A Neuro-Prosthetic Device for Substituting Sensory Functions during Stance Phase of the Gait
Abstract
:1. Introduction
1.1. A Background on Functional Electrical Stimulation Devices and Application of Sensors in Gait Event Detection and Control
1.2. Innovation and Motivation for Current Study
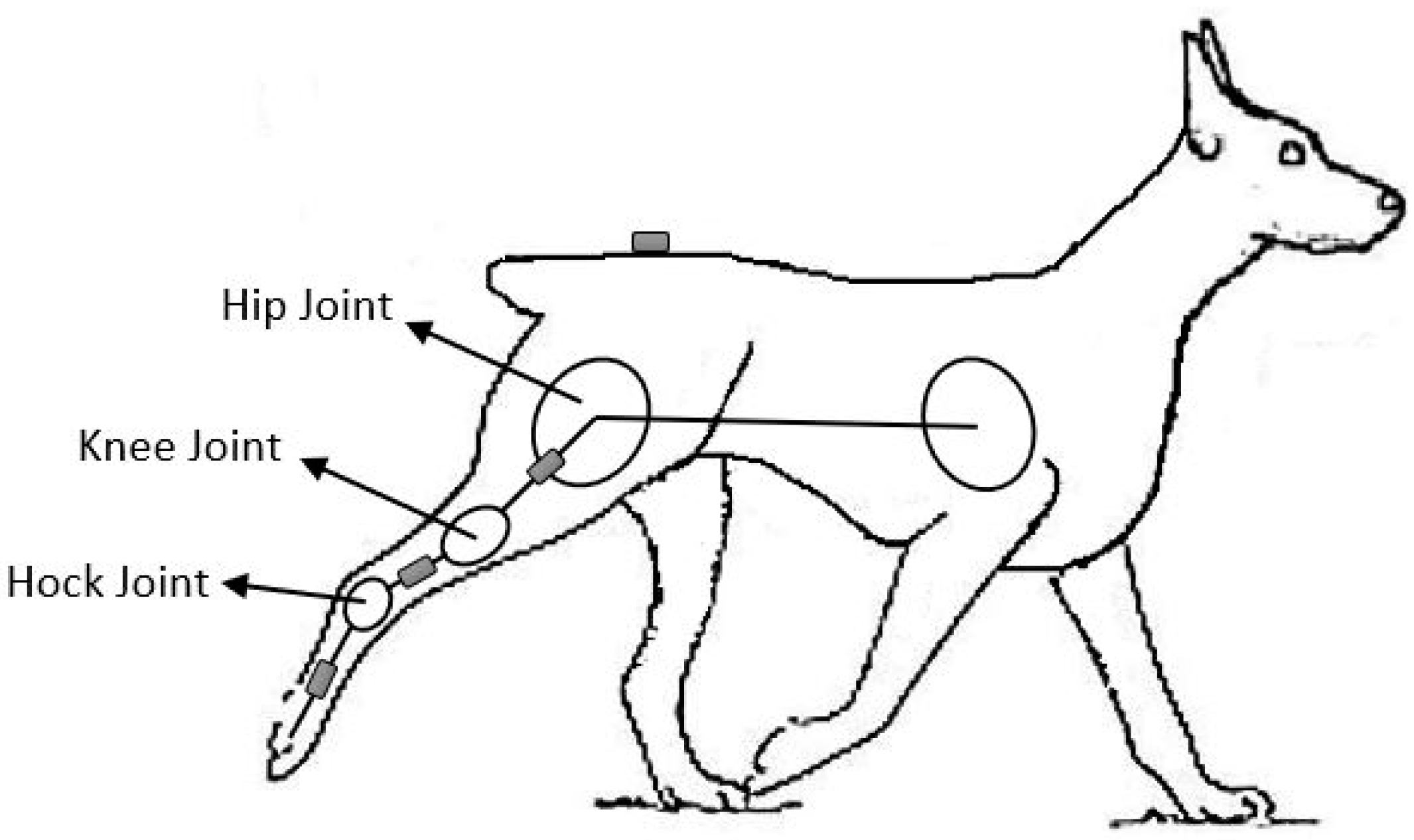
2. Materials and Methods
2.1. Hardware Set-Up
2.2. Function 1: Stance/Swing Phase Recognition
2.3. Function 2: Recognition of Body Tilt Direction
2.4. Function 3: Software Programing and Balancing Strategies
Balancing Strategies
3. Results and Discussion
3.1. Stance/Swing Phase Recognition
3.2. Results of Balancing Strategies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Besson, T.; Debayle, D.; Diochot, S.; Salinas, M.; Lingueglia, E. Low cost venom extractor based on arduino® board for electrical venom extraction from arthropods and other small animals. Toxicon 2016, 118, 156–161. [Google Scholar] [CrossRef] [PubMed]
- D’Ausilio, A. Arduino: A low-cost multipurpose lab equipment. Behav. Res. Methods 2012, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kornuta, J.A.; Nipper, M.E.; Dixon, J.B. Low-cost microcontroller platform for studying lymphatic biomechanics in vitro. J. Biomech. 2013, 46, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Bures, J. Electrophysiological Methods in Biological Research; Academic Press: New York, NY, USA, 1960. [Google Scholar]
- Melo, P.L.; Silva, M.T.; Martins, J.M.; Newman, D.J. Technical developments of functional electrical stimulation to correct drop foot: Sensing, actuation and control strategies. Clin. Biomech. 2015, 30, 101–113. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Müller, G.R.; Pfurtscheller, J.; Gerner, H.J.; Rupp, R. ‘Thought’—Control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci. Lett. 2003, 351, 33–36. [Google Scholar] [CrossRef]
- Davis, G.M.; Hamzaid, N.A.; Hasnan, N. Functional electrical stimulation in clinical applications: Fitness and cardiovascular health. In Proceedings of the 2014 IEEE 19th International Functional Electrical Stimulation Society Annual Conference (IFESS), Kuala Lumpur, Malaysia, 17–19 September 2014; pp. 1–4. [Google Scholar]
- Yang, C.C.; Hsu, Y.L. A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 2010, 10, 7772–7788. [Google Scholar] [CrossRef]
- Kesar, T.M.; Perumal, R.; Jancosko, A.; Reisman, D.S.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Novel patterns of functional electrical stimulation have an immediate e_ect on dorsiflexor muscle function during gait for people poststroke. Phys. Ther. 2010, 90, 55–66. [Google Scholar] [CrossRef]
- Kesar, T.M.; Perumal, R.; Reisman, D.S.; Jancosko, A.; Rudolph, K.S.; Higginson, J.S.; Binder-Macleod, S.A. Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: E_ects on poststroke gait. Stroke 2009, 40, 3821–3827. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, H.; Yan, T.; Jin, D.; He, X.; Zheng, X.; Xu, S.; Tan, C. The e_ectiveness of functional electrical stimulation based on a normal gait pattern on subjects with early stroke: A randomized controlled trial. BioMed Res. Int. 2014, 2014, 545408. [Google Scholar] [CrossRef]
- Burridge, J.H.; Taylor, P.N.; Hagan, S.A.; Wood, D.E.; Swain, I.D. The effects of common peroneal stimulation on the effort and speed walking: A randomized controlled trial with chronic hemiplegic patients. Clin. Rehabil. 1997, 11, 201–210. [Google Scholar] [CrossRef]
- Kottink, A.I.R.; Oostendorp, L.J.M.; Buurke, J.H.; Nene, A.V.; Hermens, H.J.; IJzerman, M.J. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patient with a dropped foot: A systematic review. Artif. Organs 2004, 28, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Luzio de Melo, P.; da Silva, M.T.; Martins, J.; Newman, D. A Microcontroller Platform for the Rapid Prototyping of Functional Electrical Stimulation-Based Gait Neuroprostheses. Artif. Organs 2015, 39, E56–E66. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, C.C.; van Riel, W.J.; Veltink, P.H. Control of triceps surae stimulation based on shank orientation using a uniaxial gyroscope during gait. Med. Biol. Eng. Comput. 2009, 47, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Zahradka, N.; Behboodi, A.; Wright, H.; Bodt, B.; Lee, S. Evaluation of Gait Phase Detection Delay Compensation Strategies to Control a Gyroscope-Controlled Functional Electrical Stimulation System During Walking. Sensors 2019, 19, 2471. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, N.; Luecke, G.R.; Jeffery, N. A bionic test-bed for sensing and balance augmentation in biological applications. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition(IMECE), Phoenix, AZ, USA, 11–17 November 2016. [Google Scholar]
- VICON. Available online: https://www.vicon.com/ (accessed on 26 November 2019).
- Taghavi, N.; Luecke, G.R.; Jeffery, N. A wearable body controlling device for application of functional electrical stimulation. Sensors 2018, 18, 1251. [Google Scholar] [CrossRef]
- Teensy. Available online: https://www.pjrc.com/teensy/ (accessed on 26 November 2019).
- Von Holst, E. Relations between the central nervous system and the peripheral organs. Br. J. Anim. Behav. 1954, 2, 89–94. [Google Scholar] [CrossRef]
- Edwards, F.A.; Gibb, A.J.; Colquhoun, D. ATP receptor-mediated synaptic currents in the central nervous system. Nature 1992, 59, 144–147. [Google Scholar] [CrossRef]
- Taghavi, N.; Luecke, G.R.; Jeffery, N.D. A Review on Electromechanical Methods and Devices for Neural Rehabilitation. Evol. Mech. Eng. 2018, 1, 1–4. [Google Scholar]
- Kraft, G.H.; Fitts, S.S.; Hammond, M.C. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch. Phys. Med. Rehabil. 1992, 73, 220–227. [Google Scholar]
- Nudo, R.J. Remodeling of cortical motor representations after stroke: Implications for recovery from brain damage. Mol. Psychiatry 1997, 2, 188–191. [Google Scholar] [CrossRef]
- Taub, E. Constraint-induced movement therapy and massed practice. Stroke 2000, 31, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.; Wall, P.D. Acupuncture and transcutaneous electrical nerve stimulation. Postgrad. Med. J. 1984, 60, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Duchenne, G.B. Physiology of Motion Demonstrated by Means of Electrical Stimulation and Clinical Observation and Applied to the Study of Paralysis and Deformities; Kaplan, E., Ed.; BWB Saunders: Philadelphia, PA, USA, 1959. [Google Scholar]
- Electroschematics. Available online: https://www.electroschematics.com/2083/electronic-muscle-stimulator/ (accessed on 26 November 2019).
- Taghavi, N.; Nahvi, H. Pull-in instability of cantilever and fixed-fixed nano-switches. Eur. J Mech. Solids 2013, 41, 123–133. [Google Scholar] [CrossRef]
- Taghavi, N.; Nahvi, H. Stability analysis of arch shape carbon nanotubes modeled by nonlocal elasticity theory. J. Comput. Theor. Nanosci. 2013, 10, 719–727. [Google Scholar] [CrossRef]
- Abbasi-Shirsavar, M.; Baghani, M.; Taghavimehr, M.; Golzar, M.; Nikzad, M.; Ansari, M.; George, D. Anexperimental-numerical study on shape memory behavior of PU/PCL/ZnO ternary blend. J. Intell. Mater. Syst. Struct. 2019, 30, 116–126. [Google Scholar] [CrossRef]
- Ansari, M.; Golzar, M.; Baghani, M.; AbbasiShirsavar, M.; Taghavimehr, M. Force recovery evaluation of thermo-induced shape-memory polymer stent: Material, process and thermo-viscoelastic characterization. Smart Mater. Struct. 2019, 28, 095022. [Google Scholar] [CrossRef]
- Pololu TB6612FNG Dual Motor Driver Carrier. Available online: https://www.pololu.com/product/713 (accessed on 26 November 2019).
- Starlino_DCM_Tutorial. Available online: http://www.starlino.com/dcm_tutorial.html (accessed on 12 October 2019).
- Pololu MinIMU-9 v5. Available online: https://www.pololu.com/product/2738/resources (accessed on 12 October 2019).
- Pololu AltIMU-10 v5. Available online: https://www.pololu.com/product/2739 (accessed on 12 October 2019).



























| Teensy Pin Number | IMU #1 Pin | IMU #2 Pin | IMU #3 Pin | IMU #4 Pin | Pot #1 Pin | Pot #2 Pin | Pot #3 Pin | Carrier #1 Pin | Carrier #2 Pin |
|---|---|---|---|---|---|---|---|---|---|
| 1 | AIN1 | ||||||||
| 2 | AIN2 | ||||||||
| 3 | PWMA | ||||||||
| 4 | PWMA | ||||||||
| 5 | PWMB | ||||||||
| 8 | AIN2 | ||||||||
| 9 | AIN1 | ||||||||
| 10 | STBY | STBY | |||||||
| 11 | BIN1 | ||||||||
| 12 | BIN2 | ||||||||
| 18 | SDA | SDA | |||||||
| 19 | SCL | SCL | |||||||
| 25 | PWMB | ||||||||
| 26 | BIN1 | ||||||||
| 27 | BIN2 | ||||||||
| 29 | SCL | SCL | |||||||
| 30 | SDA | SDA | |||||||
| A0 | WIPER PIN | ||||||||
| A1 | WIPER PIN | ||||||||
| A3 | WIPER PIN | ||||||||
| 3.3V | VIN | VIN | VIN | VIN | VIN | VIN | VIN | ||
| GND | GND | GND | GND | GND | GND | GND | GND |
| Teensy Pin Number | Pot #1 Pin | Pot #2 Pin | Pot #3 Pin | Carrier #1 Pin | Carrier #2 Pin |
|---|---|---|---|---|---|
| 0 | AIN1 | ||||
| 5 | PWMA | ||||
| 6 | PWMA | ||||
| 7 | AIN2 | ||||
| 10 | STBY | STBY | |||
| 11 | AIN1 | ||||
| 12 | AIN2 | ||||
| 14 | BIN2 | ||||
| 20 | PWMB | ||||
| 21 | BIN1 | ||||
| 22 | WIPER PIN | ||||
| 23 | WIPER PIN | ||||
| A1 | WIPER PIN | ||||
| 3.3V | VIN | VIN | VIN | ||
| GND | GND | GND | GND |
| Strategy Number 1 |  |
| Initial joint angles: , , and Final joint angles after stimulation: , , and | |
| Strategy Number 2 |  |
| Initial joint angles: , , and Final joint angles after stimulation: , , and | |
| Strategy Number 3 |  |
| Initial joint angles: , , and Final joint angles after stimulation: , , and |
| Strategy Number 4 |  |
| Initial joint angles: , , and Final joint angles after stimulation: , , and | |
| Strategy Number 5 |  |
| Initial joint angles: , , and Final joint angles after stimulation: , , and |
| Strategy | Final Pelvis Orientation Error, Degree |
|---|---|
| Strategy #1 | 1.96 |
| Strategy #2 | 1.92 |
| Strategy #3 | 2.69 |
| Strategy #4 | 1.75 |
| Strategy #5 | 2.51 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taghavi, N.; Luecke, G.R.; Jeffery, N.D. A Neuro-Prosthetic Device for Substituting Sensory Functions during Stance Phase of the Gait. Appl. Sci. 2019, 9, 5144. https://doi.org/10.3390/app9235144
Taghavi N, Luecke GR, Jeffery ND. A Neuro-Prosthetic Device for Substituting Sensory Functions during Stance Phase of the Gait. Applied Sciences. 2019; 9(23):5144. https://doi.org/10.3390/app9235144
Chicago/Turabian StyleTaghavi, Nazita, Greg R. Luecke, and Nicholas D. Jeffery. 2019. "A Neuro-Prosthetic Device for Substituting Sensory Functions during Stance Phase of the Gait" Applied Sciences 9, no. 23: 5144. https://doi.org/10.3390/app9235144
APA StyleTaghavi, N., Luecke, G. R., & Jeffery, N. D. (2019). A Neuro-Prosthetic Device for Substituting Sensory Functions during Stance Phase of the Gait. Applied Sciences, 9(23), 5144. https://doi.org/10.3390/app9235144





