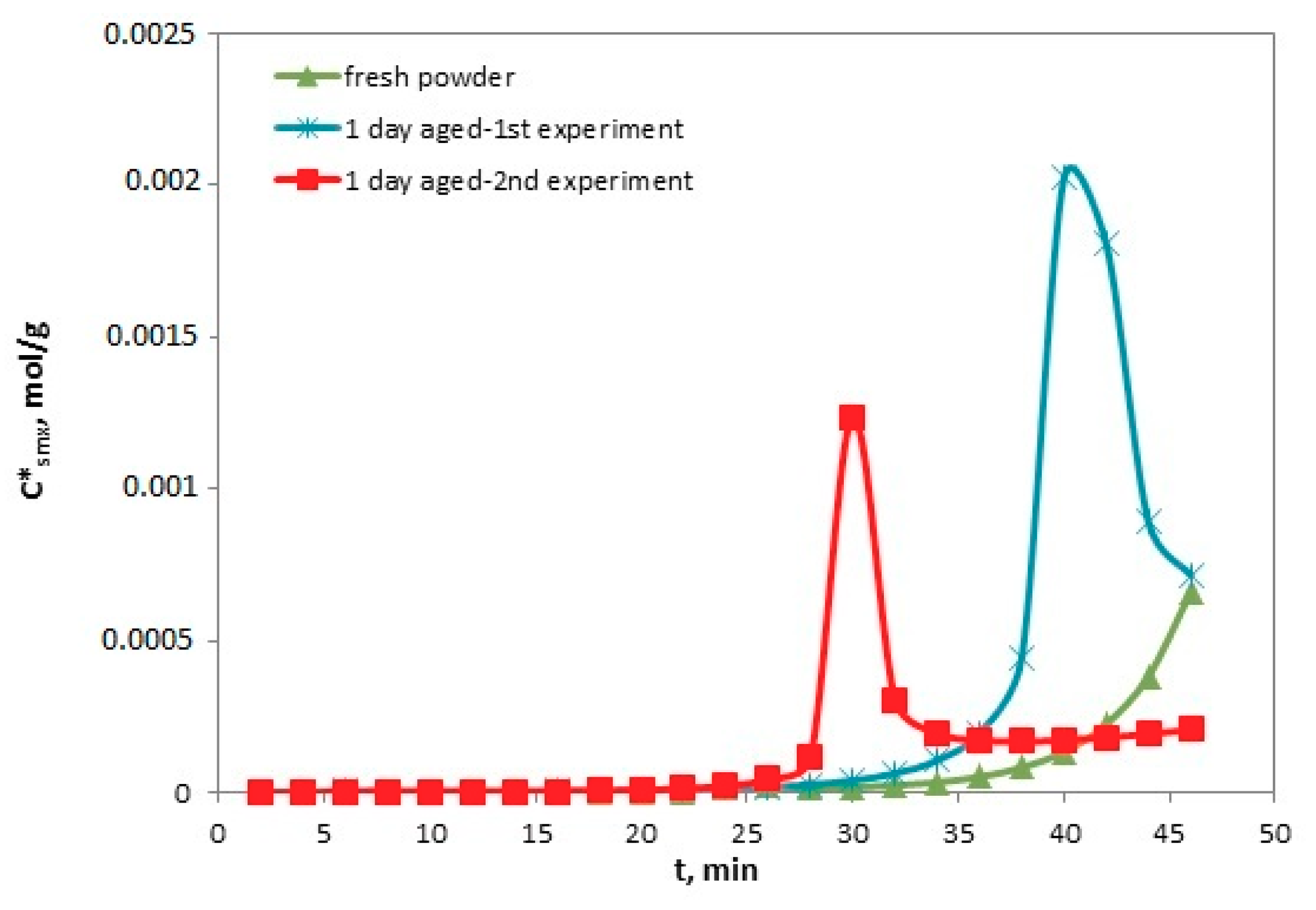
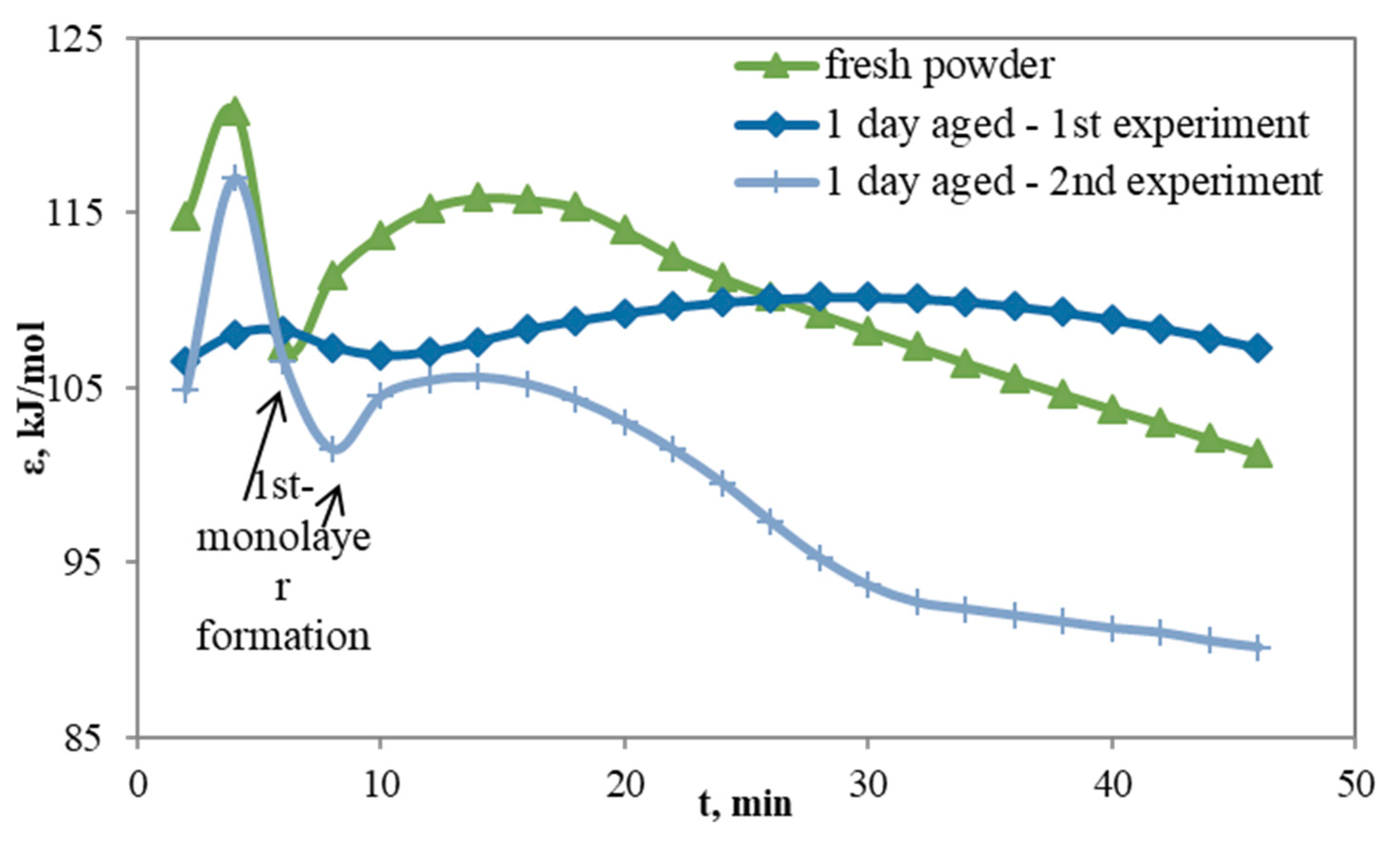
4.5. Reversed Flow – Inverse Gas Chromatography (RF-IGC)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreibig, U.; Bonnemann, H.; Hormes, J. Handbook of Surfaces and Interfaces of Materials; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Bonnemann, H.; Richards, R. Manufacture of heterogeneous mono- and bimetallic colloid catalysts and their applications in fine chemical synthesis and fuel cells. In Synthetic Methods of Organometallic Inorganic Chemistry; Herrmann, W.A., Brauer, G., Eds.; ThiemeVerlag: Stuttgart, Germany, 2002; Volume 10, pp. 209–224. [Google Scholar]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Johnson, B.F.G.; Raja, R.; Sankar, G.; Midgley, P.A. High-Performance Nanocatalysts for Single-Step Hydrogenations. Acc. Chem. Res. 2003, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Lieber, C.M. Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science 2001, 291, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Eychmuller, A. Structure and Photophysics of Semiconductor Nanocrystals. J. Phys. Chem. B 2001, 104, 6514–6528. [Google Scholar] [CrossRef]
- Puntes, V.F.; Krishnan, K.M.; Alivistos, A.P. Molecular Rulers for Scaling Down Nanostructures. Science 2001, 291, 2115–2117. [Google Scholar] [CrossRef]
- Sun, S.; Murray, C.B.; Weller, D.; Folks, L.; Moser, A. Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. Science 2000, 287, 1989–1992. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Kulkarni, G.U.; Thomas, P. Metal nanoparticles and their assemblies. Chem. Soc. Rev. 2000, 29, 27–35. [Google Scholar] [CrossRef]
- Chen, S. Two-Dimensional Crosslinked Nanoparticle Networks. Adv. Mater. 2000, 12, 186–189. [Google Scholar] [CrossRef]
- Harada, T.S.; Tai, A.O. Improvement of nickel catalyst for enantioface-differentiating (asymmetric) hydrogenation of methyl-acetoacetate. Chem. Lett. 1977, 10, 1131–1132. [Google Scholar] [CrossRef]
- Kimijima, K.; Sugimoto, T.J. Effects of the water content on the growth rate of AgCl nanoparticles in a reversed micelle system. J. Colloid Interface Sci. 2005, 286, 520–525. [Google Scholar] [CrossRef]
- Medford, J.A.; Johnston-Peck, A.C.; Tracy, J.B. Nanostructural transformations during the reduction of hollow and porous nickel oxide nanoparticles. Nanoscale 2013, 5, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.-Y.; Shih, P.-S.; Chan, T.-S.; Ma, Y.-R.; Wu, S.Y. Magnetic properties of cluster glassy Ni/NiO core–shell nanoparticles: An investigation of their static and dynamic magnetization. Nanoscale Res. Lett. 2015, 10, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Banerjee, S.; Menon, K.S.R. Core-shell model of the vacancy concentration and magnetic behavior for antiferromagnetic nanoparticle. Phys. Rev. B 2009, 80, 214420. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution combustion synthesis of nanoscale materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Patil, K.C.; Aruna, S.T.; Mimani, T. Combustion synthesis: An update. Curr. Opin. Solid State Mater. Sci. 2002, 6, 507–512. [Google Scholar] [CrossRef]
- Deganello, F.; Tyagi, A.K. Solution combustion synthesis, energy and environment: Best parameters for better materials. Prog. Cryst. Growth Charact. Mater. 2018, 64, 23–61. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Vekinis, G.; Chroneos, A. Parametric Optimisation of Solution Combustion Synthesis Catalysts and Their Application for the Aqueous Hydrogenation of Maleic Acid. Catal. Lett. 2018, 148, 764–778. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Vekinis, G.; Chroneos, A. Review of Recent Studies on Solution Combustion Synthesis of Nanostructured Catalysts. Adv. Eng. Mater. 2018, 20, 1800047. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Thoda, O.; Roslyakov, S.; Steinman, A.; Kovalev, D.; Levashov, E.; Vekinis, G.; Sytschev, A.; Chroneos, A. Solution combustion synthesis of nano-catalysts with a hierarchical structure. J. Catal. 2018, 364, 112–124. [Google Scholar] [CrossRef]
- Hadke, S.; Kalimila, M.T.; Rathkanthiwar, S.; Gour, S.; Sonkusare, R.; Ballal, A. Role of fuel and fuel-to-oxidizer ratio in combustion synthesis of nano-crystalline nickel oxide powders. Ceram. Int. 2015, 41, 14949–14957. [Google Scholar] [CrossRef]
- Kumar, A.; Wolf, E.E.; Mukasyan, A.S. Solution combustion synthesis of metal nanopowders: Nickel—Reaction pathways. AlChE J. 2011, 57, 2207–2214. [Google Scholar] [CrossRef]
- Wen, W.; Wu, J.-M. Nanomaterials via solution combustion synthesis: A step nearer to controllability. RSC Adv. 2014, 4, 58090–58100. [Google Scholar] [CrossRef]
- Jung, C.H.; Jalota, S.; Bhaduri, S.B. Quantitative effects of fuel on the synthesis of Ni/NiO particles using a microwave-induced solution combustion synthesis in air atmosphere. Mater. Lett. 2005, 59, 2426–2432. [Google Scholar] [CrossRef]
- Erri, P.; Nader, J.; Varma, A. Controlling combustion wave propagation for transition metal/alloy/cermet foam synthesis. Adv. Mater. 2008, 20, 1243–1245. [Google Scholar] [CrossRef]
- Kumar, A.; Cross, A.; Manukyan, K.; Bhosale, R.R.; van den Broeke, L.J.P.; Miller, J.T.; Mukasyan, A.S.; Wolf, E.E. Combustion synthesis of copper–nickel catalysts for hydrogen production from ethanol. Chem. Eng. J. 2015, 278, 46–54. [Google Scholar] [CrossRef]
- Cross, A.; Kumar, A.; Wolf, E.E.; Mukasyan, A.S. Combustion Synthesis of a Nickel Supported Catalyst: Effect of Metal Distribution on the Activity during Ethanol Decomposition. Ind. Eng. Chem. Res. 2012, 51, 12004–12008. [Google Scholar]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of nanomaterials in the environment. Environ. Sci. Technol. 2012, 46, 6893–6899. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Motellier, S.; Clavaguera, S.; Nowack, B. Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ. Int. 2015, 77, 132–147. [Google Scholar] [CrossRef]
- Kenning, G.G.; Heidt, C.; Barnes, A.; Martin, J.; Grove, B.; Madden, M. Thermally activated magnetization and resistance decay during near ambient temperature aging of Co nanoflakes in a confining semi-metallic environment. J. Appl. Phys. 2011, 110, 114312. [Google Scholar] [CrossRef]
- Thurber, A.P.; Alanko, G.; Beausoleil, G.L., II; Dodge, K.N.; Hanna, C.; Punnoose, A. Unusual crystallite growth and modification of ferromagnetism due to aging in pure and doped ZnO nanoparticles. J. Appl. Phys. 2012, 111, 07C319. [Google Scholar] [CrossRef]
- Ali, M.; Winterer, M. ZnO nanocrystals: Surprisingly ‘alive’. Chem. Mater. 2009, 22, 85–91. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Pettibone, J.M.; Grassian, V.H. Environmental implications of nanoparticle aging in the processing and fate of copper-based nanomaterials. Environ. Sci. Technol. 2012, 46, 7001–7010. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wang, Z.W.; Palmer, R.E. Ageing of mass-selected Cu/Au and Au/Cu core/shell clusters probed with atomic resolution. J. Exp. Nanosci. 2012, 7, 703–710. [Google Scholar] [CrossRef]
- Fetisov, A.V.; Kozhina, G.A.; Estemirova, S.K.; Mitrofanov, V.Y. On the room-temperature aging effects in YBa2Cu3O6+δ. Phys. C 2015, 515, 54–61. [Google Scholar] [CrossRef]
- Katsanos, N.A.; Karaiskakis, G. Time-Resolved Inverse Gas Chromatography and Its Practical Applications; HNB Publishing: New York, NY, USA, 2004; Volume 1. [Google Scholar]
- Metaxa, E.; Kolliopoulos, A.; Agelakopoulou, T.; Roubani-Kalantzopoulou, F. The role of surface heterogeneity and lateral interactions in the adsorption of volatile organic compounds on rutile surface. Appl. Surf. Sci. 2009, 255, 6468–6478. [Google Scholar] [CrossRef]
- Arvaniti, I.; Netos, V.; Siokos, V.; Metaxa, E.; Kalantzopoulou, F.R. Relation between adsorption and catalysis in the case of NiO and Co3O4. Appl. Surf. Sci. 2010, 256, 5559–5565. [Google Scholar] [CrossRef]
- Xanthopoulou, G.; Thoda, O.; Metaxa, E.D.; Vekinis, G.; Chroneos, A. Influence of atomic structure on the nano-nickel-based catalyst activity produced by solution combustion synthesis in the hydrogenation of maleic acid. J. Catal. 2017, 348, 9–21. [Google Scholar] [CrossRef]
- Furstenau, R.; McDougall, G.; Langell, M. Initial stages of hydrogen reduction of NiO (100). Surf. Sci. 1985, 150, 55–79. [Google Scholar] [CrossRef]
- Rodriguez, A.; Hanson, J.C.; Frenkel, A.I.; Kim, J.Y.; Pérez, M. Experimental and Theoretical Studies on the Reaction of H2 with NiO: Role of O Vacancies and Mechanism for Oxide Reduction. J. Am. Chem. Soc. 2002, 124, 346–354. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Vekinis, G.; Chroneos, A. The effect of the precursor solution’s pretreatment on the properties and microstructure of the SCS final nanomaterials. Appl. Sci. 2019, 9, 1200. [Google Scholar] [CrossRef]
- Thoda, O.; Xanthopoulou, G.; Prokof’ev, V.; Roslyakov, S.; Vekinis, G.; Chroneos, A. Influence of preheating temperature on Solution Combustion Synthesis of Ni–NiO nanocomposites: Mathematical model and experiment. Int. J. Self-Propagating High-Temp. Synth. 2018, 27, 207–215. [Google Scholar] [CrossRef]
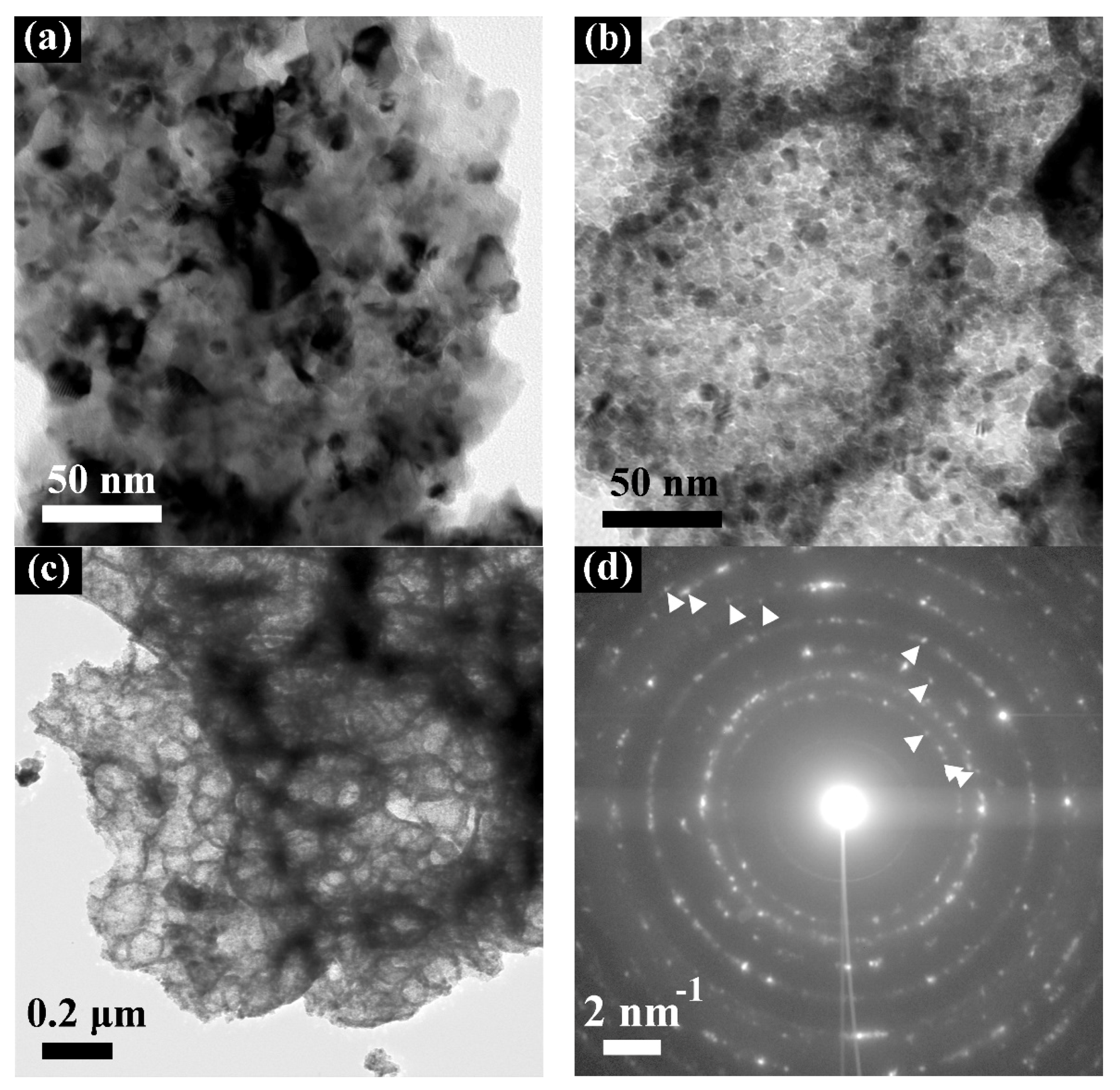

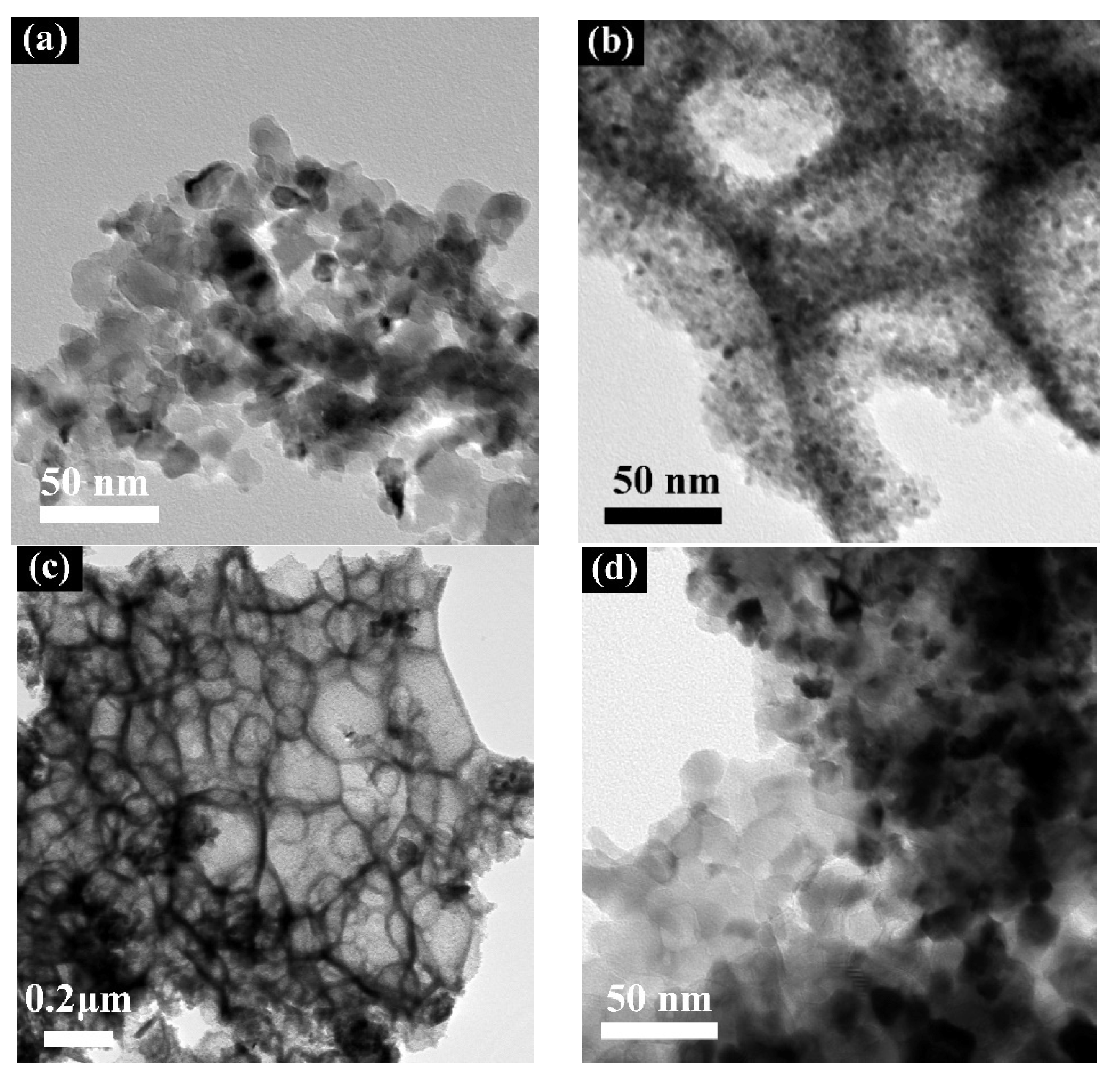
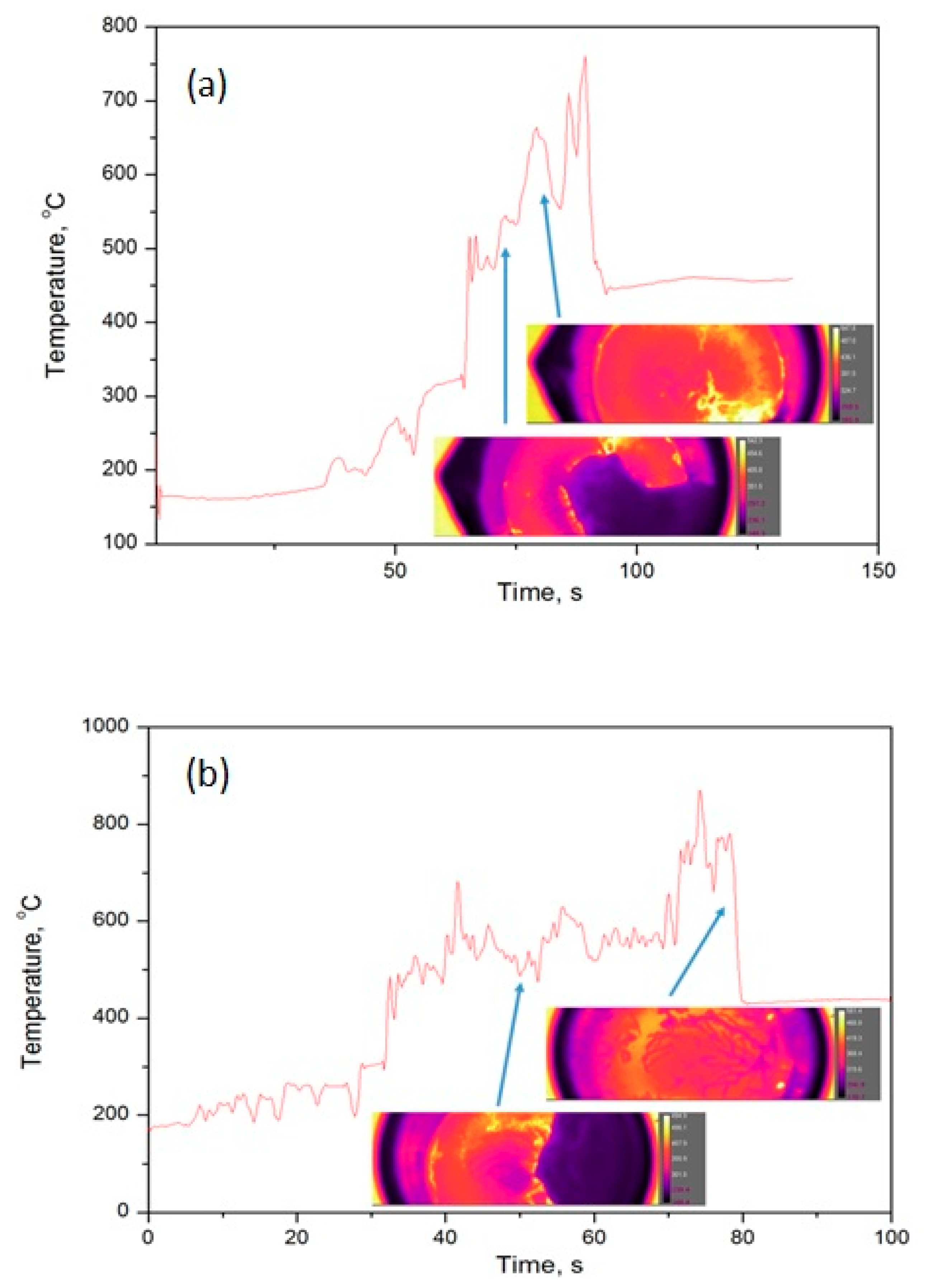
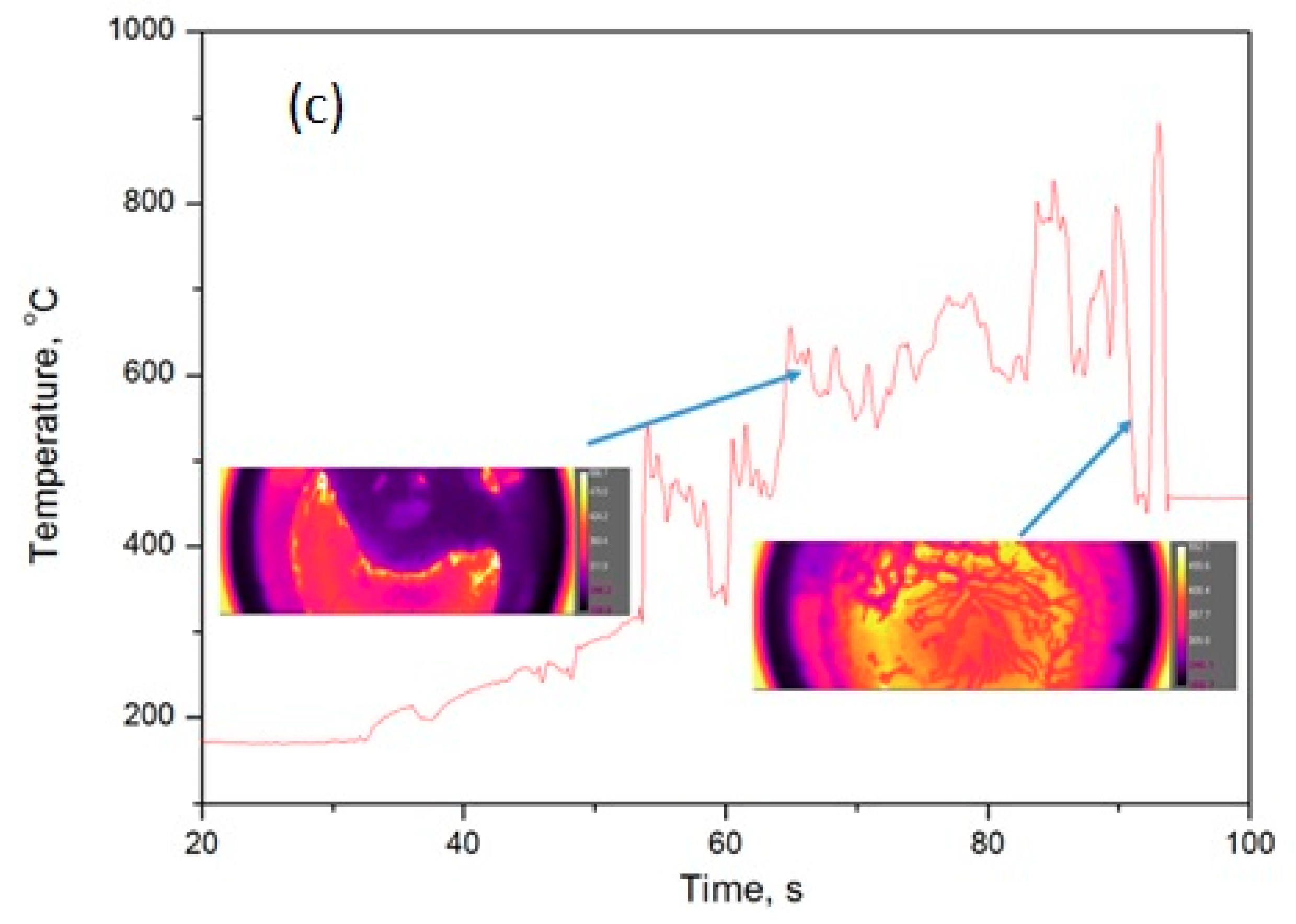



| Material | Manufacturer | Assay/Purity |
|---|---|---|
| Nickel (II) nitrate hexahydrate for analysis (Ni(NO3)2·6H2O) | Merck | 99.0%–102.0% |
| Glycine for synthesis (CH2NH2COOH) | PanReacAppliChem | 99.0% |
| Maleic acid (HOOCCH=CHCOOH) | Riedel-de Haën | 99.0% |
| Hydrogen (H2) | Air Liquide | 99.999% |
| Number of Experiment | Water Added in the Precursor Solution |
|---|---|
| 25 | 50 mL |
| 27 | 75 mL |
| 28 | 25 mL |
| 29 | 100 mL |
| Water Volume, mL | Ni, % | O, % |
|---|---|---|
| 25 | 19.70 | 80.30 |
| 50 | 21.10 | 78.90 |
| 75 | 22.50 | 77.50 |
| 100 | 23.30 | 76.70 |
| Water Volume, mL | Ni 2p3/2 | Ni 2p1/2 | ||
|---|---|---|---|---|
| Ni2+ | Ni3+ | Ni2+ | Ni3+ | |
| 25 | 854.2 ± 0.2 eV | 856.2 ± 0.2 eV | 872.1 ± 0.2 eV | 874.5 ± 0.2 eV |
| 50 | 854.1 ± 0.2 eV | 856.1 ± 0.2 eV | 872.1 ± 0.2 eV | 874.4 ± 0.2 eV |
| 75 | 854.3 ± 0.2 eV | 856.2 ± 0.2 eV | 872.2 ± 0.2 eV | 874.2 ± 0.2 eV |
| 100 | 854.3 ± 0.2 eV | 856.3 ± 0.2 eV | 872.1 ± 0.2 eV | 874.2 ± 0.2 eV |
| O1s | ||||
|---|---|---|---|---|
| O:Ni2+ | O:Ni3+ | O:NiOOH | Ni2+–O:Ni3+–O | |
| 25 | 529.8 ± 0.2 eV | 531.8 ± 0.2 eV | 532.5 ± 0.2 eV | 4.3 |
| 50 | 529.7 ± 0.2 eV | 531.7 ± 0.2 eV | - | 1.8 |
| 75 | 529.8 ± 0.2 eV | 531.8 ± 0.2 eV | - | 1.9 |
| 100 | 529.8 ± 0.2 eV | 531.9 ± 0.2 eV | - | 1.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulou, G.; Thoda, O.; Boukos, N.; Krishnamurthy, S.; Dey, A.; Roslyakov, S.; Vekinis, G.; Chroneos, A.; Levashov, E. Effects of Precursor Concentration in Solvent and Nanomaterials Room Temperature Aging on the Growth Morphology and Surface Characteristics of Ni–NiO Nanocatalysts Produced by Dendrites Combustion during SCS. Appl. Sci. 2019, 9, 4925. https://doi.org/10.3390/app9224925
Xanthopoulou G, Thoda O, Boukos N, Krishnamurthy S, Dey A, Roslyakov S, Vekinis G, Chroneos A, Levashov E. Effects of Precursor Concentration in Solvent and Nanomaterials Room Temperature Aging on the Growth Morphology and Surface Characteristics of Ni–NiO Nanocatalysts Produced by Dendrites Combustion during SCS. Applied Sciences. 2019; 9(22):4925. https://doi.org/10.3390/app9224925
Chicago/Turabian StyleXanthopoulou, Galina, Olga Thoda, Nikos Boukos, Satheesh Krishnamurthy, Avishek Dey, Sergey Roslyakov, George Vekinis, Alexandros Chroneos, and Evgeny Levashov. 2019. "Effects of Precursor Concentration in Solvent and Nanomaterials Room Temperature Aging on the Growth Morphology and Surface Characteristics of Ni–NiO Nanocatalysts Produced by Dendrites Combustion during SCS" Applied Sciences 9, no. 22: 4925. https://doi.org/10.3390/app9224925
APA StyleXanthopoulou, G., Thoda, O., Boukos, N., Krishnamurthy, S., Dey, A., Roslyakov, S., Vekinis, G., Chroneos, A., & Levashov, E. (2019). Effects of Precursor Concentration in Solvent and Nanomaterials Room Temperature Aging on the Growth Morphology and Surface Characteristics of Ni–NiO Nanocatalysts Produced by Dendrites Combustion during SCS. Applied Sciences, 9(22), 4925. https://doi.org/10.3390/app9224925









