Packed Bed Photoreactor for the Removal of Water Pollutants Using Visible Light Emitting Diodes
Abstract
:1. Introduction
2. Results and Discussion
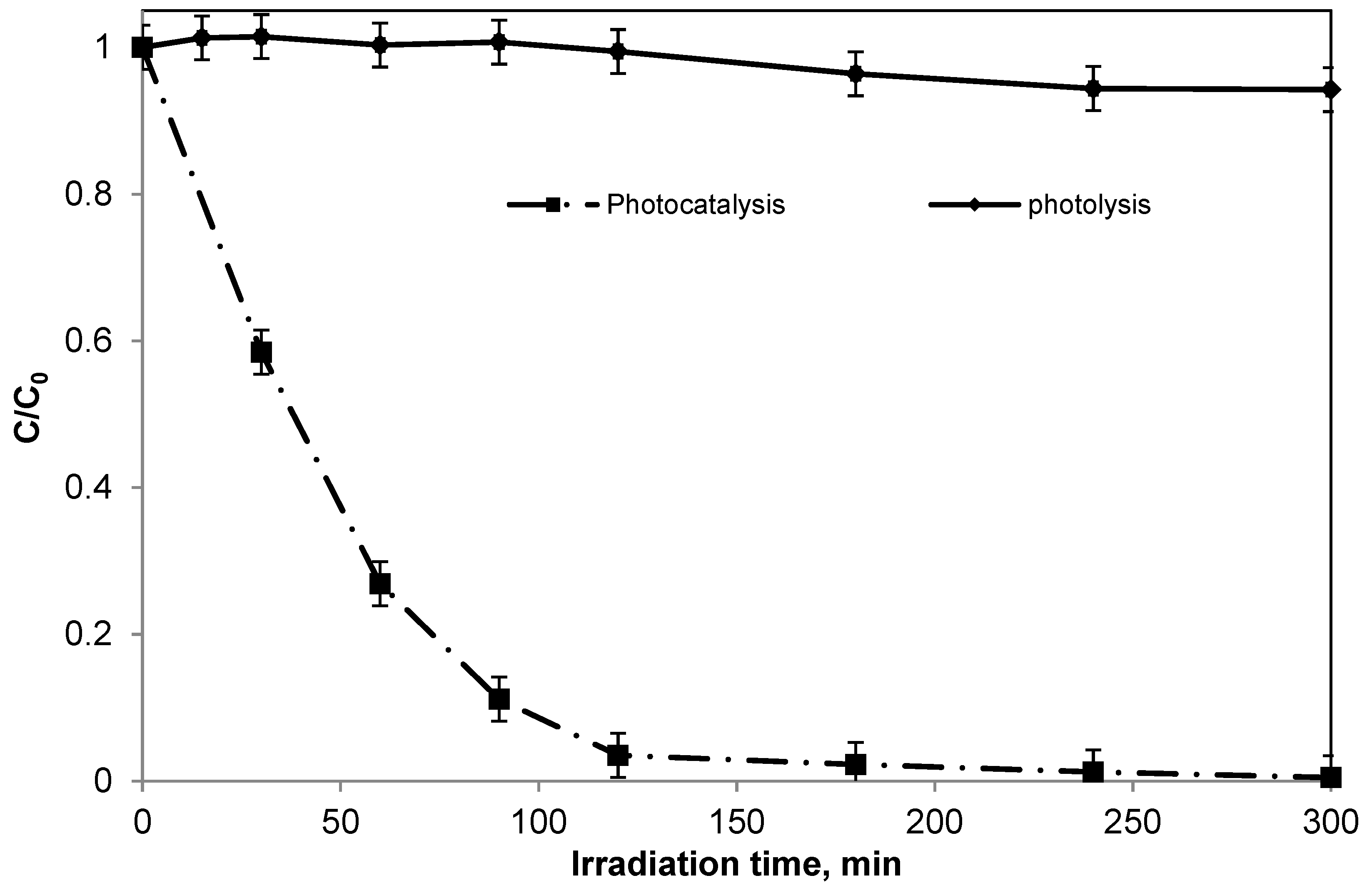
2.1. Discoloration and Mineralization of Methylene Blue with the Packed Bed Photoreactor Operating in Batch Mode
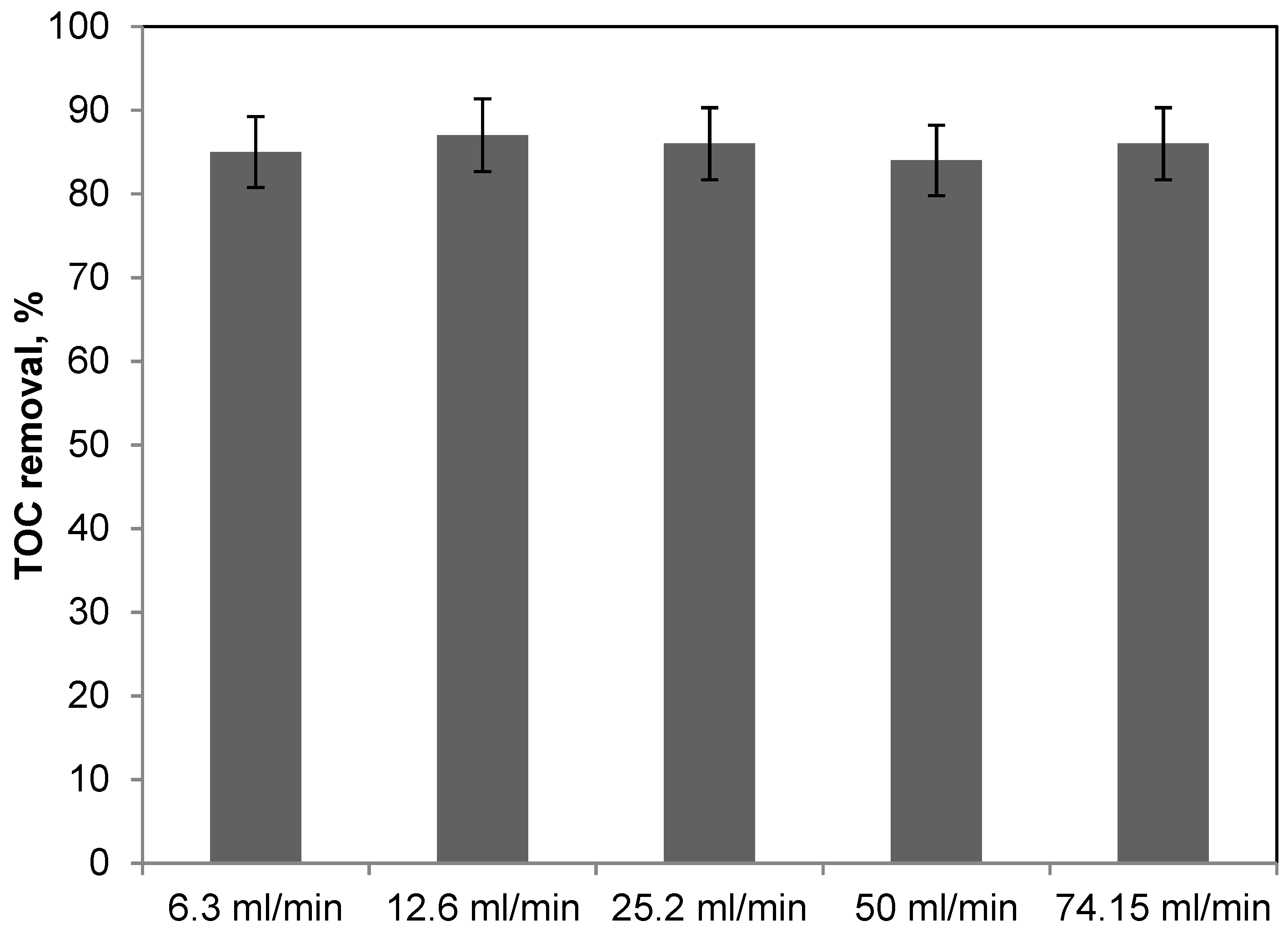
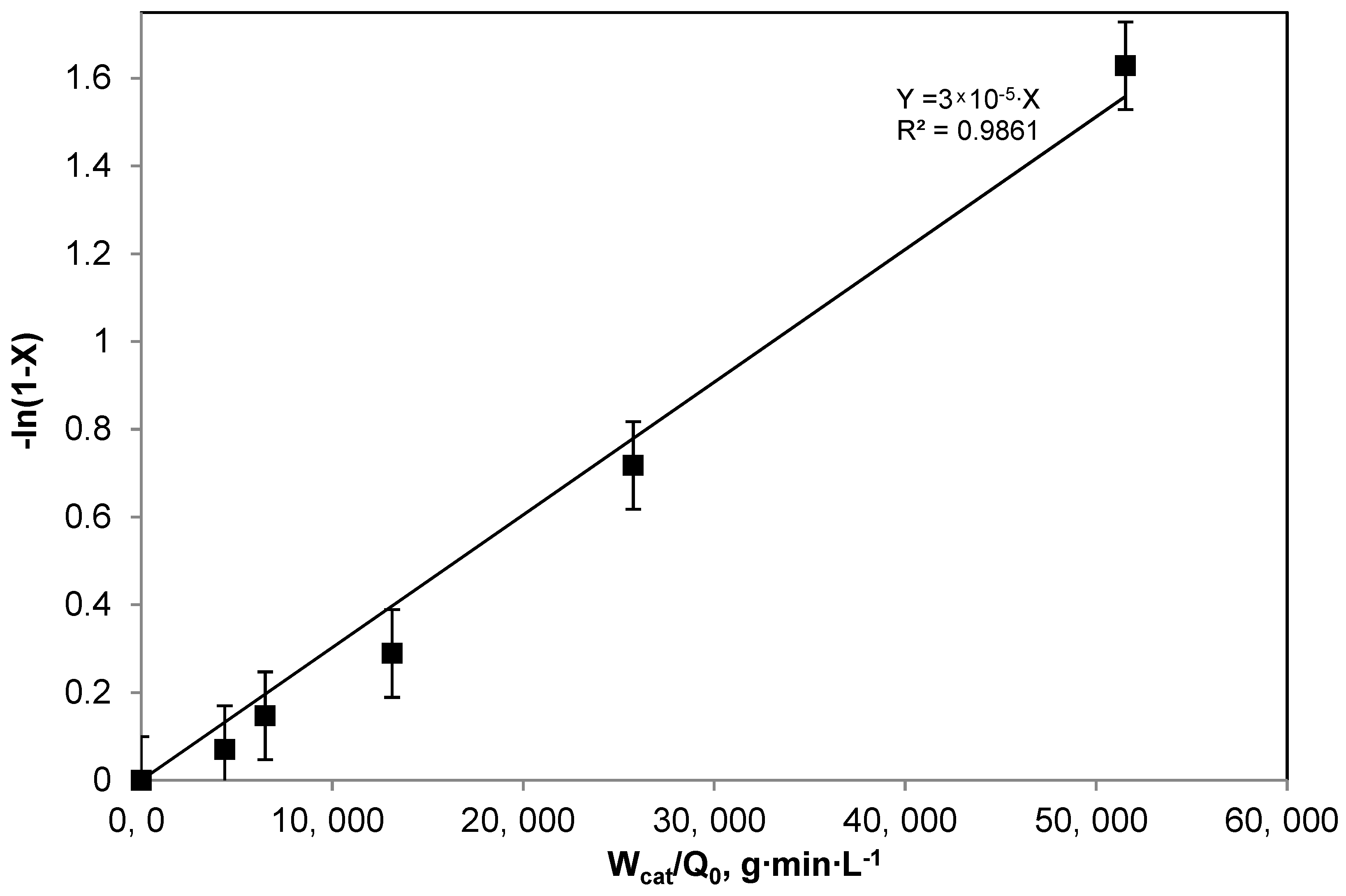
2.2. Photocatalytic Results on Methylene Blue Degradation with the Packed Bed photoreactor: Kinetic Evaluation and Mathematical Modelling
- Q0 = liquid flow rate, L/min;
- K = apparent kinetic constant, L/(g min);
- X = MB conversion;
- Wcat = structured catalyst amount, g.
- Wcat = 0 : X = 0.
- kkinetic = kinetic constant, L cm2/(g min mW);
- I = light intensity, mW/cm2.
- t = 0; C = C0,
- C0 = MB initial concentration, mg/L;
- C = MB concentration at the generic irradiation time, mg/L;
- V = treated solution volume, L;
- Wcat = structured catalyst amount, g.
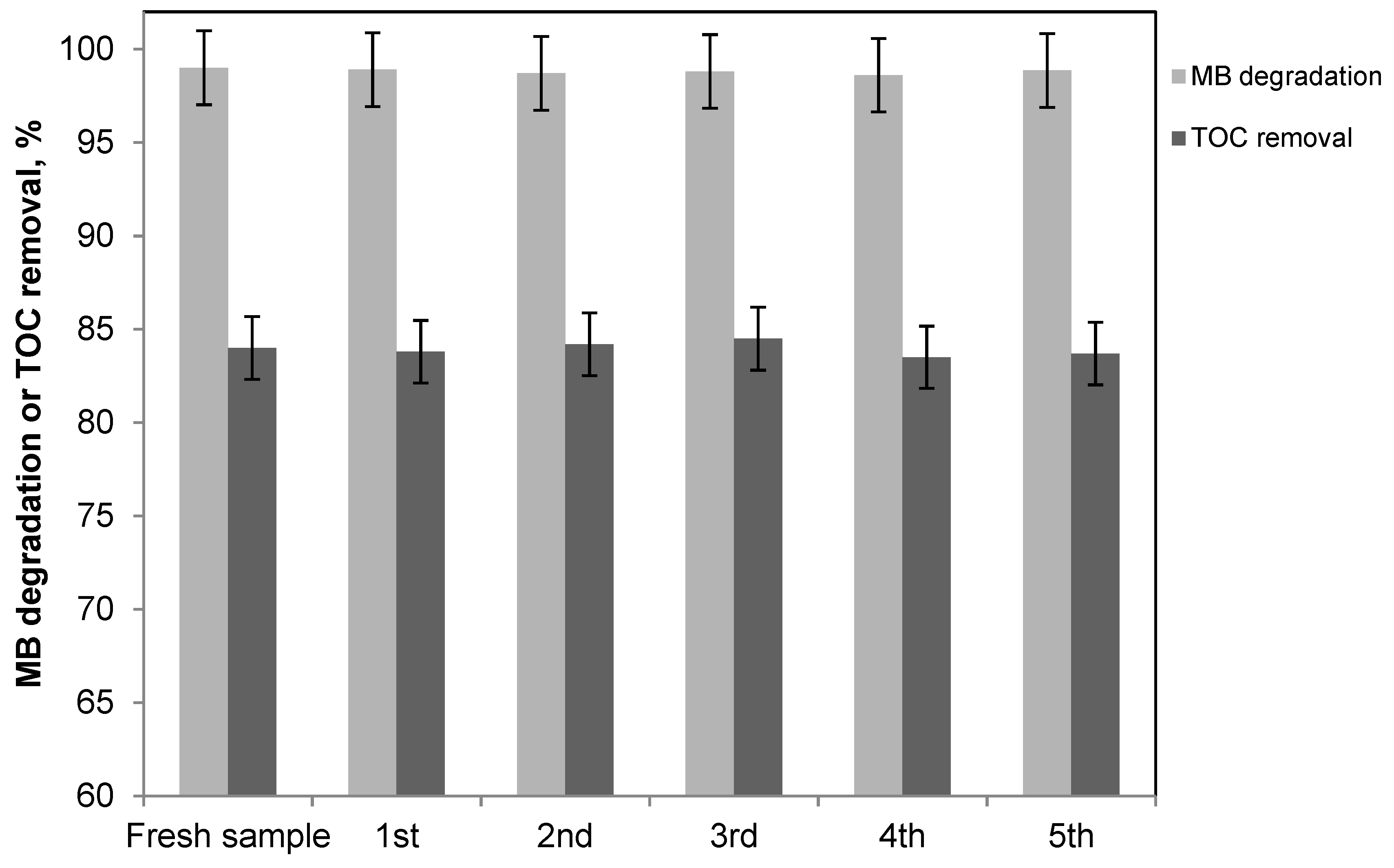
2.3. Degradation and Mineralization of Ceftriaxone, Paracetamol, and Caffeine with the Packed Bed Photoreactor
3. Materials and Methods
3.1. Preparation of the Structured Photocatalyst
3.2. Photocatalytic Tests with the Packed Bed Photoreactor at Semi-Pilot Scale
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ho, Y.C.; Show, K.Y.; Guo, X.X.; Norli, I.; Abbas, F.M.A.; Morad, N. Industrial Discharge and Their Effect to the Environment; InTech: London, UK, 2012; pp. 1–32. [Google Scholar]
- Koch, M.; Yediler, A.; Lienert, D.; Insel, G.; Kettrup, A. Ozonation of hydrolyzed azo dye reactive yellow 84 (ci). Chemosphere 2002, 46, 109–113. [Google Scholar] [CrossRef]
- Can, O.T.; Kobya, M.; Demirbas, E.; Bayramoglu, M. Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 2006, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment. Ii: Hybrid methods. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Panizza, M.; Brillas, E.; Comninellis, C. Application of boron-doped diamond electrodes for wastewater treatment. J. Environ. Eng. Manag. 2008, 18, 139–153. [Google Scholar]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Isupova, L.A. Mathematical modelling of the heterogeneous photo-fenton oxidation of acetic acid on structured catalysts. Chem. Eng. J. 2013, 224, 53–58. [Google Scholar] [CrossRef]
- Yu, L.; Chen, J.; Liang, Z.; Xu, W.; Chen, L.; Ye, D. Degradation of phenol using Fe3O4-GO nanocomposite as a heterogeneous photo-fenton catalyst. Sep. Purif. Technol. 2016, 171, 80–87. [Google Scholar] [CrossRef]
- Saleh, R.; Taufik, A. Degradation of methylene blue and congo-red dyes using fenton, photo-fenton, sono-fenton, and sonophoto-fenton methods in the presence of iron(ii,iii) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Rajasulochana, P.; Preethy, V. Comparison on efficiency of various techniques in treatment of waste and sewage water—A comprehensive review. Resour. Effic. Technol. 2016, 2, 175–184. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Z.; Wu, S.; Liu, F. Treatment of municipal wastewater by a magnetic activated sludge device. Desalin. Water Treat. 2015, 53, 909–918. [Google Scholar] [CrossRef]
- Maicu, M.; Hidalgo, M.C.; Colón, G.; Navío, J.A. Comparative study of the photodeposition of pt, au and pd on pre-sulphated TiO2 for the photocatalytic decomposition of phenol. J. Photochem. Photobiol. A Chem. 2011, 217, 275–283. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Matarangolo, M. Zno supported on zeolite pellets as efficient catalytic system for the removal of caffeine by adsorption and photocatalysis. Sep. Purif. Technol. 2018, 193, 303–310. [Google Scholar] [CrossRef]
- Kim, S.-H.; Ngo, H.H.; Shon, H.K.; Vigneswaran, S. Adsorption and photocatalysis kinetics of herbicide onto titanium oxide and powdered activated carbon. Sep. Purif. Technol. 2008, 58, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Ray, A.K. A new photocatalytic reactor for destruction of toxic water pollutants by advanced oxidation process. Catal. Today 1998, 44, 357–368. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Jafarikojour, M.; Dabir, B.; Sohrabi, M.; Royaee, S.J. Application of a new immobilized impinging jet stream reactor for photocatalytic degradation of phenol: Reactor evaluation and kinetic modelling. J. Photochem. Photobiol. A Chem. 2018, 364, 613–624. [Google Scholar] [CrossRef]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Kamble, S.P.; Sawant, S.B.; Pangarkar, V.G. Photocatalytic and photochemical degradation of nitrobenzene using artificial ultraviolet light. Chem. Eng. J. 2004, 102, 283–290. [Google Scholar] [CrossRef]
- Borges, M.E.; Sierra, M.; Esparza, P. Solar photocatalysis at semi-pilot scale: Wastewater decontamination in a packed-bed photocatalytic reactor system with a visible-solar-light-driven photocatalyst. Clean Technol. Environ. Policy 2017, 19, 1239–1245. [Google Scholar] [CrossRef]
- Rönnelid, M.; Perers, B.; Karlsson, B. Construction and testing of a large-area cpc-collector and comparison with a flat plate collector. Sol. Energy 1996, 57, 177–184. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with solar energy at a pilot-plant scale: An overview. Appl. Catal. B Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- Blanco, J.; Fernandez-Ibanez, P.; Malato-Rodríguez, S. Solar Photocatalytic Detoxification and Disinfection of Water: Recent Overview. J. Sol. Energy Eng. 2006, 129, 4–15. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Jo, W.-K.; Tayade, R.J. New generation energy-efficient light source for photocatalysis: Leds for environmental applications. Ind. Eng. Chem. Res. 2014, 53, 2073–2084. [Google Scholar] [CrossRef]
- Gan, W.Y.; Friedmann, D.; Amal, R.; Zhang, S.; Chiang, K.; Zhao, H. A comparative study between photocatalytic and photoelectrocatalytic properties of pt deposited TiO2 thin films for glucose degradation. Chem. Eng. J. 2010, 158, 482–488. [Google Scholar] [CrossRef]
- Casado, C.; Timmers, R.; Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Bowen, C.R.; van Grieken, R.; Marugán, J. Design and validation of a led-based high intensity photocatalytic reactor for quantifying activity measurements. Chem. Eng. J. 2017, 327, 1043–1055. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Favoni, O.; Fava, G.; Ruello, M.L. A novel method for the combined photocatalytic activity determination and bandgap estimation. Methods Protoc. 2018, 1, 22. [Google Scholar] [CrossRef]
- Hajaghazadeh, M.; Vaiano, V.; Sannino, D.; Kakooei, H.; Sotudeh-Gharebagh, R. Influence of operating parameters on gas phase photocatalytic oxidation of methyl-ethyl-ketone in a light emitting diode (led)-fluidized bed reactor. Korean J. Chem. Eng. 2015, 32, 636–642. [Google Scholar] [CrossRef]
- Romero, R.L.; Alfano, O.M.; Cassano, A.E. Cylindrical photocatalytic reactors. Radiation absorption and scattering effects produced by suspended fine particles in an annular space. Ind. Eng. Chem. Res. 1997, 36, 3094–3109. [Google Scholar] [CrossRef]
- Ata, R.; Sacco, O.; Vaiano, V.; Rizzo, L.; Tore, G.Y.; Sannino, D. Visible light active N-doped TiO2 immobilized on polystyrene as efficient system for wastewater treatment. J. Photochem. Photobiol. A Chem. 2017, 348, 255–262. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Sutisna; Rokhmat, M.; Wibowo, E.; Murniati, R.; Khairurrijal; Abdullaha, M. Novel solar photocatalytic reactor for wastewater treatment. IOP Conf. Ser. Mater. Sci. Eng. 2017, 214, 012010. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Nady, N.; Abdelwahab, N.A.; Mohamed, W.A.A.; Abdel-Mottaleb, M.S.A. Flexible bench-scale recirculating flow cpc photoreactor for solar photocatalytic degradation of methylene blue using removable TiO2 immobilized on pet sheets. Int. J. Photoenergy 2016, 2016, 9270499. [Google Scholar] [CrossRef]
- Cunha, D.L.; Marques, M.; Kuznetsov, A.; Achete, C.A.; Machado, A.E.D.H. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. PeerJ 2018, 6, e4464. [Google Scholar] [CrossRef] [PubMed]
- Sutisna; Rokhmat, M.; Wibowo, E.; Khairurrijal; Abdullah, M. Prototype of a flat-panel photoreactor using tio2 nanoparticles coated on transparent granules for the degradation of methylene blue under solar illumination. Sustain. Environ. Res. 2017, 27, 172–180. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Pisano, D.; Sannino, D.; Ciambelli, P. From the design to the development of a continuous fixed bed photoreactor for photocatalytic degradation of organic pollutants in wastewater. Chem. Eng. Sci. 2015, 137, 152–160. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Kinetic modelling for the photocatalytic degradation of phenol by using TiO2-coated glass raschig rings under simulated solar light. J. Chem. Technol. Biotechnol. 2016, 91, 346–352. [Google Scholar] [CrossRef]
- Vaiano, V.; Sarno, G.; Sacco, O.; Sannino, D. Degradation of terephthalic acid in a photocatalytic system able to work also at high pressure. Chem. Eng. J. 2017, 312, 10–19. [Google Scholar] [CrossRef]
- Sacco, O.; Stoller, M.; Vaiano, V.; Ciambelli, P.; Chianese, A.; Sannino, D. Photocatalytic degradation of organic dyes under visible light on N-doped TiO2 photocatalysts. Int. J. Photoenergy 2012, 2012, 626759. [Google Scholar] [CrossRef]
- Perego, C.; Peratello, S. Experimental methods in catalytic kinetics. Catal. Today 1999, 52, 133–145. [Google Scholar] [CrossRef]
- De Lasa, H.; Serrano, B.; Salaices, M. Photocatalytic Reaction Engineering; Springer Science+Business Media: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Mahdizadeh, F.; Aber, S.; Karimi, A. Synthesis of nano zinc oxide on granular porous scoria: Application for photocatalytic removal of pharmaceutical and textile pollutants from synthetic and real wastewaters. J. Taiwan Inst. Chem. Eng. 2015, 49, 212–219. [Google Scholar] [CrossRef]
- Shaykhi, Z.M.; Zinatizadeh, A.A.L. Statistical modeling of photocatalytic degradation of synthetic amoxicillin wastewater (saw) in an immobilized TiO2 photocatalytic reactor using response surface methodology (rsm). J. Taiwan Inst. Chem. Eng. 2014, 45, 1717–1726. [Google Scholar] [CrossRef]
- Zuccato, E.; Calamari, D.; Natangelo, M.; Fanelli, R. Presence of therapeutic drugs in the environment. Lancet 2000, 355, 1789–1790. [Google Scholar] [CrossRef]
- Ethiraj, R.; Sampath, V.S.; Vahid, A.; Raj, J.; Thiruvengadam, E. Development and validation of stability indicating spectroscopic method for content analysis of ceftriaxone sodium in pharmaceuticals. Int. Sch. Res. Not. 2014, 2014, 278173. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Shokri, M.; Isapour, G.; Shamsvand, S.; Kavousi, B. Photocatalytic Degradation of Ceftriaxone in Aqueous Solutions by Immobilized TiO2 and ZnO Nanoparticles: Investigating Operational Parameters. J. Mater. Environ. Sci. 2016, 7, 2843–2851. [Google Scholar]
- Rizzo, L.; Sannino, D.; Vaiano, V.; Sacco, O.; Scarpa, A.; Pietrogiacomi, D. Effect of solar simulated N-doped TiO2 photocatalysis on the inactivation and antibiotic resistance of an E. coli strain in biologically treated urban wastewater. Appl. Catal. B Environ. 2014, 144, 369–378. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production. In Scattering Methods and the Properties of Polymer Materials; Stribeck, N., Smarsly, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–14. [Google Scholar]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on mg doped ZnO-Al2O3 heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Wang, Y.; Shi, H.; Liu, E.; Fan, J.; Hu, X. Degradation and removal of ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 photocatalyst. J. Colloid Interface Sci. 2018, 523, 7–17. [Google Scholar] [CrossRef] [PubMed]






| Catalyst | Catalyst Size | Initial MB Concentration (mg/L) | Light Source | Treatment Time * | Reusability | References |
|---|---|---|---|---|---|---|
| TiO2/plastic granules | 3.5 mm | 25 | Solar light | >48 h | Not reported | [34] |
| TiO2/PET | 2 × 7 cm | 5 | Solar light | 5 h | Not reported | [35] |
| TiO2/glass spheres | 5 mm | 10 | Solar simulator | >1.5 h | Decrease of activity | [36] |
| TiO2/polypropylene | 3.5 mm | 25 | Solar light | 6 h | Not reported | [37] |
| N-TiO2/glass spheres | 1 cm | 10 | Visible light lamps | >6.5 h | Not reported | [38] |
| Liquid Flow Rate (Q0), mL/min | MB Conversion (X) |
|---|---|
| 74.15 | 0.068 |
| 50 | 0.136 |
| 25.2 | 0.258 |
| 12.6 | 0.512 |
| 6.3 | 0.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sacco, O.; Sannino, D.; Vaiano, V. Packed Bed Photoreactor for the Removal of Water Pollutants Using Visible Light Emitting Diodes. Appl. Sci. 2019, 9, 472. https://doi.org/10.3390/app9030472
Sacco O, Sannino D, Vaiano V. Packed Bed Photoreactor for the Removal of Water Pollutants Using Visible Light Emitting Diodes. Applied Sciences. 2019; 9(3):472. https://doi.org/10.3390/app9030472
Chicago/Turabian StyleSacco, Olga, Diana Sannino, and Vincenzo Vaiano. 2019. "Packed Bed Photoreactor for the Removal of Water Pollutants Using Visible Light Emitting Diodes" Applied Sciences 9, no. 3: 472. https://doi.org/10.3390/app9030472
APA StyleSacco, O., Sannino, D., & Vaiano, V. (2019). Packed Bed Photoreactor for the Removal of Water Pollutants Using Visible Light Emitting Diodes. Applied Sciences, 9(3), 472. https://doi.org/10.3390/app9030472







