Low-Energy Strawberry Fruits of Joly Cultivar, the First Step Towards a Novel, Food-Based Solution for the Obese Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Moisture Content
2.3. Total Soluble Sugars and Carbohydrates
2.4. Glucose, Fructose and Sucrose
2.5. Total Proteins
2.6. Total Dietary Fibers
2.7. Total Lipids
2.8. Total Ash and Energy Value
2.9. Elemental Analysis
2.10. The Preparation of Polyphenolic Extracts of Selected Strawberry Breeds
2.11. Total Phenolic Content (TPC) of the Strawberry Breed Selected
2.12. Total Anthocyanin Content (TAC) of the Strawberry Breed Selected
2.13. HPMC Assay
2.14. DPPH Assay
2.15. Anti-α Glucosidase Activity
2.16. Statistical Analysis
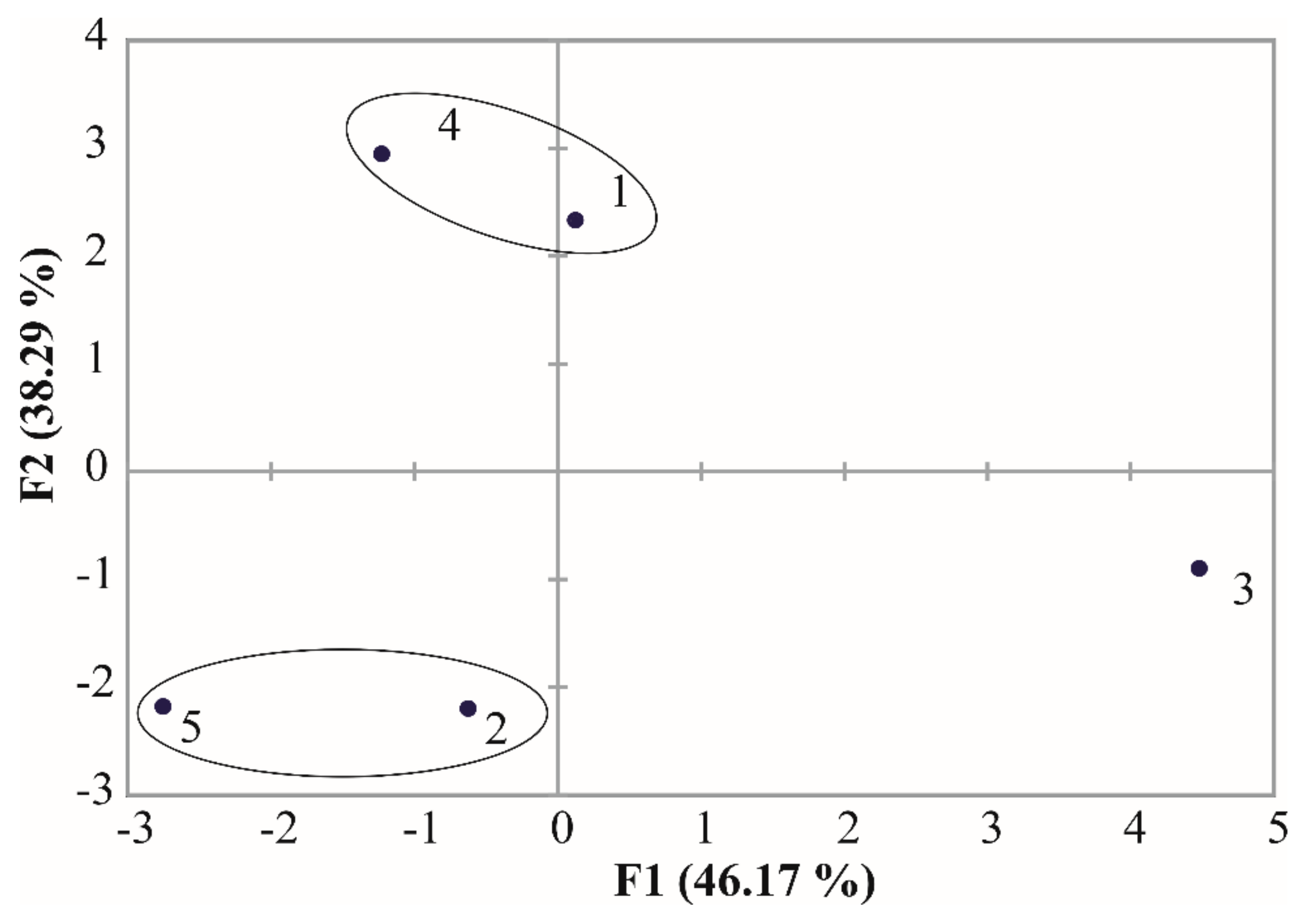
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 September 2019).
- Drewnowski, A. Obesity and the food environment: Dietary energy density and diet costs. Am. J. Prev. Med. 2004, 27, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Bechthold, A. A food energy density and body weight. Ernahr. Umsch. 2014, 6, 2–11. [Google Scholar]
- Ello-Martin, J.A.; Ledikwe, J.H.; Rolls, B.J. The influence of food portion size and energy density on energy intake: Implications for weight management. Am. J. Clin. Nutr. 2005, 82, 236–241. [Google Scholar] [CrossRef]
- Stelmach-Mardas, M.; Mardas, M.; Walkowiak, J.; Boeing, H. Long-term weight status in regainers after weight loss by lifestyle intervention: Status and challenges. Proc. Nutr. Soc. 2014, 73, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Stelmach-Mardas, M.; Mardas, M.; Warchoł, W.; Jamka, M.; Walkowiak, J. Successful maintenance of body weight reduction after individualized dietary counseling in obese subjects. Sci. Rep. 2014, 4, 6620. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Almiron-Roig, E.; Marmonier, C.; Lluch, A. Dietary energy density and body weight: Is there a relationship? Nutr. Rev. 2004, 62, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Hegedűs, A.; Stefanovits-Bányai, É. Application of and correlation among antioxidant and antiradical assays for characterizing antioxidant capacity of berries. Sci. Hortic. 2010, 125, 332–336. [Google Scholar] [CrossRef]
- Celikel, G.; Demirsoy, L.; Demirsoy, H. The strawberry tree (Arbutus unedo L.) selection in Turkey. Sci. Hortic. 2008, 118, 115–119. [Google Scholar] [CrossRef]
- Chaves, V.C.; Calvete, E.; Reginatto, F.H. Quality properties and antioxidant activity of seven strawberry (Fragaria x ananassa duch) cultivars. Sci. Hortic. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Čakar, U.; Grozdanić, N.; Pejin, B.; Vasić, V.; Čakar, M.; Petrović, A.; Djordjević, B. Impact of vinification procedure on fruit wine inhibitory activity against α-glucosidase. Food Biosci. 2018, 25, 1–7. [Google Scholar] [CrossRef]
- Mandave, P.C.; Pawar, P.K.; Ranjekar, P.K.; Mantri, N.; Kuvalekar, A.A. Comprehensive evaluation of in vitro antioxidant activity, total phenols and chemical profiles of two commercially important strawberry varieties. Sci. Hortic. 2014, 172, 124–134. [Google Scholar] [CrossRef]
- Parra-Palma, C.; Úbeda, C.; Gil, M.; Ramos, P.; Castro, R.I.; Morales-Quintana, L. Comparative study of the volatile organic compounds of four strawberry cultivars and it relation to alcohol acyltransferase enzymatic activity. Sci. Hortic. 2019, 251, 65–72. [Google Scholar] [CrossRef]
- Simonovic, M.; Kojic, V.; Jakimov, D.; Glumac, M.; Pejin, B. Raspberry seeds extract selectively inhibits the growth of human lung cancer cells in vitro. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Zhang, J.; Zhao, R.; Dai, H.; Zhang, Z. Application of vermicompost improves strawberry growth and quality through increased photosynthesis rate, free radical scavenging and soil enzymatic activity. Sci. Hortic. 2018, 233, 132–140. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Ren, J.; Gu, P.; Li, T.; Wu, Y.; Zhang, B. Comparison on evolution of volatile compounds and aroma attributes in different pH adjusted fermented bog bilberry syrup wines during bottle-aging period. Food Biosci. 2018, 22, 121–128. [Google Scholar] [CrossRef]
- Galetto, C.D.; Verdini, R.A.; Zorrilla, S.E.; Rubiolo, A.C. Freezing of strawberries by immersion in CaCl2 solutions. Food Chem. 2010, 123, 243–248. [Google Scholar] [CrossRef]
- Roos, Y.H. Thermal analysis, state transitions and food quality. J. Therm. Anal. Calorim. 2003, 71, 197–203. [Google Scholar] [CrossRef]
- FAO Food and Nutrition Paper 77. In Proceedings of the Report of a Technical Workshop, Rome, Italy, 3–6 December 2002.
- Hall, M.B.; Hoover, W.H.; Jennings, J.P.; Webster, T.K.M. A method for partitioning neutral detergent-soluble carbohydrates. J. Sci. Food Agric. 1999, 79, 2079–2086. [Google Scholar] [CrossRef]
- Steegmans, M.; Iliaens, S.; Hoebregs, H. Enzymatic, spectrophotometric determination of glucose, fructose, sucrose, and inulin/oligofructose in foods. J. AOAC Int. 2004, 87, 1200–1207. [Google Scholar]
- Warburg, O.; Christian, W. Isolierung und kristallisation des garungsferments enolase. Naturwissenschaften 1941, 29, 589–590. [Google Scholar] [CrossRef]
- Phillips, K.M.; Tarragó-Trani, M.T.; Grove, T.M.; Grün, I.; Lugogo, R.; Harris, R.F.; Stewart, K.K. Simplified gravimetric determination of total fat in food composites after chloroform-methanol extraction. J. Am. Oil Chem. Soc. 1997, 74, 137–142. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Oxford, UK, 1994. [Google Scholar]
- Matić, I.; Žižak, Ž.; Simonović, M.; Simonović, B.; Gođevac, D.; Šavikin, K.; Juranić, Z. Cytotoxic effect of wine polyphenolic extracts and resveratrol against human carcinoma cells and normal peripheral blood mononuclear cells. J. Med. Food 2010, 13, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Sužnjević, D.Ž.; Pastor, F.T.; Gorjanović, S.Ž. Polarographic study of hydrogen peroxide anodic current and its application to antioxidant activity determination. Talanta 2011, 85, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- McCue, P.; Kwon, Y.I.; Shetty, K. Anti-amylase, anti-glucosidase and anti angiotensin I converting enzyme potential of selected foods. J. Food Biochem. 2005, 29, 278–294. [Google Scholar] [CrossRef]
- Sójka, M.; Klimczak, E.; Macierzyński, J.; Kołodziejczyk, K. Nutrient and polyphenolic composition of industrial strawberry press cake. Eur. Food Res. Technol. 2013, 237, 995–1007. [Google Scholar] [CrossRef]
- Blasco, A.J.; Crevillén, A.G.; González, M.C.; Escarpa, A. Direct electrochemical sensing and detection of natural antioxidants and antioxidant capacity in vitro systems. Electroanalysis 2007, 19, 2275–2286. [Google Scholar] [CrossRef]
- Janjušević, L.; Pejin, B.; Kaišarević, S.; Gorjanović, S.; Pastor, F.; Tešanović, K.; Karaman, M. Trametes versicolor ethanol extract, a promising candidate for health-promoting food supplement. Nat. Prod. Res. 2018, 32, 963–967. [Google Scholar] [CrossRef]
- Karaman, M.; Tesanovic, K.; Gorjanovic, S.; Pastor, F.T.; Simonovic, M.; Glumac, M.; Pejin, B. Polarography as a technique of choice for the evaluation of total antioxiant activity: The case study of selected Coprinus comatus extracts and quinic acid, their antidiabetic ingredient. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef]
- Simonovic, M.; Ostojic, S.; Micic, D.; Pejin, B. Low sugar jellies of berry fruits: The impact of low vs. high temperature regime on their chemical composition and antioxidativity. Nat. Prod. Res. 2019, in press. [Google Scholar] [CrossRef]
- Simonovic, M.; Simonovic, B.R.; Ostojic, S.; Pezo, L.; Micic, D.; Stanisavljevic, N.; Pejin, B. A contribution to the estimation of berry fruits quality. Sci. Hortic. 2019, 258, 108776. [Google Scholar] [CrossRef]
- Cakar, U.; Grozdanic, N.; Petrovic, A.; Pejin, B.; Nastasijevic, B.; Markovic, B.; Dordevic, B. Fruit wines inhibitory activity against α-glucosidase. Curr. Pharm. Biotechnol. 2017, 18, 1264–1272. [Google Scholar] [CrossRef]
- Dimitrić Marković, J.M.; Pejin, B.; Milenković, D.; Amić, D.; Begović, N.; Mojović, M.; Marković, Z.S. Antiradical activity of delphinidin, pelargonidin and malvin towards hydroxyl and nitric oxide radicals: The energy requirements calculations as a prediction of the possible antiradical mechanisms. Food Chem. 2017, 218, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Potipiranun, T.; Worawalai, W.; Phuwapraisirisan, P. Lamesticumin G, a new α-glucosidase inhibitor from the fruit peels of Lansium parasiticum. Nat. Prod. Res. 2018, 32, 1881–1886. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, R.; Kusuma, I.W.; Amirta, R.; Worawalai, W.; Phuwapraisirisan, P. A new 4–arylflavan from the pericarps of Horsfieldia motleyi displaying dual inhibition against α-glucosidase and free radicals. Nat. Prod. Res. 2018, 32, 2676–2682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, Y.S.; Cui, H.H.; Yin, Y.Q.; Pan, J.T.; Yu, B.W. Three new resin glycosides compounds from Argyreia acuta and their α-glucosidase inhibitory activity. Nat. Prod. Res. 2017, 31, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Braunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Bolen, S.; Feldman, L.; Vassy, J.; Wilson, L.; Yeh, H.C.; Marinopoulos, S.; Brancati, F.L. Systematic review: Comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann. Intern. Med. 2007, 147, 386–399. [Google Scholar] [CrossRef]
- Sarkar, D.; Orwat, J.; Hurburt, T.; Woods, F.; Pitts, J.A.; Shetty, K. Evaluation of phenolic bioactive-linked functionality of blackberry cultivars targeting dietary management of early stages type-2 diabetes using in vitro models. Sci. Hortic. 2016, 212, 193–202. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Hogan, S.; Chung, H.; Welbaum, G.E.; Zhou, K. Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic composition. Food Chem. 2010, 119, 592–599. [Google Scholar] [CrossRef]
- Yao, Y.; Sang, W.; Zhou, M.; Ren, G. Antioxidant and alpha-glucosidase inhibitory activity of colored grains in China. J. Agric. Food. Chem. 2010, 58, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Lemos, G.; Lia Barbieri, R.; Franzon, R.C.; Vizzotto, M. In vitro assessment of the antihyperglycemic and antioxidant properties of araçá, butiá and pitanga. Food Biosci. 2017, 19, 92–100. [Google Scholar]
- Moraga, G.; Talens, P.; Moraga, M.J.; Martínez-Navarrete, N. Implication of water activity and glass transition on the mechanical and optical properties of freeze-dried apple and banana slices. J. Food Eng. 2011, 106, 212–219. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R.; Nambi, V.E.; Gupta, R.K. Postharvest changes in antioxidant capacity, enzymatic activity, and microbial profile of strawberry fruits treated with enzymatic and divalent ions. Food Bioprocess Technol. 2014, 7, 2060–2070. [Google Scholar] [CrossRef]
| Parameters | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Total soluble sugars (g/100 g) | 7.20 ± 0.60 ab | 5.60 ± 0.70 ab | 6.60 ± 0.80 ab | 7.4 0 ± 0.80 a | 5.40 ± 0.80 b |
| Carbohydrates (g/100 g) | 7.70 ± 0.70 ab | 6.20 ± 0.90 ab | 7.30 ± 0.80 ab | 8.10 ± 0.60 a | 5.90 ± 0.70 b |
| Glucose (g/100 g) | 2.60 ± 0.28 a | 2.10 ± 0.31 a | 2.50 ± 0.28 a | 2.70 ± 0.30 a | 2.00 ± 0.28 a |
| Fructose (g/100 g) | 3.00 ± 0.39 a | 2.50 ± 0.38 a | 2.90 ± 0.38 a | 3.20 ± 0.39 a | 2.40 ± 0.39 a |
| Sucrose (g/100 g) | 1.60 ± 0.20 a | 1.00 ± 0.19 b | 1.20 ± 0.14 ab | 1.50 ± 0.16 a | 1.00 ± 0.13 b |
| Total dietary fibers (g/100 g) | 2.09 ± 0.23 a | 2.36 ± 0.26 a | 2.44 ± 0.23 a | 2.14 ± 0.25 a | 1.85 ± 0.22 a |
| Total lipids (g/100 g) | 0.20 ± 0.02 c | 0.30 ± 0.02 b | 0.20 ± 0.02 c | 0.30 ± 0.02 b | 0.40 ± 0.02 a |
| Total proteins (g/100 g) | 0.40 ± 0.03 b | 0.40 ± 0.02 b | 0.40 ± 0.02 b | 0.40 ± 0.02 b | 0.50 ± 0.03 a |
| Water (g/100 g) | 90.50 ± 7.20 a | 90.40 ± 7.20 a | 91.00 ± 7.30 a | 89.80 ± 7.30 a | 90.10 ± 7.30 a |
| Ash (g/100 g) | 0.30 ± 0.02 a | 0.30 ± 0.03 a | 0.30 ± 0.03 a | 0.30 ± 0.03 a | 0.30 ± 0.02 a |
| Energy value (kJ/100 g) | 142 ± 5 ab | 143 ± 4 ab | 130 ± 6 b | 155 ± 5 a | 138 ± 5 b |
| Potassium (mg/kg) | 1246 ± 94 b | 1283 ± 98 b | 2011 ± 126 a | 1243 ± 124 b | 1157 ± 94 b |
| Sodium (mg/kg) | 23.30 ± 2.60 a | <10 | 13.50 ± 2.80 b | <10 | <10 |
| Magnesium (mg/kg) | 129 ± 14 b | 137 ± 17 b | 202 ± 16 a | 129 ± 15 b | 124 ± 16 b |
| Calcium (mg/kg) | 204 ± 25 b | 243 ± 22 b | 332 ± 25 a | 205 ± 30 b | 252 ± 29 b |
| Phosphorus (mg/kg) | 31.0 ± 3.2 a | 27.3 ± 3.2 a | 29.5 ± 3.0 a | 33.8 ± 3.1 a | 32.3 ± 3.0 a |
| Zinc (mg/kg) | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 |
| Copper (mg/kg) | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 |
| Iron (mg/kg) | 2.48 ± 0.25 b | 2.30 ± 0.26 b | 3.21 ± 0.29 a | 2.49 ± 0.27 b | 2.21 ± 0.24 b |
| Manganese (mg/kg) | 2.02 ± 0.29 b | 2.60 ± 0.31 b | 3.35 ± 0.21 a | 2.31 ± 0.28 b | 2.10 ± 0.29 b |
| Lead (mg/kg) | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Cadmium (mg/kg) | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
| Mercury (mg/kg) | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 |
| Arsenic (mg/kg) | <0.10 | <0.10 | <0.10 | <0.10 | <0.10 |
| Parameters | PC1 | PC2 |
|---|---|---|
| Total dietary fibres | 0.7865 | −0.1029 |
| Potassium | 0.9562 | −0.1682 |
| Phosphorus | −0.4186 | 0.5994 |
| Glucose | 0.4111 | 0.9052 |
| Fructose | 0.3442 | 0.9191 |
| Calcium | 0.7333 | −0.5861 |
| Sucrose | 0.1173 | 0.9734 |
| Iron | 0.9597 | 0.0912 |
| Magnesium | 0.9461 | −0.2241 |
| Manganese | 0.8588 | −0.3366 |
| Water | 0.8702 | −0.3188 |
| Sodium | 0.5468 | 0.3992 |
| Carbohydrates | 0.3480 | 0.9284 |
| Total soluble sugars | 0.3050 | 0.9511 |
| Total lipids | −0.8086 | −0.4356 |
| Energy value | −0.6016 | 0.6195 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonovic, M.; Ostojic, S.; Micic, D.; Pesakovic, M.; Pejin, B. Low-Energy Strawberry Fruits of Joly Cultivar, the First Step Towards a Novel, Food-Based Solution for the Obese Population. Appl. Sci. 2019, 9, 5140. https://doi.org/10.3390/app9235140
Simonovic M, Ostojic S, Micic D, Pesakovic M, Pejin B. Low-Energy Strawberry Fruits of Joly Cultivar, the First Step Towards a Novel, Food-Based Solution for the Obese Population. Applied Sciences. 2019; 9(23):5140. https://doi.org/10.3390/app9235140
Chicago/Turabian StyleSimonovic, Mladen, Sanja Ostojic, Darko Micic, Marijana Pesakovic, and Boris Pejin. 2019. "Low-Energy Strawberry Fruits of Joly Cultivar, the First Step Towards a Novel, Food-Based Solution for the Obese Population" Applied Sciences 9, no. 23: 5140. https://doi.org/10.3390/app9235140
APA StyleSimonovic, M., Ostojic, S., Micic, D., Pesakovic, M., & Pejin, B. (2019). Low-Energy Strawberry Fruits of Joly Cultivar, the First Step Towards a Novel, Food-Based Solution for the Obese Population. Applied Sciences, 9(23), 5140. https://doi.org/10.3390/app9235140





