Theoretical Study on Pressure Damage Based on Clinical Purpura during the Laser Irradiation of Port Wine Stains with Real Complex Vessels
Abstract
1. Introduction
2. Materials and Methods
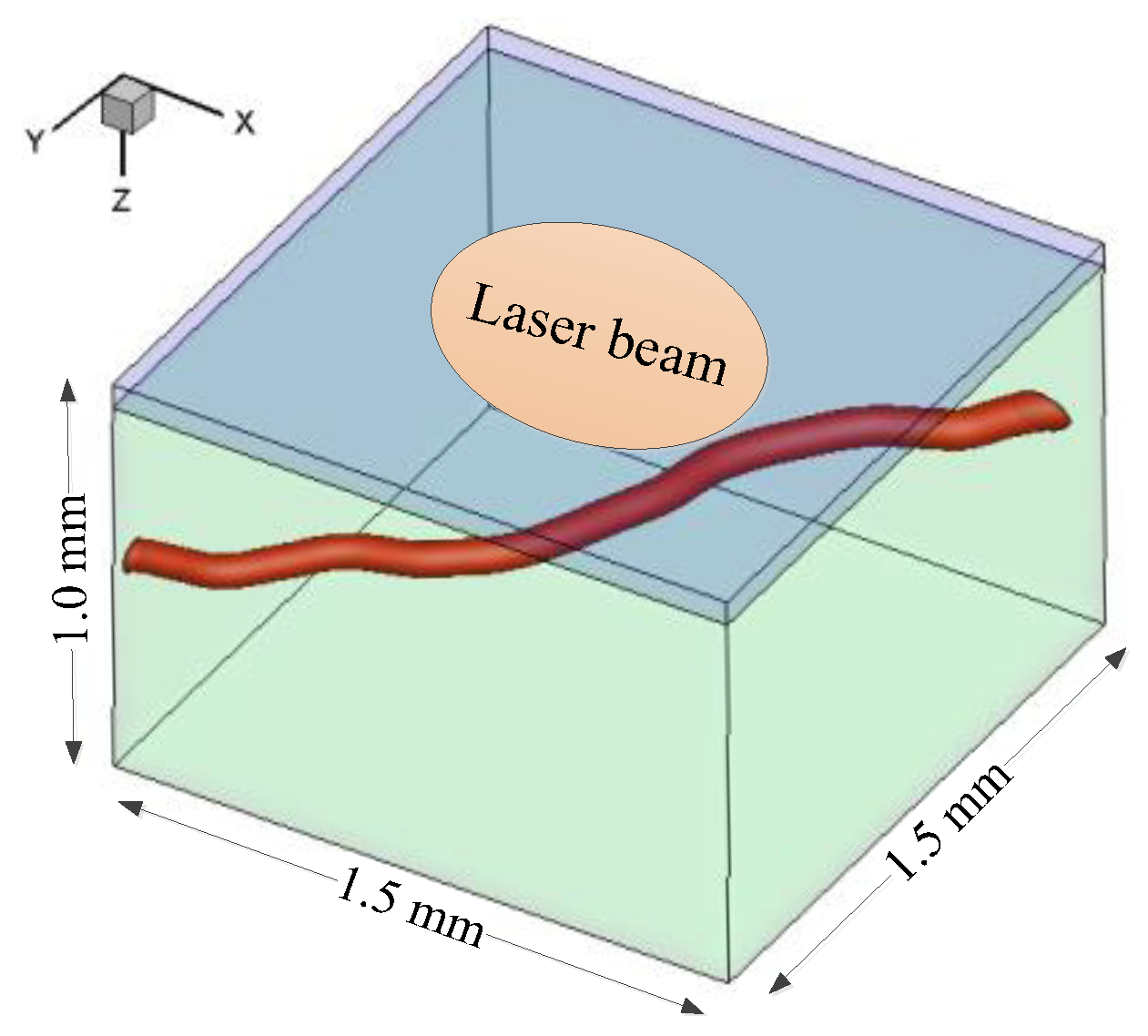
2.1. Problem Description and Basic Assumptions
- (1)
- The epidermis of a rat was removed in the DSC model for microscopic observation [12,13]. For convenience, the epidermal layer was excluded from the simulation to validate the theoretical model by the in vivo results from the DSC model. Afterward, the whole skin model comprising the epidermis and the dermis was adopted to study the effects of epidermal melanin content and vessel depth on laser fluence. The surfaces of the epidermis and the dermis were considered planar.
- (2)
- The tetrahedron-based Monte Carlo (TMC) method was employed to simulate light propagation and energy deposition because a tetrahedron mesh is body fitted for a complex vessel shape [28]. The optical properties of different tissues are assumed to be constant and uniform within a given component. The following parameters were disregarded during the short laser pulse duration: changes in the density, heat capacity, and thermal conductivity of skin tissues; metabolic heat generation; and biochemical reactions.
- (3)
- The epidermis and the dermis were treated as solid phases because of the low water content [14], and only the energy equation was involved. Thermal damage represented by temperature was used to characterize the safety of the epidermis and the dermis.
- (4)
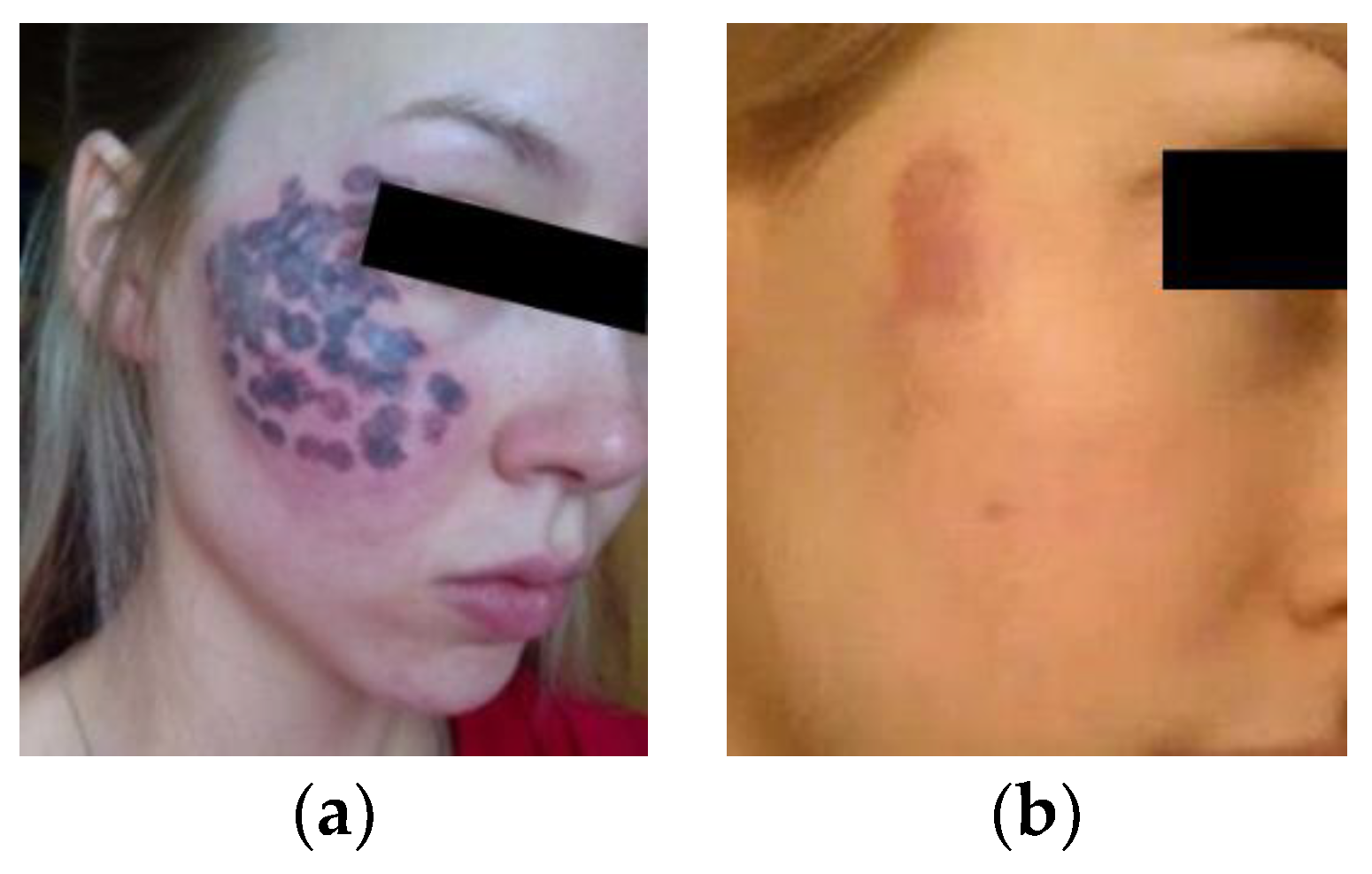
- The purpura area was assumed to be equal to the bleeding area in the DSC model and directly proportional to the volume fraction of blood vessels in the lesion under the same treatment parameters in accordance with the approximate relationship between the clinical treatment and the animal experiment. The purpura image in clinical settings was analyzed to provide the appropriate area ratio of purpura for setting the laser parameters in simulation.
2.2. Governing Equations
2.3. Boundary Conditions
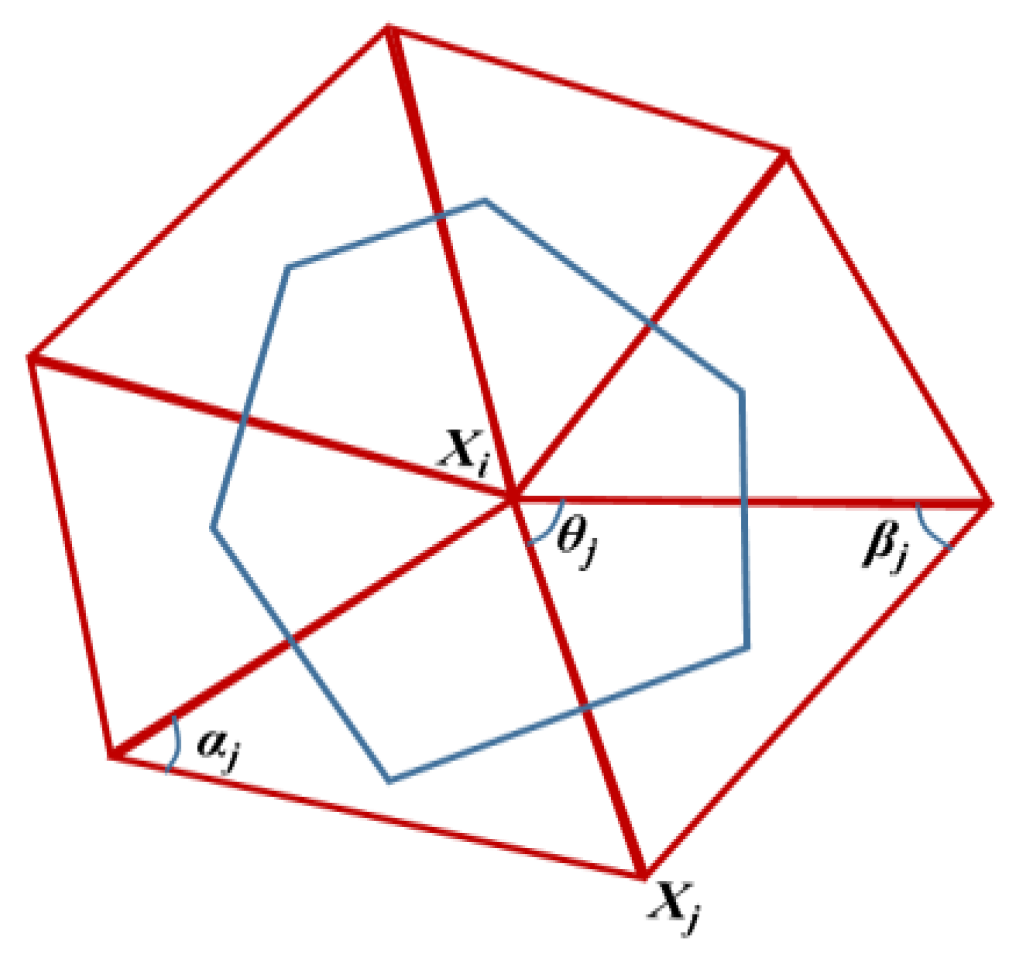
2.4. CCPD Model
3. Validation of CCPD Model
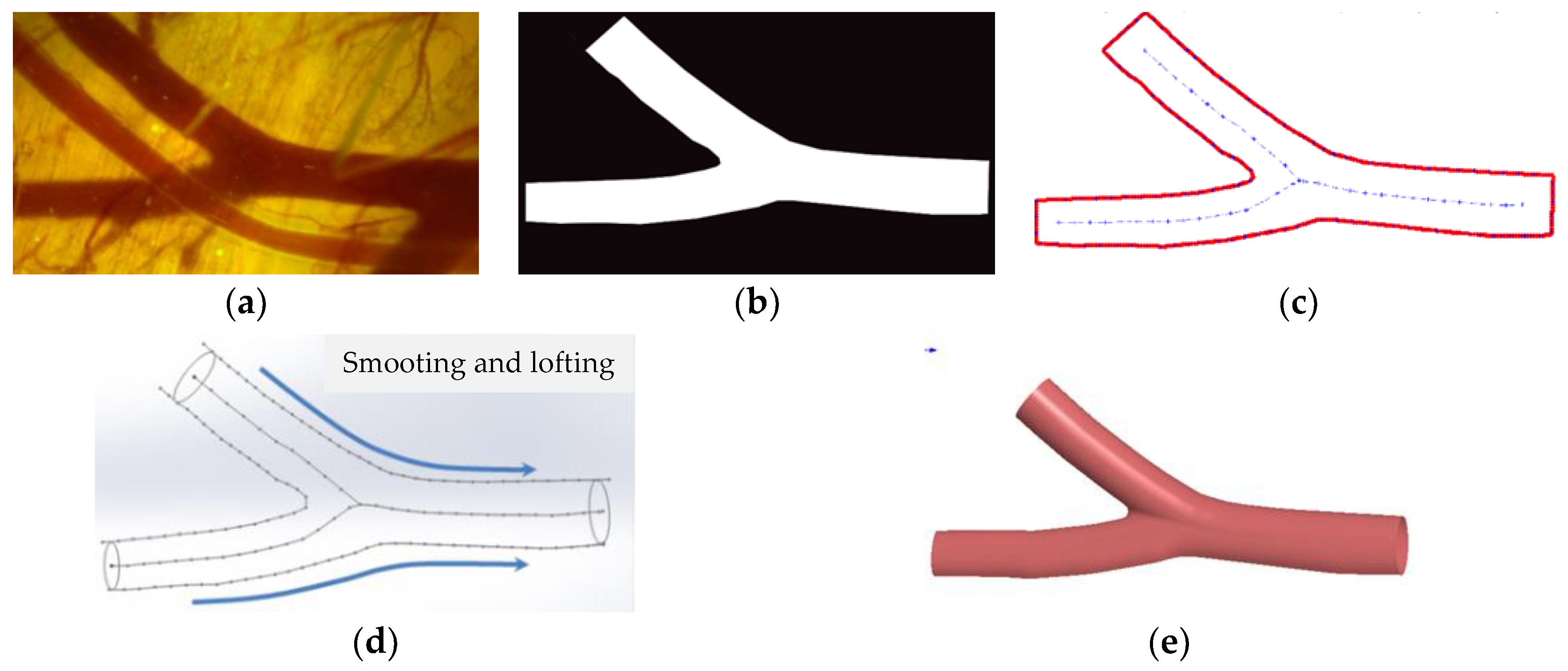
3.1. 3D Reconstruction of Real Blood Vessels
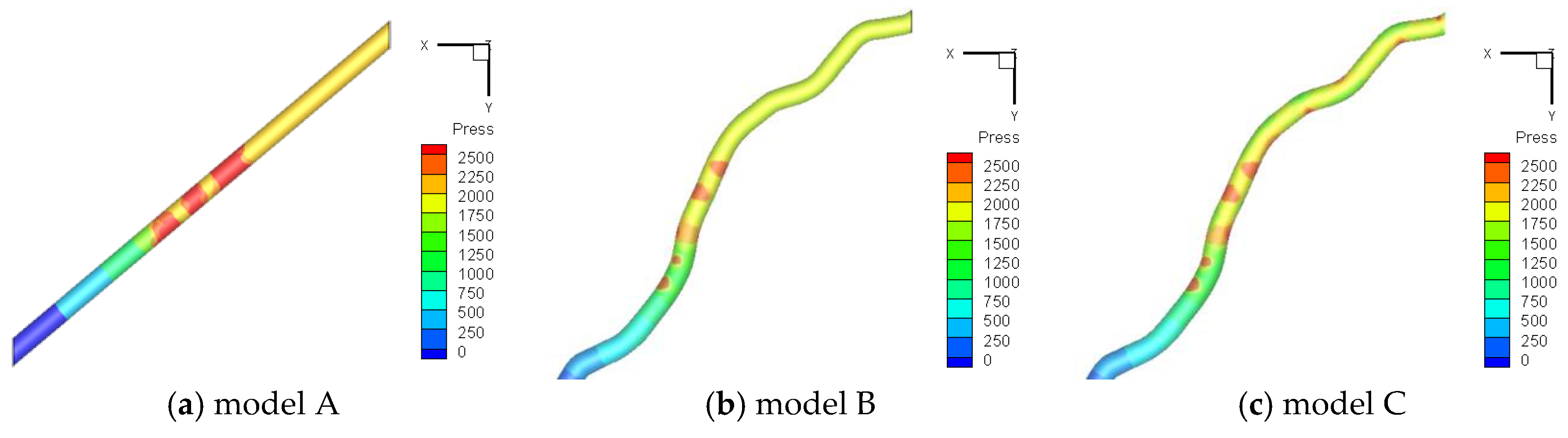
3.2. Grid Independence Test
3.3. Validation of the CCPD Model
4. Results and Discussion
4.1. Energy Threshold of Vessel Hemorrhage
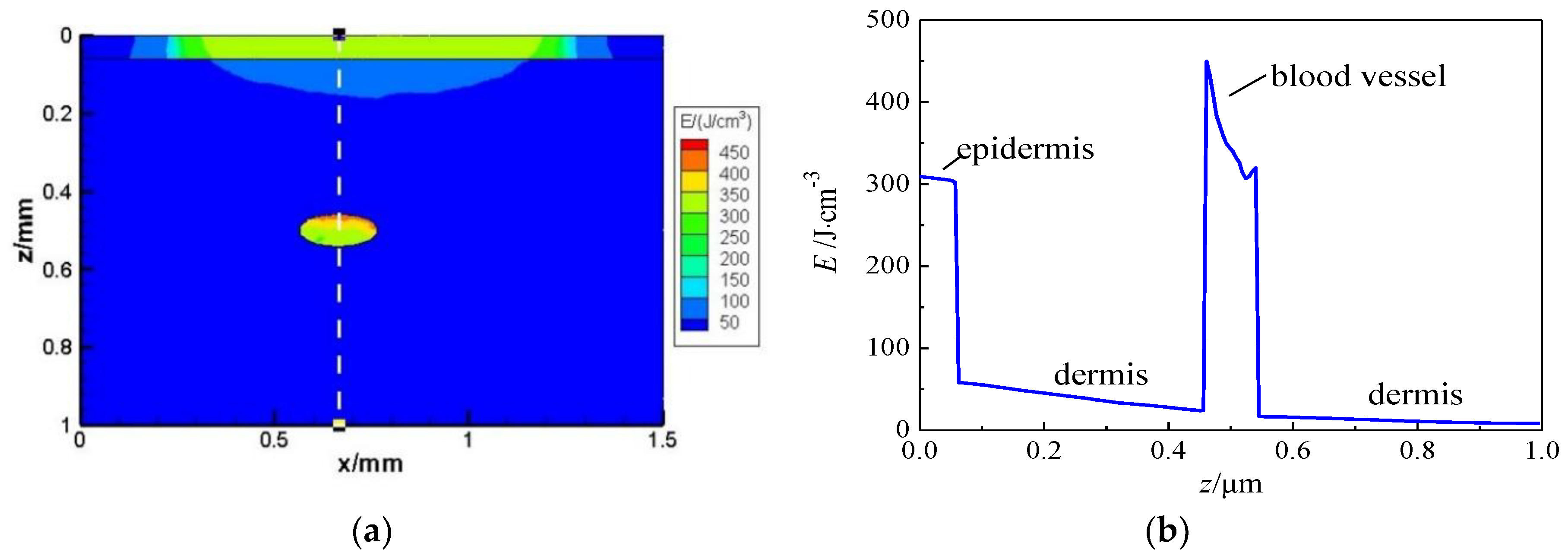
4.2. Effect of Laser Transport in Skin with PWS Vessel by TMC
4.3. Effect of Melanin Content in the Epidermis on Laser Fluence Selection
4.4. Effect of Vessel Depth on the Selection of Laser Fluence
5. Conclusions
- (1)
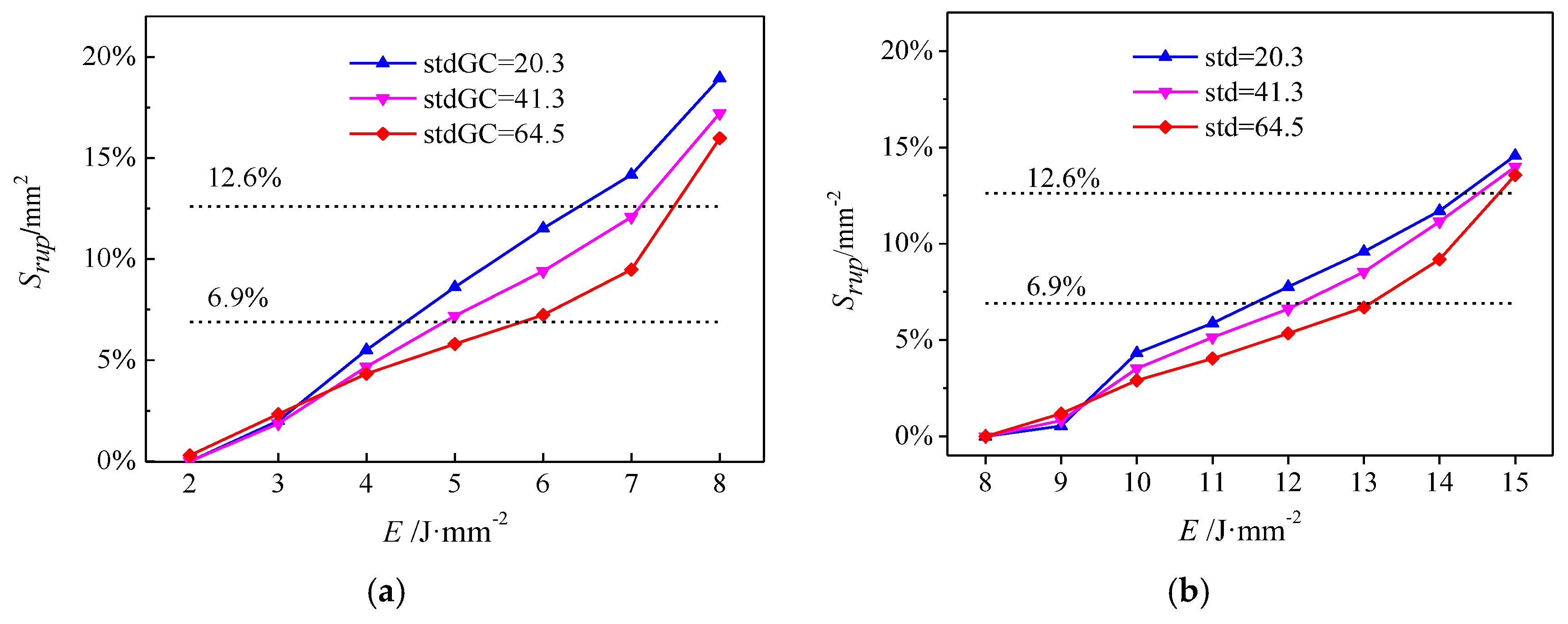
- Vascular lesions with a high stdGC are prone to hemorrhage and purpura formation but have difficulty in achieving Srpu to damage vascular lesions. This observation reveals one possible reason for the poor treatment of PWS in clinic, that is, purpura formation is simply regarded as the TEP, which leads to insufficient treatment and a high recurrence rate of vascular lesions with a large stdGC. An appropriate Srpu should be adopted as the TEP that is higher than Srpu of marked effects and lower than Srpu of side effects. With the ability to provide appropriate Srpu, Eeff is recommended to precisely treat vascular lesions.
- (2)
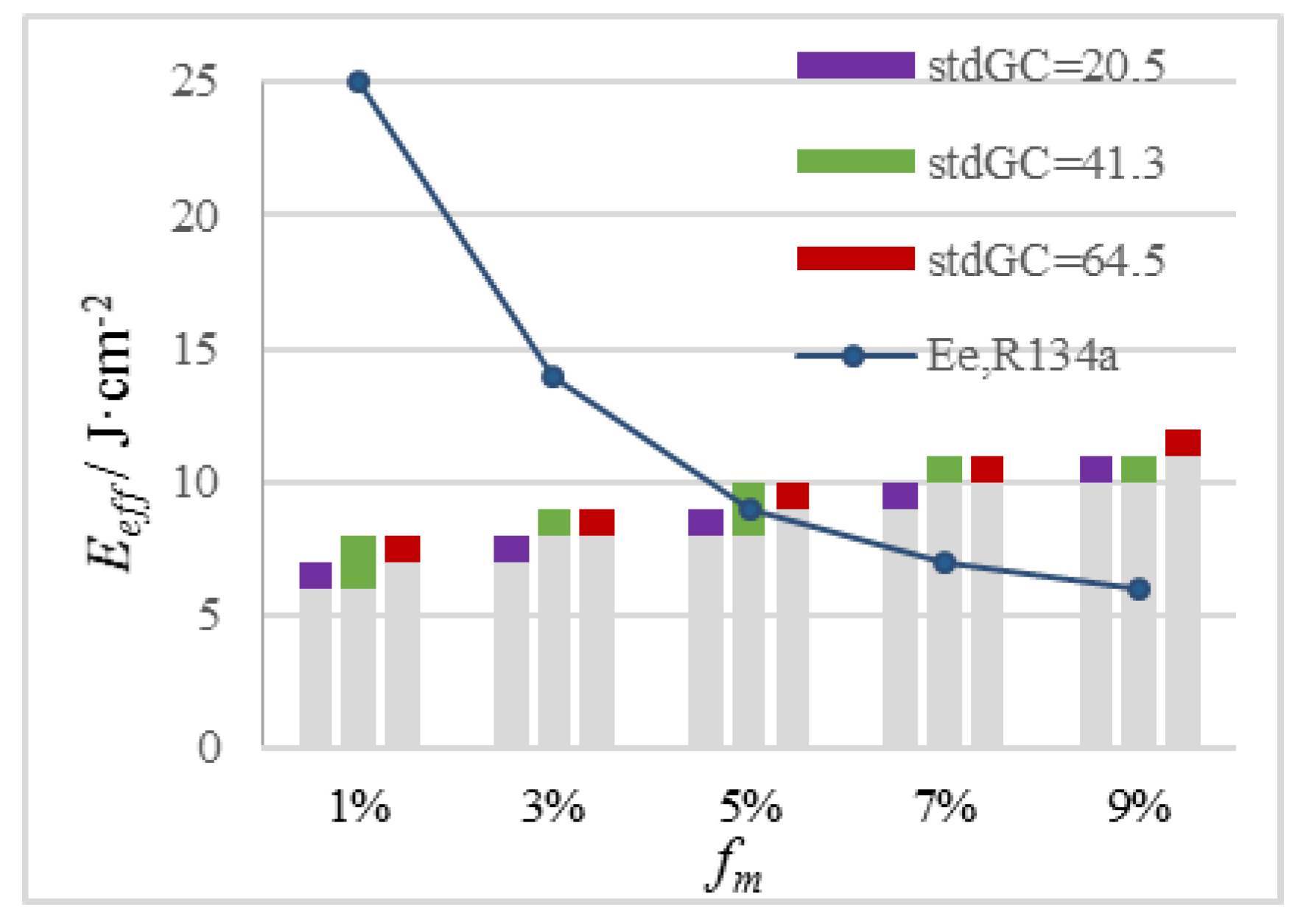
- Our simulation results reveal the quantitative correlations of Eeff of PDL with epidermal melanin content, vessel curvature, and depth for individualized and precise guidance on clinical treatments. To PWS patients with different melanin levels, different treatment strategies are suggested. PDL should be used carefully in treating light-yellow people (fm = 3%). Cryogen R404A with a good cooling capacity is suggested to protect the epidermis with fm = 5%. For brown (fm = 7%) and black (fm = 9%) people, the implementation of Eeff of blood vessels in PDL severely harms the epidermis; thus, near-infrared lasers are recommended because they minimally absorb melanin.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aguilar, G.; Choi, B.; Broekgaarden, M.; Yang, O.; Yang, B.; Ghasri, P.; Chen, J.K.; Bezemer, R.; Nelson, J.S.; van Drooge, A.M.; et al. An overview of three promising mechanical, optical, and biochemical engineering approaches to improve selective photothermolysis of refractory port wine stains. Ann. Biomed. Eng. 2012, 40, 486–506. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Maza, A.; Fontas, E.; Toubel, G.; Vabres, P.; Livideanu, C.; Mazer, J.-M.; Rossi, B.; Boukari, F.; Harmelin, Y.; et al. Treatment of port wine stains with pulsed dye laser and topical timolol: A multicenter randomized controlled trial. Br. J. Dermatol. 2014, 170, 1350–1353. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.K.; Choi, B.; Ramirez-San-Juan, J.C.; Nelson, J.S.; Osann, K.; Kelly, K.M. Microvascular blood flow dynamics associated with photodynamic therapy, pulsed dye laser irradiation and combined regimens. Lasers Surg. Med. 2006, 38, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, B.; Wu, W.J.; Wang, G.X.; He, Y.L.; Ying, Z.X. Experimental study on the vascular thermal response to visible laser pulses. Lasers Med. Sci. 2015, 30, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.M.; Choi, B.S.; Motosue, A.; Jung, B.; Khan, M.H.; Jc, R.S.J.; Nelson, J.S. Description and analysis of treatments for port-wine stain birthmarks. Arch. Facial Plast. Surg. 2005, 7, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Chen, B.; Li, D. Criteria of pressure and thermal damage during laser irradiation of port wine stains: Which is dominant to vascular lesions? Int. J. Heat Mass Transf. 2019, 132, 848–860. [Google Scholar] [CrossRef]
- Li, D.; Farshidi, D.; Wang, G.X.; He, Y.L.; Kelly, K.M.; Wu, W.J.; Chen, B.; Ying, Z.X. A comparison of microvascular responses to visible and near-infrared lasers. Lasers Surg. Med. 2014, 46, 479–487. [Google Scholar] [CrossRef]
- Goldman, M.P. Cutaneous and Cosmetic Laser Surgery; Elsevier Pte Ltd.: Singapore, 2006. [Google Scholar]
- Goldberg, D.J. Laser and Lights; Saunders/Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Chen, B.; Thomsen, S.L.; Thomas, R.J.; Oliver, J.; Welch, A.J. Histological and modeling study of skin thermal injury to 2.0 μm laser irradiation. Lasers Surg. Med. 2008, 40, 358–370. [Google Scholar] [CrossRef]
- Jia, W.; Choi, B.; Franco, W.; Lotfi, J.; Majaron, B.; Aguilar, G.; Nelson, J.S. Treatment of cutaneous vascular lesions using multiple-intermittent cryogen spurts and two-wavelength laser pulses: Numerical and animal studies. Lasers Surg. Med. 2007, 39, 494–503. [Google Scholar] [CrossRef]
- Welch, A.J.; Gemert, M.J.C.V. Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Dordrecht, The Netherlands, 2011; pp. 579–589. [Google Scholar]
- Zhang, J.Z.; Zhang, X.X.; Audette, M. A photothermal model of selective photothermolysis with dynamically changing vaporization temperature. Lasers Med. Sci. 2011, 26, 633. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, G.X.; He, Y.L.; Kelly, K.M.; Wu, W.J.; Wang, Y.X.; Ying, Z.X. A two-temperature model for selective photothermolysis laser treatment of port wine stains. Appl. Therm. Eng. 2013, 59, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Suthamjariya, K.; Farinelli, W.A.; Koh, W.; Anderson, R.R. Mechanisms of microvascular response to laser pulses. J. Investig. Dermatol. 2004, 122, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, A.; Xu, L.X. A study on mechanical damage of tumor microvasculature induced by alternate cooling and heating. J. Therm. Sci. Eng. Appl. 2009, 1, 031002. [Google Scholar] [CrossRef]
- Han, H.-C.; Chesnutt, J.K.W.; Garcia, J.R.; Liu, Q.; Wen, Q. Artery buckling: New phenotypes, models, and applications. Ann. Biomed. Eng. 2013, 41, 1399–1410. [Google Scholar] [CrossRef]
- Smithies, D.J.; Butler, P.H. Modelling the distribution of laser light in port-wine stains with the Monte Carlo method. Phys. Med. Biol. 1995, 40, 701. [Google Scholar] [CrossRef]
- Pfefer, T.J.; Barton, J.K.; Smithies, D.J. Modeling Laser Treatment of Port Wine Stains With a Computer-Reconstructed Biopsy. Lasers Surg. Med. 1999, 24, 151–166. [Google Scholar] [CrossRef]
- Daly, S.M.; Leahy, M.J. ‘Go with the flow’: A review of methods and advancements in blood flow imaging. J. Biophotonics 2013, 6, 217–255. [Google Scholar] [CrossRef]
- Barton, J.K.; Frangineas, G.; Pummer, H.; Black, J.F. Cooperative phenomena in two-pulse, two-color laser photocoagulation of cutaneous blood vessels. Photochem. Photobiol. 2001, 73, 642–650. [Google Scholar] [CrossRef]
- Babilas, P.; Shafirstein, G.; Bäumler, W.; Baier, J.; Landthaler, M.; Szeimies, R.M.; Abels, C. Selective Photothermolysis of Blood Vessels Following Flashlamp-Pumped Pulsed Dye Laser Irradiation: In Vivo Results and Mathematical Modelling Are in Agreement. J. Investig. Dermatol. 2005, 20, 343–352. [Google Scholar] [CrossRef]
- Li, D.; Li, R.; Jia, H.; Chen, B.; Wu, W.; Ying, Z. Experimental and numerical investigation on the transient vascular thermal response to multi-pulse Nd:YAG laser. Lasers Surg. Med. 2017, 49, 852–865. [Google Scholar] [CrossRef] [PubMed]
- Moy, A.J.; White, S.M.; Indrawan, E.S.; Lotfi, J.; Nudelman, M.J.; Costantini, S.J.; Agarwal, N.; Jia, W.; Kelly, K.M.; Sorg, B.S.; et al. Wide-field functional imaging of blood flow and hemoglobin oxygen saturation in the rodent dorsal window chamber. Microvasc. Res. 2011, 82, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.M.; Fontanella, A.N.; Shan, S.; Hanna, G.; Zhang, G.; Fraser, C.L.; Dewhirst, M.W. In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat. Protoc. 2011, 6, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.Z.; Shao, H.W.; He, Y.; Oda, T.; Jia, X.M. Construction of anatomically accurate finite element models of the human hand and a rat kidney. J. Mech. Med. Biol. 2012, 11, 1141–1164. [Google Scholar] [CrossRef]
- Jia, H.; Chen, B.; Li, D.; Zhang, Y. Boundary discretization in the numerical simulation of light propagation in skin tissue: Problem and strategy. J. Biomed. Opt. 2015, 20, 25007. [Google Scholar] [CrossRef] [PubMed]
- Pfefer, T.J.; Barton, J.K.; Chan, E.K.; Ducros, M.G. A three-dimensional modular adaptable grid numerical model for light propagation during laser irradiation of skin tissue. IEEE J. Sel. Top. Quantum Electron. 1996, 2, 934–942. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Wu, W.J.; Wang, G.X.; He, Y.L. Multi-scale modeling of tissue freezing during cryogen spray cooling with R134a, R407c and R404a. Appl. Therm. Eng. 2014, 73, 1489–1500. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, B.; Wu, W.J.; Zhang, Y.; Jia, H. An integral MPS model of blood coagulation by laser irradiation: Application to the optimization of multi-pulse Nd:YAG laser treatment of port-wine stains. Int. J. Heat Mass Transf. 2017, 114, 1220–1233. [Google Scholar] [CrossRef]
- Meyer, M.; Desbrun, M.; Schröder, P.; Barr, A.H. Discrete Differential-Geometry Operators for Triangulated 2-Manifolds. Vis. Math. 2003, 35–57. [Google Scholar]
- Bowyer, A. Computing Dirichlet tessellations. Comput. J. 1981, 24, 162–166. [Google Scholar] [CrossRef]
- Watson, D.F. Computing the n-dimensional Delaunay tessellation with application to Voronoi polytopes. Comput. J. 1981, 24, 167–172. [Google Scholar] [CrossRef]
- Ruiz, d.G.S.; Cazón, A.; Antón, R.; Finol, E.A. A Methodology for Verifying Abdominal Aortic Aneurysm Wall Stress. J. Biomech. Eng. 2017, 139, 011006. [Google Scholar] [CrossRef] [PubMed]
- Koster, P.H.; van der Horst, C.M.; van der Wal, A.C. Histologic evaluation of skin damage after overlapping and nonoverlapping flashlamp pumped pulsed dye laser pulses: A study on normal human skin as a model for port wine stains. Lasers Surg. Med. 2001, 28, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Tanghetti, E.; Sherr, E.A.; Sierra, R.; Mirkov, M. The effects of pulse dye laser double-pass treatment intervals on depth of vessel coagulation. Lasers Surg. Med. 2006, 38, 16–21. [Google Scholar] [CrossRef]
- Milanic, M.; Majaron, B. Three-dimensional Monte Carlo model of pulsed-laser treatment of cutaneous vascular lesions. J. Biomed. Opt. 2011, 16, 128002. [Google Scholar] [CrossRef]
- Li, D.; Chen, B.; Wang, G.X.; Wu, W.; He, Y.L. A New Theoretical Model of Selectively Photothermolysis to Aid Laser Treatment of Poor Responding Port Wine Stain Blood Vessels. Begel House Inc 2014, 6993–7006. [Google Scholar]
- Li, D.; Chen, B.; Wu, W.J.; Ying, Z.X. Influence of laser wavelength on the thermal responses of port wine stain lesions in light, moderate and heavy pigmented skin. Appl. Therm. Eng. 2017, 117, 193–203. [Google Scholar] [CrossRef]
- Yang, J.; Shen, Y.; Liu, Y.; Hemphill, A.S.; Wang, L.V. Focusing light through scattering media by polarization modulation based generalized digital optical phase conjugation. Appl. Phys. Lett. 2017, 111, 201108. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; He, S.; Wang, L.V. Angular-spectrum modeling of focusing light inside scattering media by optical phase conjugation. Optica 2019, 6, 250–256. [Google Scholar] [CrossRef]














| Property | Dermis | Blood | |
|---|---|---|---|
| Optical properties | Absorption coefficient, µa/cm−1 | 20 | 49.3 |
| Scattering coefficient, µs/cm−1 | 460 | 466 | |
| Anisotropy index, g | 0.8 | 0.995 | |
| Refractive index, n | 1.37 | 1.33 | |
| Thermal properties | Density, ρ/kg·m−3 | 1090 | 1060 |
| Thermal conductivity, k/kW·m−1·K−1 | 0.41 | 0.55 | |
| Specific heat, c/J·kg−1·K−1 | 3500 | 3600 | |
| Property | Epidermis (No Melanin) | Melanin | Epidermis with fm Melanin |
|---|---|---|---|
| Absorption coefficient, µa/cm−1 | 0.371 | 402.63 | μa,m × fm + μa,e × (1 − fm) |
| Scattering coefficient, µs/cm−1 | 470 | ------ | 470 |
| Anisotropy index, g | 0.79 | ------ | 0.79 |
| Refractive index, n | 1.37 | ------ | 1.37 |
| Density, ρ/kg·m−3 | 1120 | ------ | 1120 |
| Thermal conductivity, k/kW·m−1·K−1 | 0.34 | ------ | 0.34 |
| Specific heat, c/J·kg−1·K−1 | 3200 | ------ | 3200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Chen, B.; Li, D. Theoretical Study on Pressure Damage Based on Clinical Purpura during the Laser Irradiation of Port Wine Stains with Real Complex Vessels. Appl. Sci. 2019, 9, 5478. https://doi.org/10.3390/app9245478
Jia H, Chen B, Li D. Theoretical Study on Pressure Damage Based on Clinical Purpura during the Laser Irradiation of Port Wine Stains with Real Complex Vessels. Applied Sciences. 2019; 9(24):5478. https://doi.org/10.3390/app9245478
Chicago/Turabian StyleJia, Hao, Bin Chen, and Dong Li. 2019. "Theoretical Study on Pressure Damage Based on Clinical Purpura during the Laser Irradiation of Port Wine Stains with Real Complex Vessels" Applied Sciences 9, no. 24: 5478. https://doi.org/10.3390/app9245478
APA StyleJia, H., Chen, B., & Li, D. (2019). Theoretical Study on Pressure Damage Based on Clinical Purpura during the Laser Irradiation of Port Wine Stains with Real Complex Vessels. Applied Sciences, 9(24), 5478. https://doi.org/10.3390/app9245478




