On-Line Tendency Control of Dissolved Oxygen Concentration during Aerobic Fed-Batch Fermentations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corynebacterium Glutamicum Fermentation
2.1.1. Fermentation Conditions
2.1.2. Analytical Methods
2.2. Pichia pastoris Fermentation
2.2.1. Microorganism and Fermentation Conditions
2.2.2. Analytical Methods
2.3. DO Control Model
2.3.1. DO Tendency Control Model
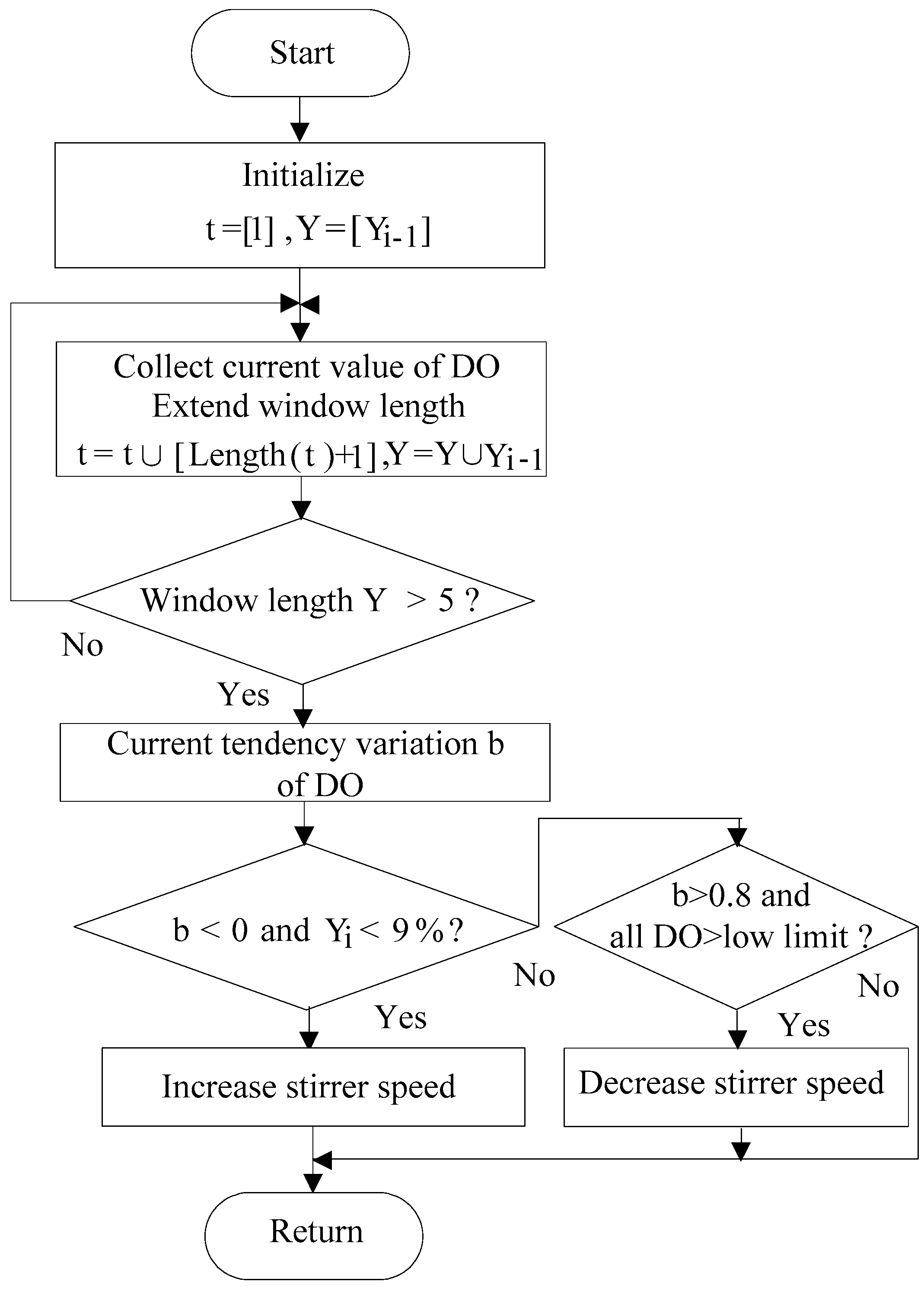
2.3.2. DO Control Algorithm
3. Results and Discussion
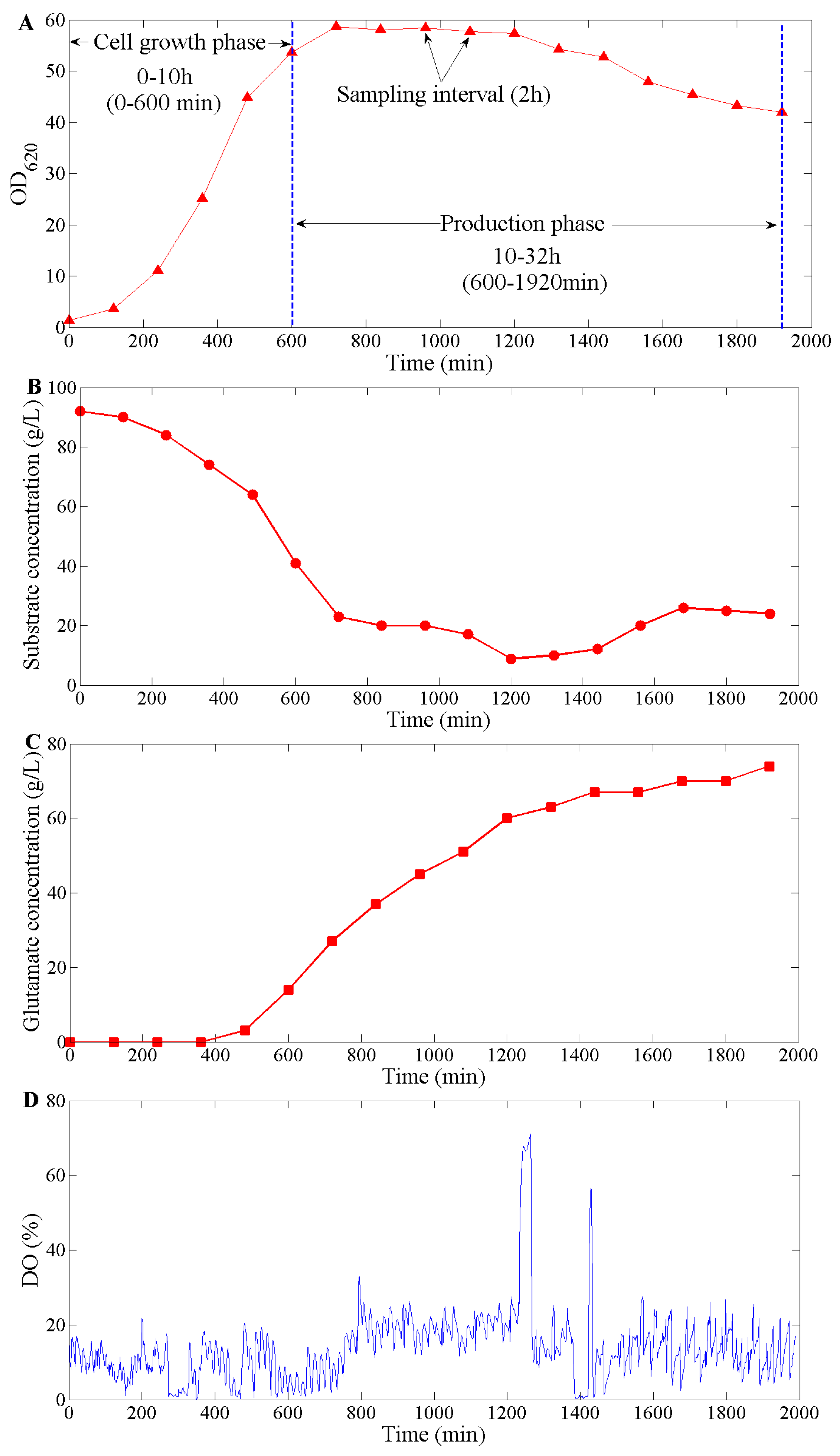
3.1. Performance during C. glutamicum Fermentation
3.1.1. DO Tendency Control in Cell Growth Phase
3.1.2. DO Tendency Control in Production Phase
3.2. Performance during P. pastoris Fermentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendes, C.E.; Badino, A.C. Oxygen transfer in different pneumatic bioreactors containing viscous Newtonian fluids. Chem. Eng. Res. Des. 2015, 94, 456–465. [Google Scholar] [CrossRef]
- Nuñez, S.; Garelli, F.; Battista, H.D. Decentralized control with minimum dissolved oxygen guaranties in aerobic Fed-Batch cultivations. Ind. Eng. Chem. Res. 2013, 52, 18014–18021. [Google Scholar] [CrossRef]
- Giefer, L.A.; Lütjen, M.; Rohde, A.K.; Freitag, M. Determination of the Optimal State of Dough Fermentation in Bread Production by Using Optical Sensors and Deep Learning. Appl. Sci. 2019, 9, 4266. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.Y.; Park, S.; Middelberg, A.P.J. Control of fed-batch fermentations. Biotechnol. Adv. 1999, 17, 29–48. [Google Scholar] [CrossRef]
- Ertunc, S.; Akay, B.; Boyacioglu, H.; Hapoglu, H. Self-tuning control of dissolved oxygen concentration in a batch bioreactor. Food Bioprod. Process. 2009, 87, 46–55. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Landi, C.; Cartenì, F.; de Alteriis, E.; Giannino, F.; Paciello, L.; Parascandola, P. A novel process-based model of microbial growth: Self-inhibition in Saccharomyces cerevisiae aerobic fed-batch cultures. Microb. Cell Fact. 2015, 14, 109. [Google Scholar] [CrossRef]
- Johnsson, O.; Sahlin, D.; Linde, J.; Lidén, G.; Hägglund, T. A mid-ranging control strategy for non-stationary processes and its application to dissolved oxygen control in a bioprocess. Control Eng. Pract. 2015, 42, 89–94. [Google Scholar] [CrossRef]
- Rocha-Valadez, J.A.; Albiter, V.; Caro, M.A.; Serrano-Carreón, L.; Galindo, E. A fermentation system designed to independently evaluate mixing and/or oxygen tension effects in microbial processes: Development, application and performance. Bioproc. Biosyst. Eng. 2007, 30, 115–122. [Google Scholar] [CrossRef]
- Wang, X.F.; Chen, J.D.; Liu, C.B.; Pan, F. Hybrid modeling of penicillin fermentation process based on least square support vector machine. Chem. Eng. Res. Des. 2010, 88, 415–420. [Google Scholar] [CrossRef]
- Kazemi, M.A.; Bamdad, H.; Papari, S.; Yaghmaei, S. Modeling and control of dissolved oxygen concentration in the fermentation of Glucose to Gluconic Acid. Period. Polytech.-Chem. 2013, 57, 63–70. [Google Scholar] [CrossRef]
- Ha, S.; Kim, H.M.; Chun, H.H.; Hwang, I.M.; Lee, J.-H.; Kim, J.-C.; Kim, I.S.; Park, H.W. Effect of Oxygen Supply on Surfactin Production and Sporulation in Submerged Culture of Bacillus subtilis Y9. Appl. Sci. 2018, 8, 1660. [Google Scholar] [CrossRef]
- Jin, H.; Liu, G.Q.; Ye, X.F.; Duan, Z.Y.; Li, Z.; Shi, Z.P. Enhanced porcine interferon-α production by recombinant Pichia pastoris with a combinational control strategy of low induction temperature and high dissolved oxygen concentration. Biochem. Eng. J. 2010, 52, 91–98. [Google Scholar] [CrossRef]
- Bodizs, L.; Titica, M.; Faria, N.; Srinivasan, B.; Dochain, D.; Bonvin, D. Oxygen control for an industrial pilot-scale fed-batch filamentous fungal fermentation. J. Process Control 2007, 17, 595–606. [Google Scholar] [CrossRef]
- Kuprijanov, A.; Gnoth, S.; Simutis, R.; Lübbert, A. Advanced control of dissolved oxygen concentration in fed batch cultures during recombinant protein production. Appl. Microbiol. Biotechnol. 2009, 82, 221–229. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Shi, Z.P.; Gao, P.; Duan, Z.Y.; Mao, Z.G. On-line prediction of products concentrations in glutamate fermentation using metabolic network model and linear programming. Biochem. Eng. J. 2005, 25, 99–108. [Google Scholar] [CrossRef]
- Xiao, J.; Shi, Z.P.; Gao, P.; Feng, H.J.; Duan, Z.Y.; Mao, Z.G. On-line optimization of glutamate production based on balanced metabolic control by RQ. Bioproc. Biosyst. Eng. 2006, 29, 109–117. [Google Scholar] [CrossRef]
- Cao, Y.; Mpofu, E.; Shi, Z.P. A novel metabolic model incorporating directed signal flow diagram with enzymatic activities data for evaluating glutamate yield in glutamate fermentation. Biochem. Eng. J. 2013, 77, 136–146. [Google Scholar] [CrossRef]
- Ding, J.; Gao, M.J.; Hou, G.L.; Liang, K.X.; Yu, R.S.; Li, Z.; Shi, Z.P. Stabilizing porcine interferon-α production by Pichia pastoris with an ethanol on-line measurement based DO-Stat glycerol feeding strategy. J. Chem. Technol. Biotechnol. 2015, 89, 1948–1953. [Google Scholar] [CrossRef]
- Gao, M.J.; Zheng, Z.Y.; Wu, J.R.; Dong, S.J.; Li, Z.; Jin, H.; Zhan, X.B.; Lin, C.C. Improvement of specific growth rate of Pichia pastoris for effective porcine interferon-α production with an on-line model-based glycerol feeding strategy. Appl. Microbiol. Biotechnol. 2012, 93, 1437–1445. [Google Scholar] [CrossRef]
- Hess, A.; Lyer, H.; Malm, W. Linear trend analysis: A comparison of methods. Atmos. Environ. 2001, 35, 5211–5222. [Google Scholar] [CrossRef]
- Hu, Z.C.; Zheng, Y.G.; Shen, Y.C. Dissolved-oxygen-stat fed-batch fermentation of 1, 3-dihydroxyacetone from glycerol by Gluconobacter oxydans ZJB09112. Biotechnol. Bioproc. Eng. 2010, 15, 651–656. [Google Scholar] [CrossRef]
- Gomes, J.; Menawat, A.S. Precise control of dissolved oxygen in bioreactors-a model-based geometric algorithm. Chem. Eng. Sci. 2000, 55, 67–78. [Google Scholar] [CrossRef]
- Yuan, X.F.; Ge, Z.Q.; Song, Z.H. Spatio-temporal adaptive soft sensor for nonlinear time-varying and variable drifting processes based on moving window LWPLS and time difference model. Asia-Pac. J. Chem. Eng. 2016, 11, 209–219. [Google Scholar] [CrossRef]
- Zheng, R.J.; Pan, F. Multi-phase support vector regression soft sensor for online product quality prediction in glutamate fermentation process. Am. J. Biochem. Biotechnol. 2017, 13, 90–98. [Google Scholar] [CrossRef]
- Li, Y.P.; Chen, J.D.; Pan, F. Online monitoring of fermentation processes based on trend analysis. J. Jiangnan Univ. 2013, 12, 379–383. [Google Scholar]
- Amicarelli, A.; di Sciascio, F.; Toibero, J.M.; Alvarez, H. Including dissolved oxygen dynamics into the Bt δ-endotoxins production process model and its application to process control. Braz. J. Chem. Eng. 2010, 27, 41–62. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, C.L.; Gao, M.J.; Hou, G.L.; Liang, K.X.; Li, C.H.; Ni, J.P.; Li, Z.; Shi, Z.P. Enhanced porcine circovirus Cap protein production by Pichia pastoris with a fuzzy logic DO control based methanol/sorbitol co-feeding induction strategy. J. Biotechnol. 2014, 177, 35–44. [Google Scholar] [CrossRef]










| Different Methods | From 0 to 45 min |
|---|---|
| Manual control | 27 times |
| Tendency control | 3 times |
| Different Methods | From 1565 to 1609 min |
|---|---|
| Manual control | 27 times |
| Tendency control | 2 times |
| Different Methods | From 1220 to 1509 min |
|---|---|
| Manual control | 99 times |
| Tendency control | 2 times |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Pan, F. On-Line Tendency Control of Dissolved Oxygen Concentration during Aerobic Fed-Batch Fermentations. Appl. Sci. 2019, 9, 5232. https://doi.org/10.3390/app9235232
Zheng R, Pan F. On-Line Tendency Control of Dissolved Oxygen Concentration during Aerobic Fed-Batch Fermentations. Applied Sciences. 2019; 9(23):5232. https://doi.org/10.3390/app9235232
Chicago/Turabian StyleZheng, Rongjian, and Feng Pan. 2019. "On-Line Tendency Control of Dissolved Oxygen Concentration during Aerobic Fed-Batch Fermentations" Applied Sciences 9, no. 23: 5232. https://doi.org/10.3390/app9235232





