The Effect of Carbon Nanofibers Surface Properties in Hydrogenation and Dehydrogenation Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalysts Characterisation
2.3. Catalytic Reactions
3. Results
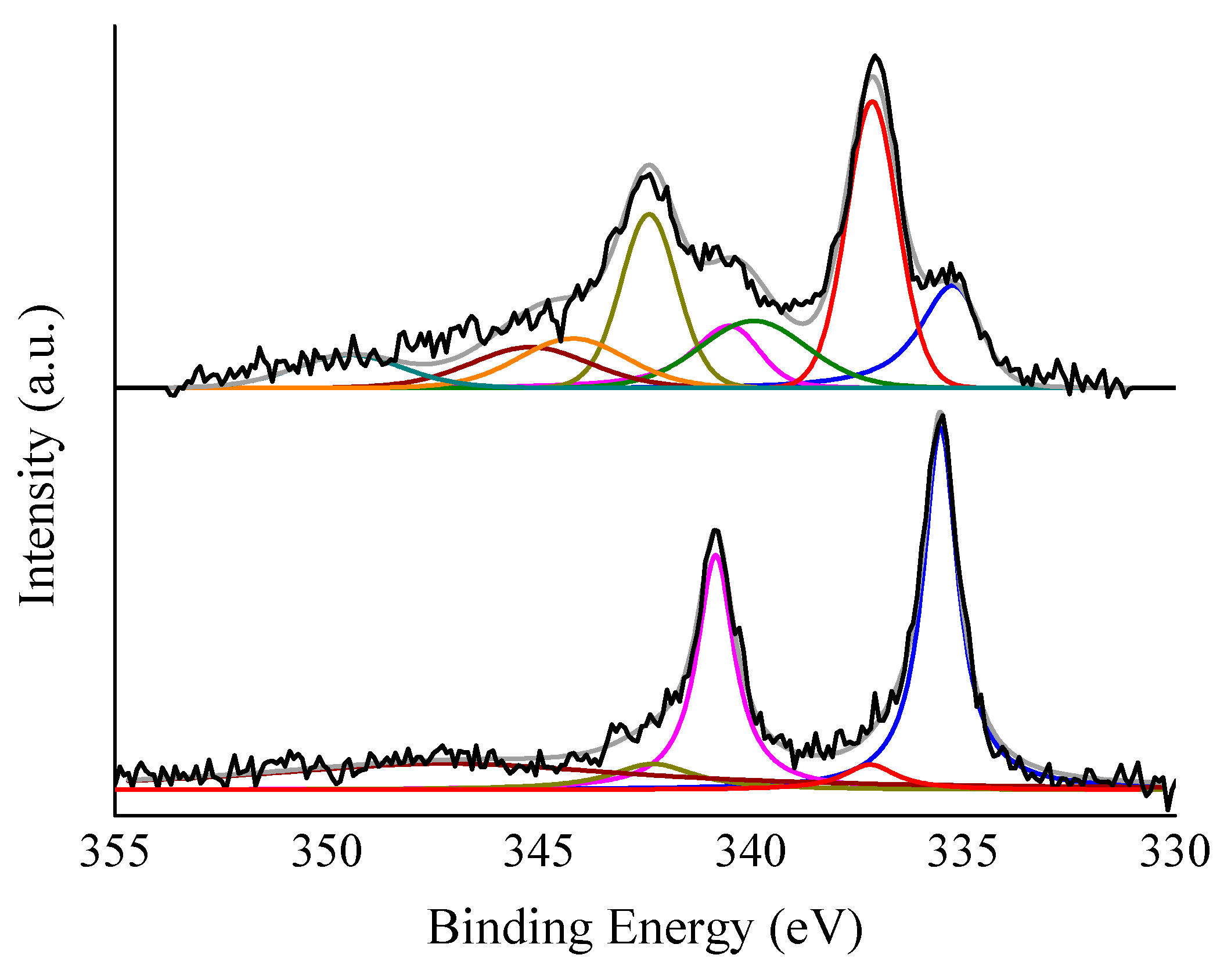
3.1. Catalysts Characterisation
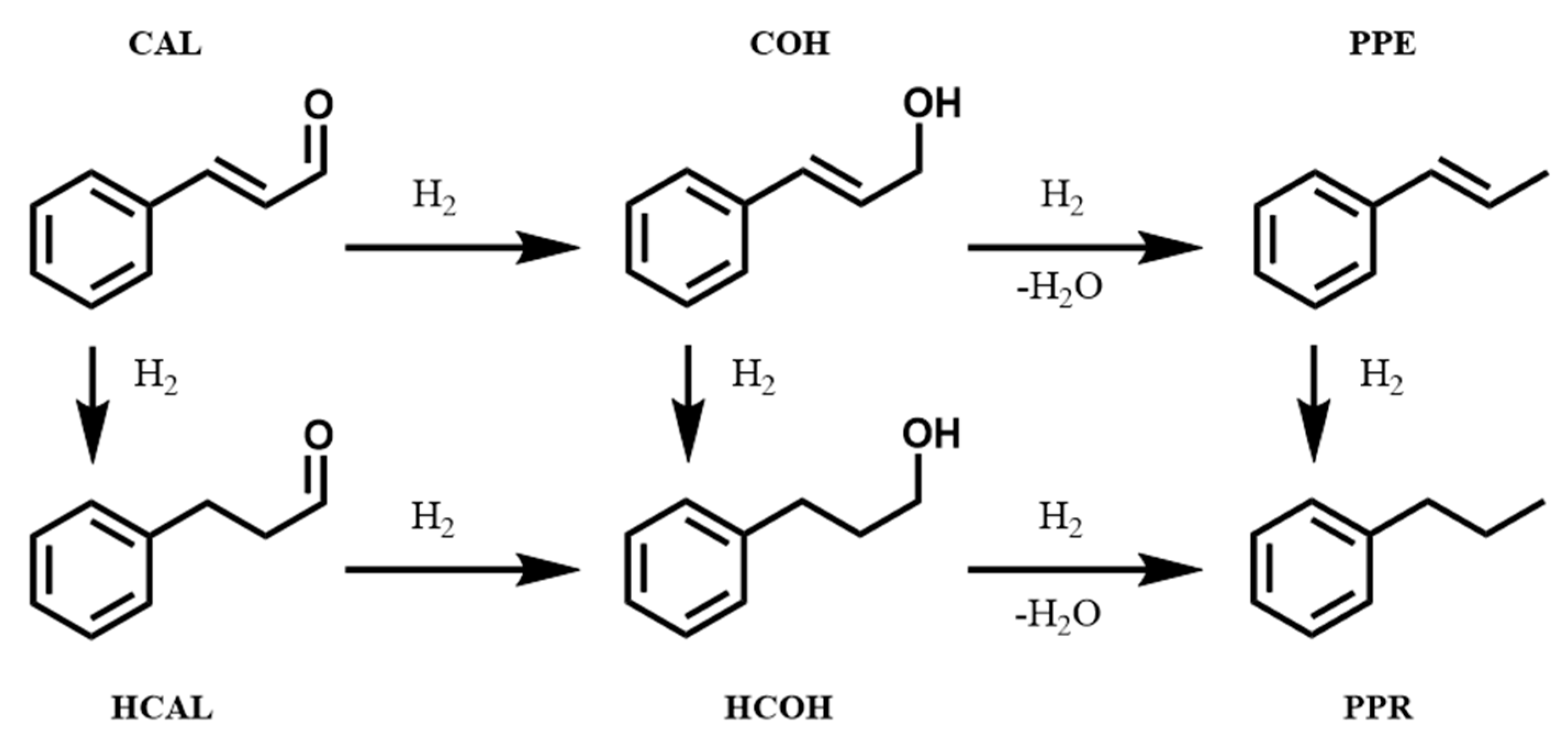
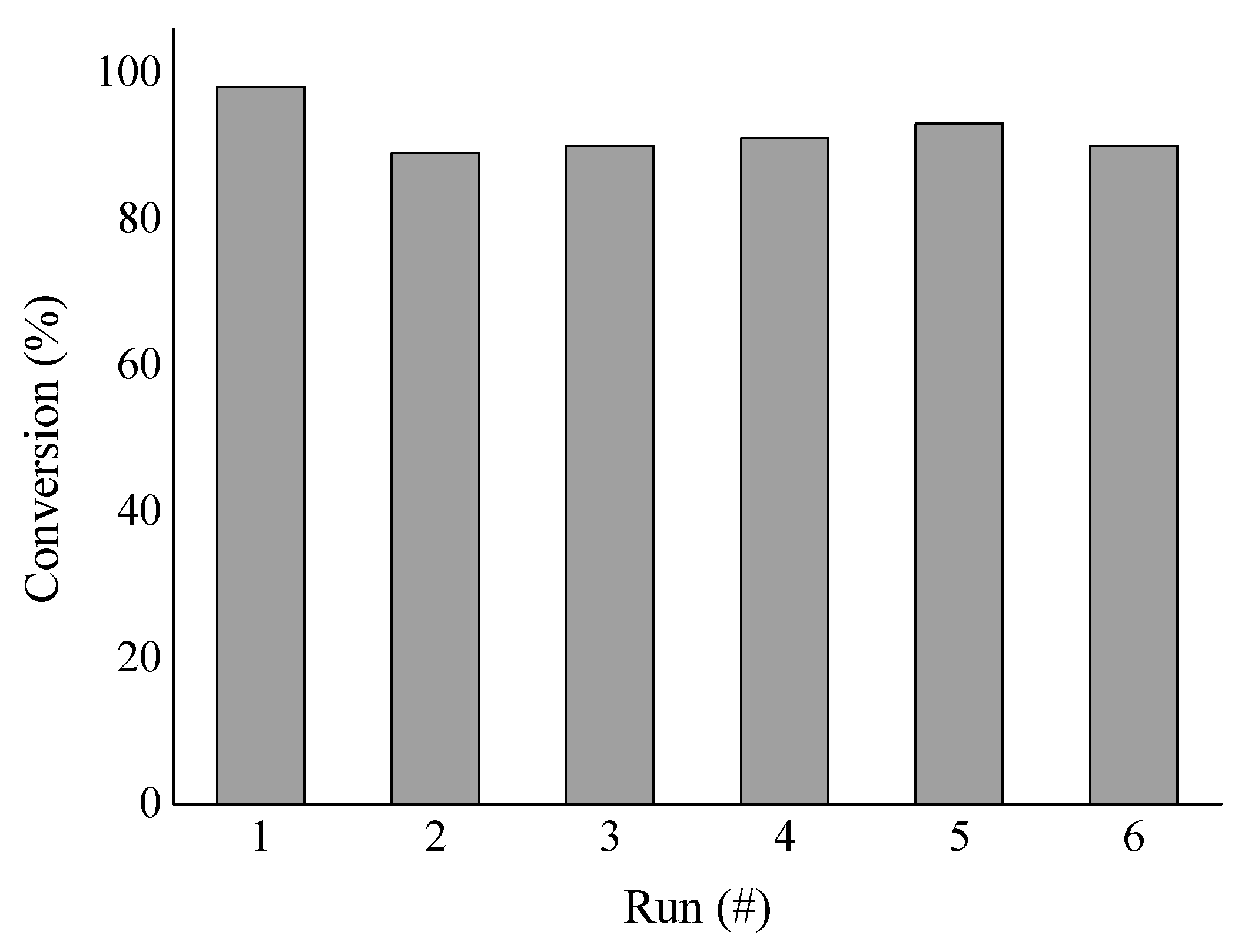
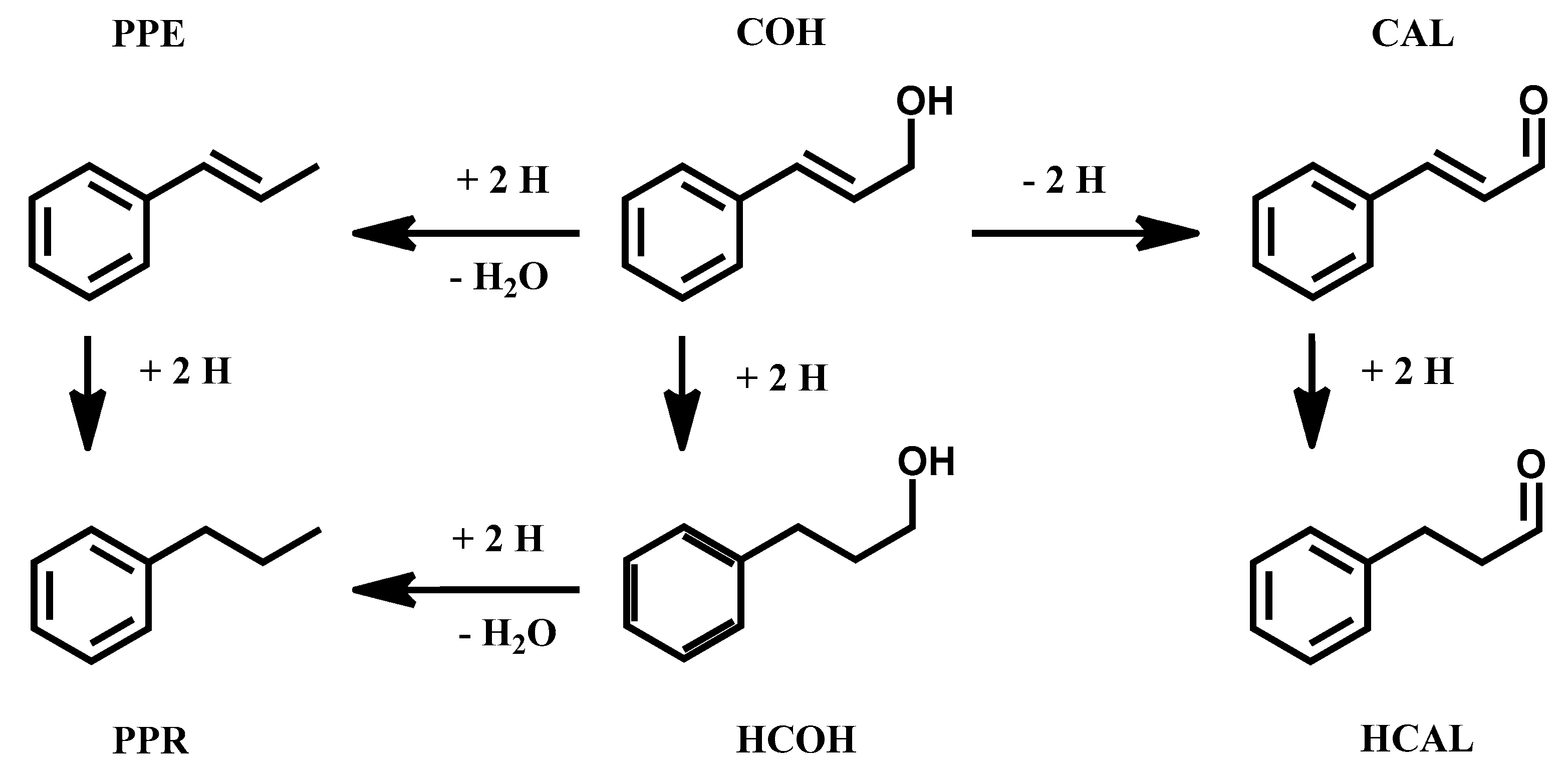
3.2. Cinnamaldehyde Hydrogenation
3.3. Cinnamyl Alcohol Dehydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Edison, T.A. Manufacture of Carbon Filaments. U.S. Patent US484185A, 11 October 1892. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Coelho, N.M.A.; Furtado, J.L.B.; Pham-Huu, C.; Vieira, R. Carbon Nanofibers: A Versatile Catalytic Support. Mater. Res. 2008, 11, 353–357. [Google Scholar] [CrossRef]
- Feng, L.; Xie, N.; Zhong, J. Carbon nanofibers and their composites: A review of synthesizing, properties and applications. Materials 2014, 7, 3919–3945. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. Sci. Eng. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Serp, P.; Corrias, M.; Kalck, P. Carbon nanotubes and nanofibers in catalysis. Appl. Catal. A Gen. 2003, 253, 337–358. [Google Scholar] [CrossRef]
- Cattaneo, S.; Naslhajian, H.; Somodi, F.; Evangelisti, C.; Villa, A.; Prati, L. Ruthenium on carbonaceous materials for the selective hydrogenation of HMF. Molecules 2018, 23, 2007. [Google Scholar] [CrossRef]
- Villa, A.; Wang, D.; Chan-Thaw, C.E.; Campisi, S.; Veith, G.M.; Prati, L. The confinement effect on the activity of Au NPs in polyol oxidation. Catal. Sci. Technol. 2016, 6, 598–601. [Google Scholar] [CrossRef]
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mater. 2007, 6, 507–511. [Google Scholar] [CrossRef]
- Jouve, A.; Cattaneo, S.; Capelli, S.; Stucchi, M.; Evangelisti, C.; Villa, A.; Prati, L. CNF-Functionalization as Versatile Tool for Tuning Activity in Cellulose-Derived Product Hydrogenation. Molecules 2019, 24, 316. [Google Scholar] [CrossRef]
- Testolin, A.; Cattaneo, S.; Wang, W.; Wang, D.; Pifferi, V.; Prati, L.; Falciola, L.; Villa, A. Cyclic Voltammetry Characterization of Au, Pd, and AuPd Nanoparticles Supported on Different Carbon Nanofibers. Surfaces 2019, 2, 16. [Google Scholar] [CrossRef]
- Campisi, S.; Chan-Thaw, C.; Villa, A. Understanding Heteroatom-Mediated Metal–Support Interactions in Functionalized Carbons: A Perspective Review. Appl. Sci. 2018, 8, 1159. [Google Scholar] [CrossRef]
- Delgado, J.J.; Vieira, R.; Rebmann, G.; Su, D.S.; Keller, N.; Ledoux, M.J.; Schlögl, R. Supported carbon nanofibers for the fixed-bed synthesis of styrene. Carbon 2006, 44, 809–812. [Google Scholar] [CrossRef]
- Chambers, A.; Nemes, T.; Rodriguez, N.M.; Baker, R.T.K. Catalytic Behavior of Graphite Nanofiber Supported Nickel Particles. 1. Comparison with Other Support Media. J. Phys. Chem. B 2002, 102, 2251–2258. [Google Scholar] [CrossRef]
- Xue, Z.; Ma, M.G.; Li, Z.; Mu, T. Advances in the conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts. RSC Adv. 2016, 6, 98874–98892. [Google Scholar] [CrossRef]
- Kohlpaintner, C.; Schulte, M.; Falbe, J.; Lappe, P.; Weber, J. Aldehydes, Araliphatic. Ullmann’s Encycl. Ind. Chem. 2005. [Google Scholar] [CrossRef]
- Konno, K.; Sakagami, H.; Kawazoe, Y.; Yamamoto, N. AIDS Therapeutic Agents Comprising Polymers Formed from Cinnamic Acid Derivatives. U.S. Patent US5632980A, 27 May 1997. [Google Scholar]
- Gallezot, P.; Richard, D. Selective Hydrogenation of α,β-Unsaturated Aldehydes. Catal. Rev. 1998, 41, 26. [Google Scholar] [CrossRef]
- Cattaneo, S.; Freakley, S.J.; Morgan, D.J.; Sankar, M.; Dimitratos, N.; Hutchings, G.J. Cinnamaldehyde hydrogenation using Au-Pd catalysts prepared by sol immobilisation. Catal. Sci. Technol. 2018, 8, 1677–1685. [Google Scholar] [CrossRef]
- Rao, R.G.; Blume, R.; Hansen, T.W.; Fuentes, E.; Dreyer, K.; Moldovan, S.; Ersen, O.; Hibbitts, D.D.; Chabal, Y.J.; Schlögl, R.; et al. Interfacial charge distributions in carbon-supported palladium catalysts. Nat. Commun. 2017, 8, 340. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Gurrala, L.; Gogoi, P.; Chilukuri, S.V. Hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over Pd nanoparticles deposited on nitrogen-doped mesoporous carbon. RSC Adv. 2016, 6, 44333–44340. [Google Scholar] [CrossRef]
- Ji, X.; Niu, X.; Li, B.; Han, Q.; Yuan, F.; Zaera, F.; Zhu, Y.; Fu, H. Selective Hydrogenation of Cinnamaldehyde to Cinnamal Alcohol over Platinum/Graphene Catalysts. ChemCatChem 2014, 6, 3246–3253. [Google Scholar] [CrossRef]
- Pham-Huu, C.; Keller, N.; Ledoux, M.J.; Charbonniere, L.J.; Ziessel, R. Carbon nanofiber supported palladium catalyst for liquid-phase reactions. An active and selective catalyst for hydrogenation of C=C bonds. Chem. Commun. 2000, 170, 1871–1872. [Google Scholar] [CrossRef]
- Toebes, M.L.; Prinsloo, F.F.; Bitter, J.H.; Van Dillen, A.J.; De Jong, K.P. Influence of oxygen-containing surface groups on the activity and selectivity of carbon nanofiber-supported ruthenium catalysts in the hydrogenation of cinnamaldehyde. J. Catal. 2003, 214, 78–87. [Google Scholar] [CrossRef]
- Toebes, M.L.; Zhang, Y.; Hájek, J.; Alexander Nijhuis, T.; Bitter, J.H.; Jos Van Dillen, A.; Murzin, D.Y.; Koningsberger, D.C.; De Jong, K.P. Support effects in the hydrogenation of cinnamaldehyde over carbon nanofiber-supported platinum catalysts: Characterization and catalysis. J. Catal. 2004, 226, 215–225. [Google Scholar] [CrossRef]
- Plomp, A.J.; Vuori, H.; Krause, A.O.I.; de Jong, K.P.; Bitter, J.H. Particle size effects for carbon nanofiber supported platinum and ruthenium catalysts for the selective hydrogenation of cinnamaldehyde. Appl. Catal. A Gen. 2008, 351, 9–15. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Keresszegi, C.; Mallat, T.; Baiker, A. In situ EXAFS study of Pd/Al2O3 during aerobic oxidation of cinnamyl alcohol in an organic solvent. J. Catal. 2003, 213, 291–295. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Structure–reactivity correlations in the selective aerobic oxidation of cinnamyl alcohol: In situ XAFS. Green Chem. 2004, 6, 37–42. [Google Scholar] [CrossRef]
- Mallat, T.; Bodnar, Z.; Hug, P.; Baiker, A. Selective Oxidation of Cinnamyl Alcohol to Cinnamaldehyde with Air over Bi-Pt/Alumina Catalysts. J. Catal. 1995, 153, 131–143. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, B.; Zhang, Q.; Deng, W.; Wang, Y.; Yang, Y. Recent advances in heterogeneous selective oxidation catalysis for sustainable chemistry. Chem. Soc. Rev. 2014, 43, 3480–3524. [Google Scholar] [CrossRef]
- Davis, S.E.; Ide, M.S.; Davis, R.J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles. Green Chem. 2013, 15, 17–45. [Google Scholar] [CrossRef]
- Rucinska, E.; Miedziak, P.J.; Pattisson, S.; Brett, G.L.; Iqbal, S.; Morgan, D.J.; Sankar, M.; Hutchings, G.J. Cinnamyl alcohol oxidation using supported bimetallic Au-Pd nanoparticles: An investigation of autoxidation and catalysis. Catal. Sci. Technol. 2018, 8, 2987–2997. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Brett, G.L.; Cao, E.; Constantinou, A.; Ellis, P.; Kuhn, S.; Hutchings, G.J.; Bethell, D.; Gavriilidis, A. Oxidation of cinnamyl alcohol using bimetallic Au-Pd/TiO2 catalysts: A deactivation study in a continuous flow packed bed microreactor. Catal. Sci. Technol. 2016, 6, 4749–4758. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Garcia, H.; Leyva, A. Catalytic activity of palladium supported on single wall carbon nanotubes compared to palladium supported on activated carbon: Study of the Heck and Suzuki couplings, aerobic alcohol oxidation and selective hydrogenation. J. Mol. Catal. A Chem. 2005, 230, 97–105. [Google Scholar] [CrossRef]
- Parlett, C.M.A.; Durndell, L.J.; Machado, A.; Cibin, G.; Bruce, D.W.; Hondow, N.S.; Wilson, K.; Lee, A.F. Alumina-grafted SBA-15 as a high performance support for Pd-catalysed cinnamyl alcohol selective oxidation. Catal. Today 2014, 229, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Takubo, M.; Makida, K.; Yanase, T.; Aoyagi, S.; Maegawa, T.; Monguchi, Y.; Sajiki, H. A simple and efficient oxidation of alcohols with ruthenium on carbon. Chem. Commun. 2009, 5159–5161. [Google Scholar] [CrossRef] [PubMed]
- Durndell, L.J.; Parlett, C.M.A.; Hondow, N.S.; Wilson, K.; Lee, A.F. Tunable Pt nanocatalysts for the aerobic selox of cinnamyl alcohol. Nanoscale 2013, 5, 5412–5419. [Google Scholar] [CrossRef] [Green Version]
- Tessonnier, J.P.; Rosenthal, D.; Hansen, T.W.; Hess, C.; Schuster, M.E.; Blume, R.; Girgsdies, F.; Pfänder, N.; Timpe, O.; Su, D.S.; et al. Analysis of the structure and chemical properties of some commercial carbon nanostructures. Carbon 2009, 47, 1779–1798. [Google Scholar] [CrossRef] [Green Version]
- Prati, L.; Villa, A.; Arrigo, R.; Wang, D.; Su, D.S. Gold catalyzed liquid phase oxidation of alcohol : The issue of selectivity. Faraday Discuss. 2011, 152, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Militello, M.C.; Simko, S.J. Elemental Palladium by XPS. Surf. Sci. Spectra 1994, 3, 387–394. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Palladium Oxide (PdO) by XPS. Surf. Sci. Spectra 1994, 3, 395–401. [Google Scholar] [CrossRef]
- Pattamakomsan, K.; Suriye, K.; Dokjampa, S.; Mongkolsiri, N.; Praserthdam, P.; Panpranot, J. Effect of mixed Al2O3 structure between θ- and α-Al2O3 on the properties of Pd/Al2O3 in the selective hydrogenation of 1,3-butadiene. Catal. Commun. 2010, 11, 311–316. [Google Scholar] [CrossRef]
- Serp, P.; Figueiredo, J.L. Carbon Materials for Catalysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470178850. [Google Scholar]
- Tessonnier, J.P.; Pesant, L.; Ehret, G.; Ledoux, M.J.; Pham-Huu, C. Pd nanoparticles introduced inside multi-walled carbon nanotubes for selective hydrogenation of cinnamaldehyde into hydrocinnamaldehyde. Appl. Catal. A Gen. 2005, 288, 203–210. [Google Scholar] [CrossRef]

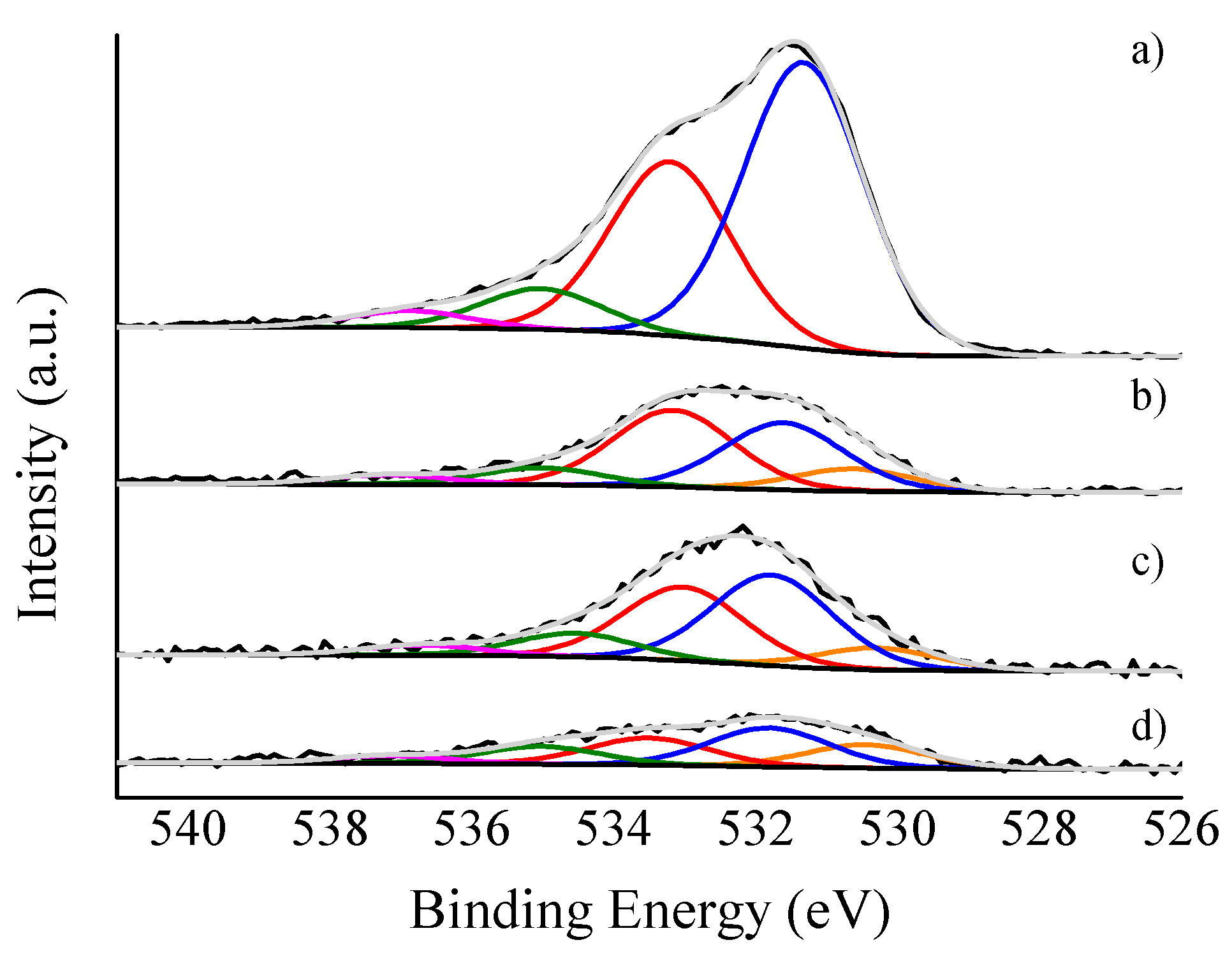
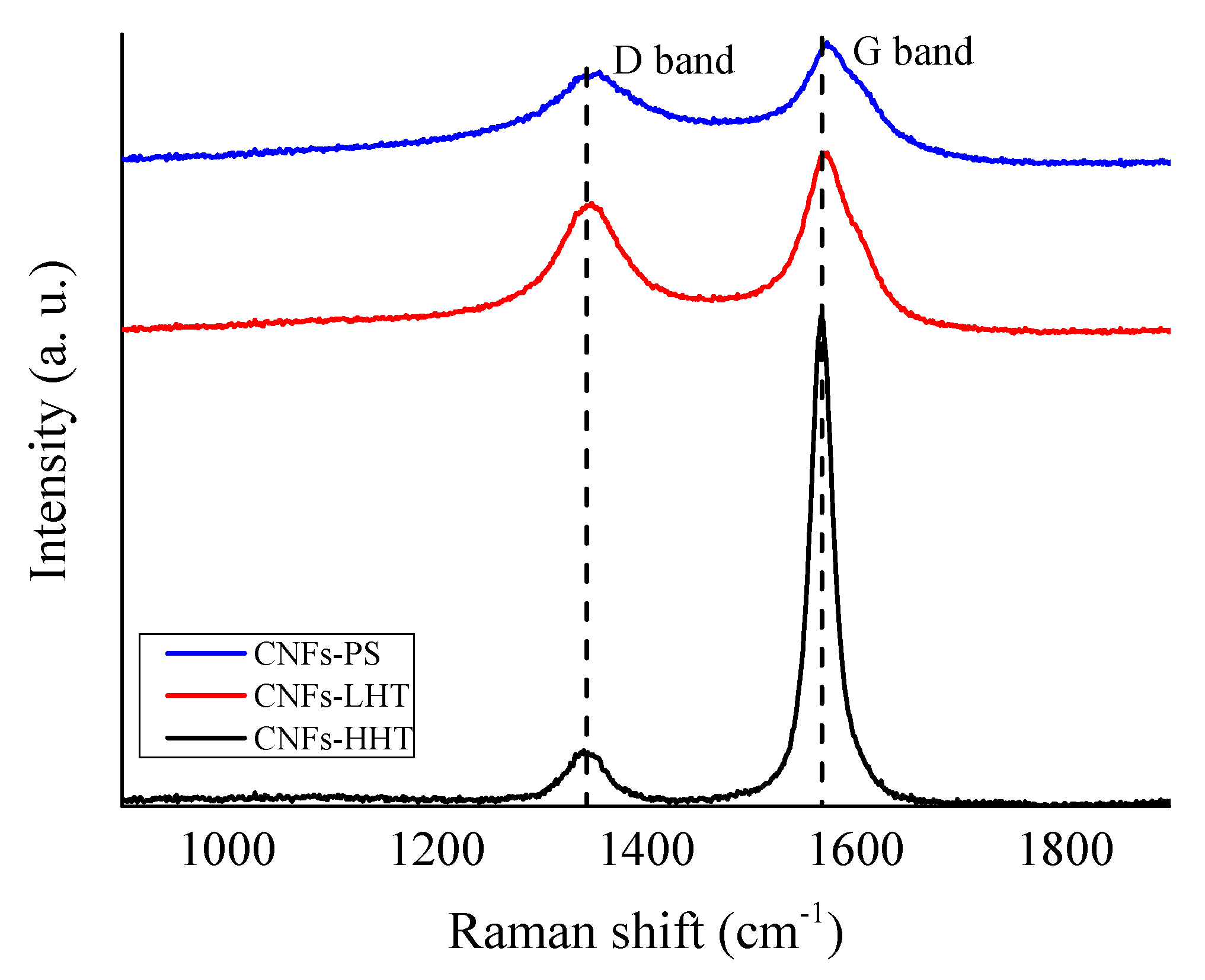
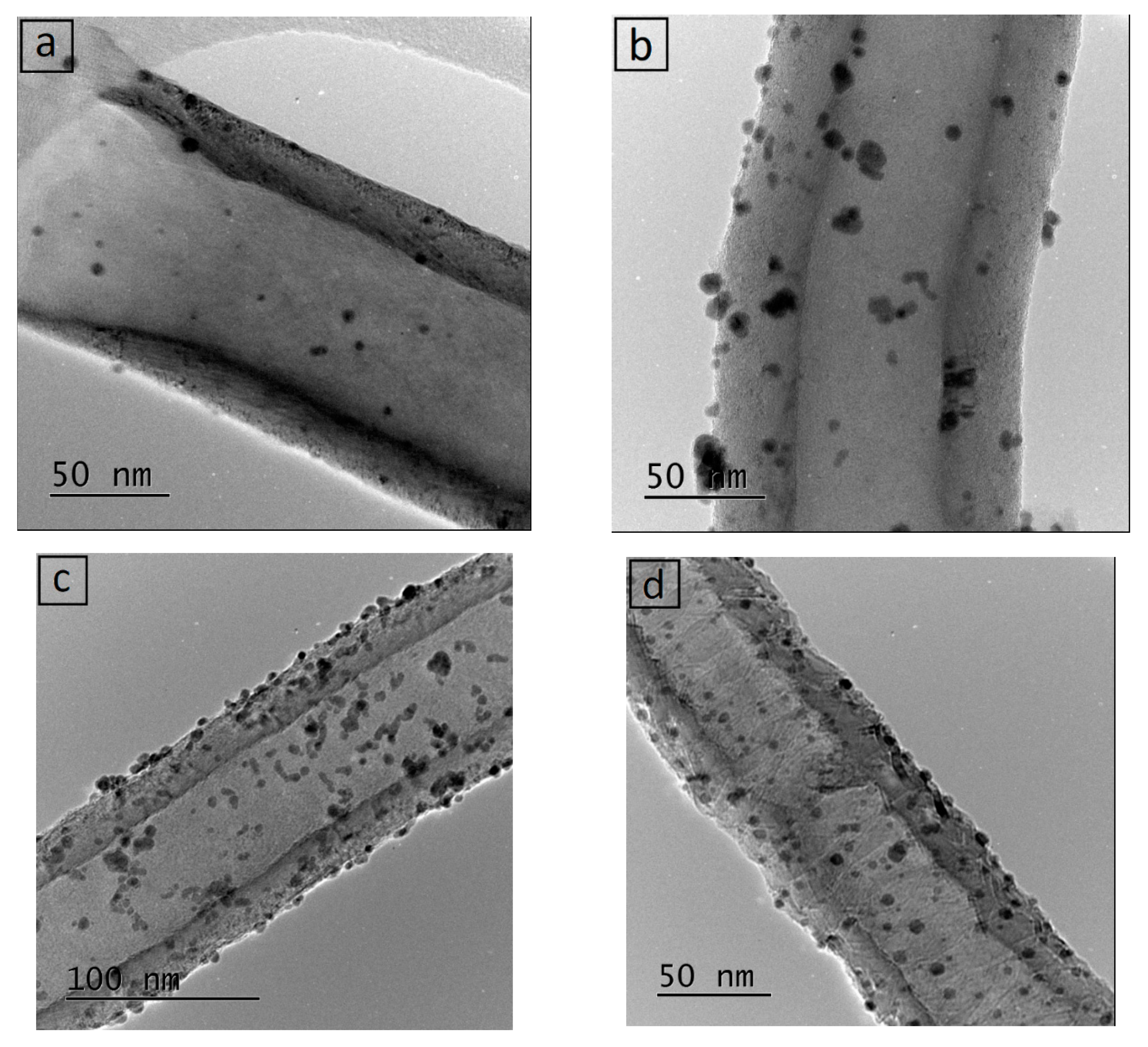
| Catalyst | C sp2 (%) | C sp3 (%) | sp2/sp3 | ID/IG |
|---|---|---|---|---|
| CNFs-PS | n.a. | n.a. | n.a. | 0.75 |
| CNFs-LHT | n.a. | n.a. | n.a. | 0.71 |
| CNFs-HHT | n.a. | n.a. | n.a. | 0.11 |
| Pd/CNFs-PS-OX | 51.8 | 34.6 | 1.5 | n.a. |
| Pd/CNFs-PS | 66.7 | 22.4 | 3.0 | n.a. |
| Pd/CNFs-LHT | 72.0 | 17.4 | 4.1 | n.a. |
| Pd/CNFs-HHT | 82.1 | 7.5 | 10.9 | n.a. |
| Catalyst | Pd Content (at. %) | Relative Pd0 Content (%) | O Content (at. %) | Cl Content (at. %) |
|---|---|---|---|---|
| Pd/CNFs-PS-OX | 0.75 | 15.6 | 12.05 | 0.04 |
| Pd/CNFs-PS | 0.40 | 58.4 | 2.94 | 0.06 |
| Pd/CNFs-LHT | 0.57 | 59.1 | 2.60 | 0.04 |
| Pd/CNFs-HHT | 0.71 | 25.9 | 0.94 | - |
| Catalyst | Fresh | Used | |||
|---|---|---|---|---|---|
| Mean (nm) | Std. Dev. (nm) | PdSURF (%) | Mean (nm) | Std. Dev. (nm) | |
| Pd/CNFs-PS-OX | 5.8 | 1.1 | 20.7 | 5.7 | 2.4 |
| Pd/CNFs-PS | 6.9 | 1.8 | 17.6 | 6.8 | 2.0 |
| Pd/CNFs-LHT | 5.7 | 1.3 | 21.1 | 7.5 | 2.8 |
| Pd/CNFs-HHT | 5.4 | 0.9 | 22.1 | 5.9 | 2.3 |
| Catalyst | Pd Loading (wt%) |
|---|---|
| Pd/CNFs-PS-OX | 1.01 |
| Pd/CNFs-PS | 1.04 |
| Pd/CNFs-LHT | 1.03 |
| Pd/CNFs-HHT | 1.03 |
| Catalyst | Conversion (%) at 5 Min | TOFTOT (s−1) | TOFSURF (s−1) | Selectivity at 90% Conversion (%) | |||
|---|---|---|---|---|---|---|---|
| HCAL | HCOH | PPR | COH | ||||
| Pd/CNFs-PS-OX | 5 | 625 | 3015 | 80 | 3 | 15 | - |
| Pd/CNFs-PS | 2 | 375 | 2126 | 83 | 10 | 6 | - |
| Pd/CNFs-LHT | 8 | 1000 | 4747 | 76 | 22 | - | - |
| Pd/CNFs-HHT | 12 | 1500 | 6776 | 68 | 30 | 1 | - |
| Temperature (°C) | TOFTOT (s−1) | TOFSURF (s−1) | Selectivity at 90% Conversion (%) | |||
|---|---|---|---|---|---|---|
| HCAL | HCOH | PPR | COH | |||
| 25 | 1500 | 6776 | 68 | 30 | 1 | - |
| 60 | 12,500 | 56,467 | 58 | 33 | 2 | 6 |
| 100 | 24,320 | 109,862 | 41 | 44 | 3 | 10 |
| Catalyst | Conversion (%) at 5 Min | TOFTOT (s−1) | TOFSURF (s−1) | Selectivity at 90% Conversion (%) | |||
|---|---|---|---|---|---|---|---|
| CAL | HCOH | PPE | Others | ||||
| Pd/CNFs-PS-OX | 4 | 680 | 3280 | 52 | 12 | 24 | 12 |
| Pd/CNFs-PS | 2 | 440 | 2494 | 58 | 17 | 15 | 10 |
| Pd/CNFs-LHT | 5 | 720 | 3418 | 63 | 23 | 9 | 5 |
| Pd/CNFs-HHT | 9 | 1360 | 6144 | 70 | 20 | 9 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattaneo, S.; Sanchez Trujillo, F.J.; Dimitratos, N.; Villa, A. The Effect of Carbon Nanofibers Surface Properties in Hydrogenation and Dehydrogenation Reactions. Appl. Sci. 2019, 9, 5061. https://doi.org/10.3390/app9235061
Cattaneo S, Sanchez Trujillo FJ, Dimitratos N, Villa A. The Effect of Carbon Nanofibers Surface Properties in Hydrogenation and Dehydrogenation Reactions. Applied Sciences. 2019; 9(23):5061. https://doi.org/10.3390/app9235061
Chicago/Turabian StyleCattaneo, Stefano, Felipe J. Sanchez Trujillo, Nikolaos Dimitratos, and Alberto Villa. 2019. "The Effect of Carbon Nanofibers Surface Properties in Hydrogenation and Dehydrogenation Reactions" Applied Sciences 9, no. 23: 5061. https://doi.org/10.3390/app9235061
APA StyleCattaneo, S., Sanchez Trujillo, F. J., Dimitratos, N., & Villa, A. (2019). The Effect of Carbon Nanofibers Surface Properties in Hydrogenation and Dehydrogenation Reactions. Applied Sciences, 9(23), 5061. https://doi.org/10.3390/app9235061







