Mixed-Unit Lattice Approach for Area Determination of Cellular and Subcellular Structures
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
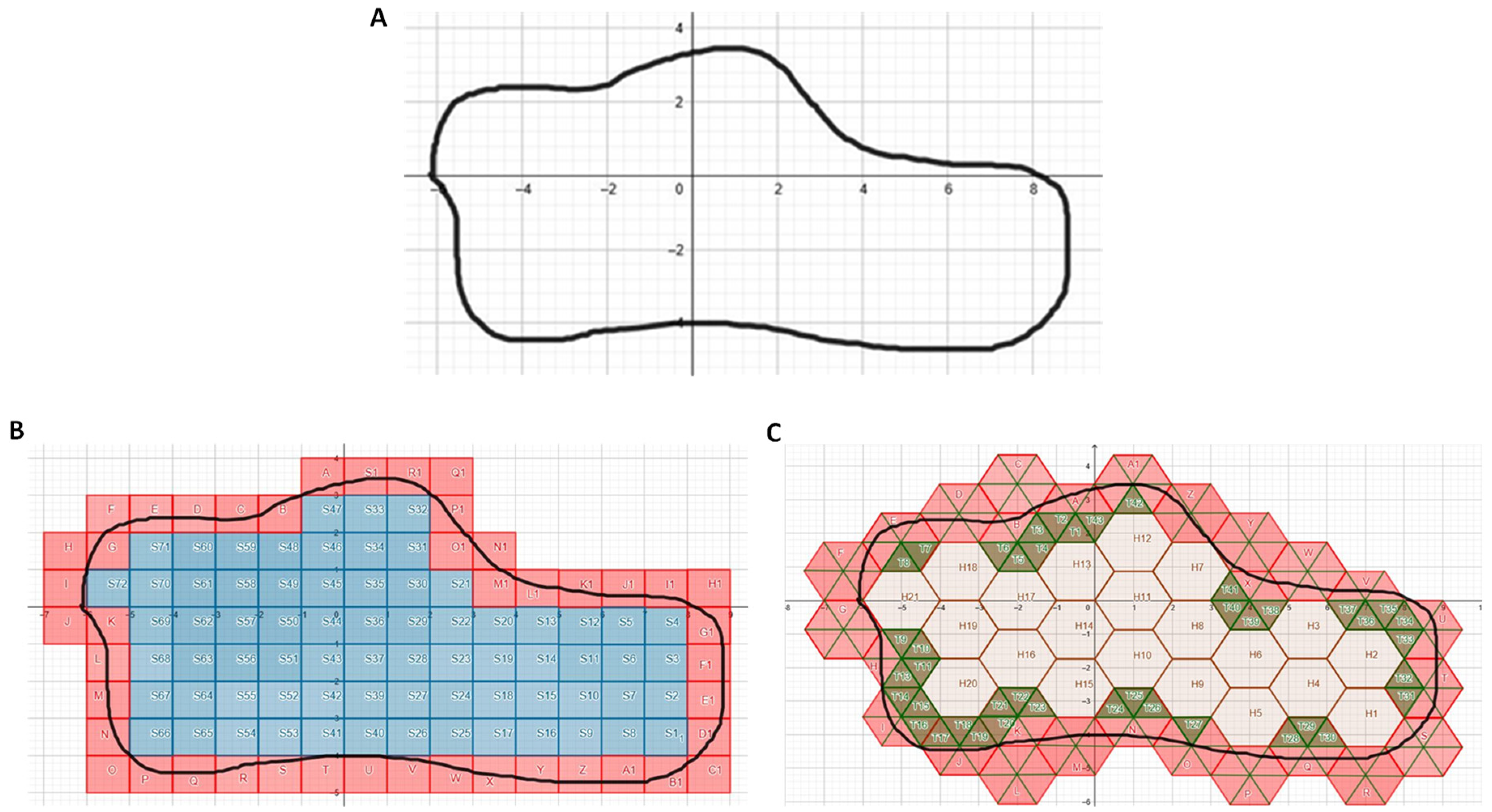
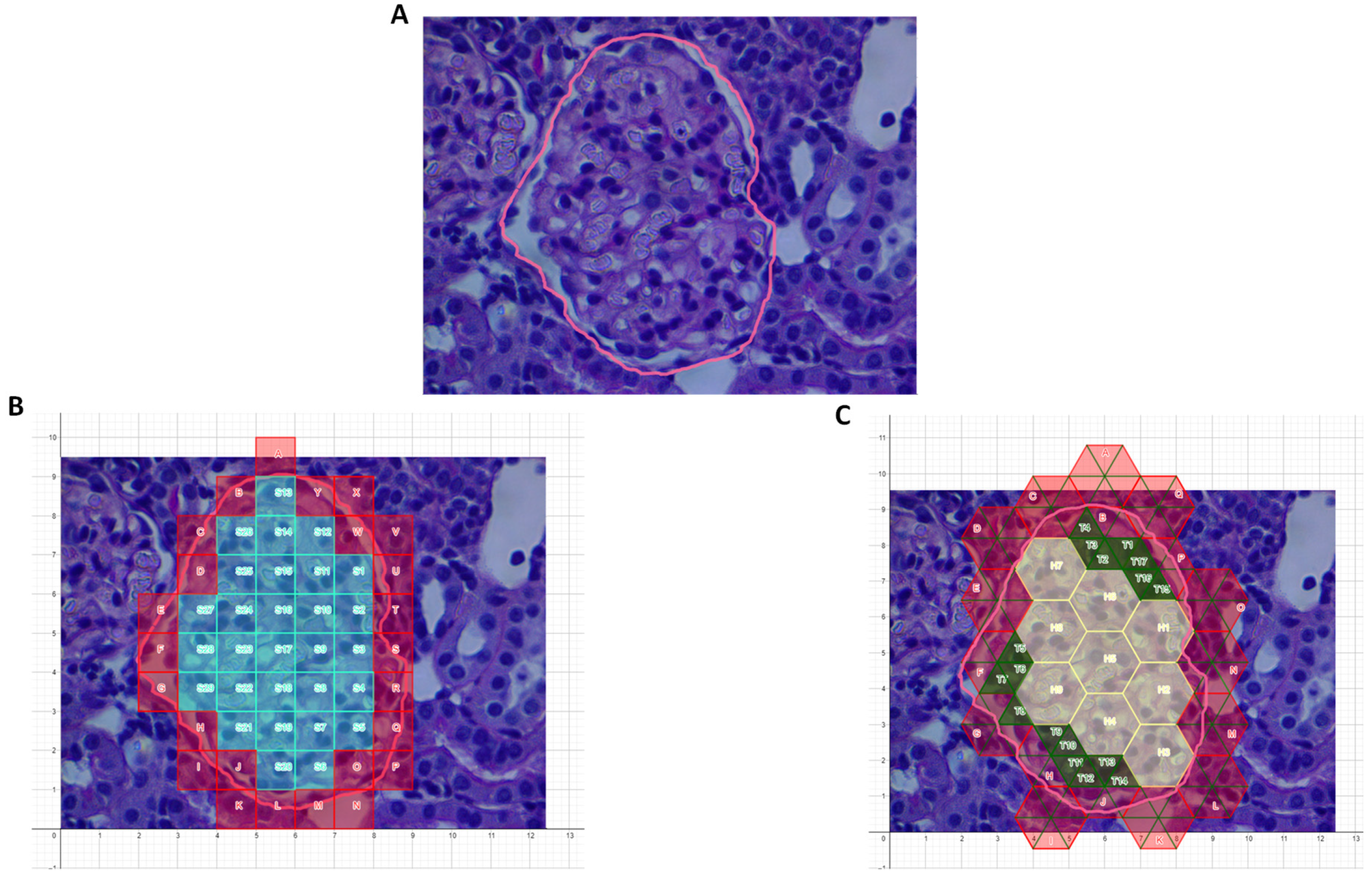
2.1. Area Determination of Curvilinear Regions, Glomeruli, and Renal Cysts
2.2. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, M.; Goldberg, I.D.; Narayan, P. Glomerular Remodeling in Proteinuric Kidney Disease. J. Am. Soc. Nephrol. 2019, 30, SA-PO0582. [Google Scholar]
- Lee, S.W.; Yu, M.Y.; Baek, S.H.; Ahn, S.Y.; Kim, S.; Na, K.Y.; Chae, D.W.; Chin, H.J. Glomerular Hypertrophy Is a Risk Factor for Relapse in Minimal Change Disease Patients. Nephron 2016, 132, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.; Hawkins, E.P.; Berry, P.L.; Glick, A.D.; Chiang, M.L.; MacDonell, R.C., Jr.; Ichikawa, I. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990, 38, 115–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughson, M.D.; Puelles, V.D.; Hoy, W.E.; Douglas-Denton, R.N.; Mott, S.A.; Bertram, J.F. Hypertension, glomerular hypertrophy and nephrosclerosis: The effect of race. Nephrol. Dial. Transpl. 2014, 29, 1399–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieto, J.A.; Yamin, M.A.; Goldberg, I.D.; Narayan, P. An Empirical Biomarker-Based Calculator for Cystic Index in a Model of Autosomal Recessive Polycystic Kidney Disease-The Nieto-Narayan Formula. PLoS ONE 2016, 11, e0163063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.woundsresearch.com/article/histogram-planimetry-method-measurement-irregular-wounds (accessed on 11 November 2019).
- Available online: https://imagej.nih.gov/ij/ (accessed on 11 November 2019).
- Chao, K.; Liao, K.; Khan, M.; Shi, C.; Li, J.; Goldberg, I.D.; Narayan, P. An Improved Method for Estimating Renal Dimensions; Implications for Management of Kidney Disease. Appl. Sci. 2019, 9, 3198. [Google Scholar] [CrossRef] [Green Version]
- Nieto, J.A.; Zhu, J.; Duan, B.; Li, J.; Zhou, P.; Paka, L.; Yamin, M.A.; Goldberg, I.D.; Narayan, P. A modified elliptical formula to estimate kidney collagen content in a model of chronic kidney disease. PLoS ONE 2018, 13, e0190815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivetti, G.; Melissari, M.; Balbi, T.; Quaini, F.; Cigola, E.; Sonnenblick, E.H.; Anversa, P. Myocyte cellular hypertrophy is responsible for ventricular remodelling in the hypertrophied heart of middle aged individuals in the absence of cardiac failure. Cardiovasc. Res. 1994, 28, 1199–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fries, J.W.; Sandstrom, D.J.; Meyer, T.W.; Rennke, R.G. Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab. Investig. 1989, 60, 205–218. [Google Scholar] [PubMed]
- Nishimoto, K.; Shiiki, H.; Nishino, T.; Uyama, H.; Iwano, M.; Dohi, K. Reversible glomerular hypertrophy in adult patients with primary focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 1997, 8, 1668–1678. [Google Scholar] [PubMed]
- Irazabal, M.V.; Mishra, P.K.; Torres, V.E.; Macura, S.I. Use of Ultra-high Field MRI in Small Rodent Models of Polycystic Kidney Disease for In Vivo Phenotyping and Drug Monitoring. J. Vis. Exp. 2015, 23, e52757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradi, V.; Gastaldon, F.; Caprara, C.; Giuliani, A.; Martino, F.; Ferrari, F.; Ronco, C. Predictors of rapid disease progression in autosomal dominant polycystic kidney disease. Minerva Med. 2017, 108, 43–56. [Google Scholar] [PubMed]
- Ta, M.H.; Schwensen, K.G.; Foster, S.; Korgaonkar, M.; Ozimek-Kulik, J.E.; Phillips, J.K.; Peduto, A.; Rangan, G.K. Effects of TORC1 Inhibition during the Early and Established Phases of Polycystic Kidney Disease. PLoS ONE 2016, 11, e0164193. [Google Scholar] [CrossRef]
- Available online: https://www.maa.org/external_archive/joma/Volume7/Aktumen/Polygon.html (accessed on 11 November 2019).



| Glomerulus | ImageJ (unit2) | Squares (unit2) | Lattice (unit2) | Improvement (%) over Unit Squares |
|---|---|---|---|---|
| 1 | 42.5 | 29 | 31 | 7 |
| 2 | 53 | 40 | 42.4 | 6 |
| 3 | 21.3 | 15 | 15.6 | 4 |
| 4 | 29.7 | 20 | 22.5 | 13 |
| Cyst | ImageJ (unit2) | Squares (unit2) | Lattice (unit2) | Improvement (%) over Unit Squares |
|---|---|---|---|---|
| 1 | 68.8 | 46 | 52.8 | 15 |
| 2 | 71.6 | 46 | 53.2 | 16 |
| 3 | 71 | 52 | 55.87 | 7 |
| 4 | 49 | 31 | 33.3 | 7 |
| 5 | 99.2 | 80 | 85.3 | 7 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayan, R. Mixed-Unit Lattice Approach for Area Determination of Cellular and Subcellular Structures. Appl. Sci. 2019, 9, 5267. https://doi.org/10.3390/app9245267
Narayan R. Mixed-Unit Lattice Approach for Area Determination of Cellular and Subcellular Structures. Applied Sciences. 2019; 9(24):5267. https://doi.org/10.3390/app9245267
Chicago/Turabian StyleNarayan, Rithika. 2019. "Mixed-Unit Lattice Approach for Area Determination of Cellular and Subcellular Structures" Applied Sciences 9, no. 24: 5267. https://doi.org/10.3390/app9245267




