The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production
Abstract
:1. Introduction
2. Nutrient Fortification, Its Relevance and Types
3. Biofortification of Crops
3.1. Crop Breeding and Genetic Modification as Biofortification Tool
3.2. Agronomic Biofortification
4. Inorganic Fertilizers
4.1. Nitrogen Fertilizers
4.2. Phosphorus Fertilizers
4.3. Potassium Fertilizers
4.4. Secondary and Micronutrients
5. Biofertilizers
6. Nanofertilizers
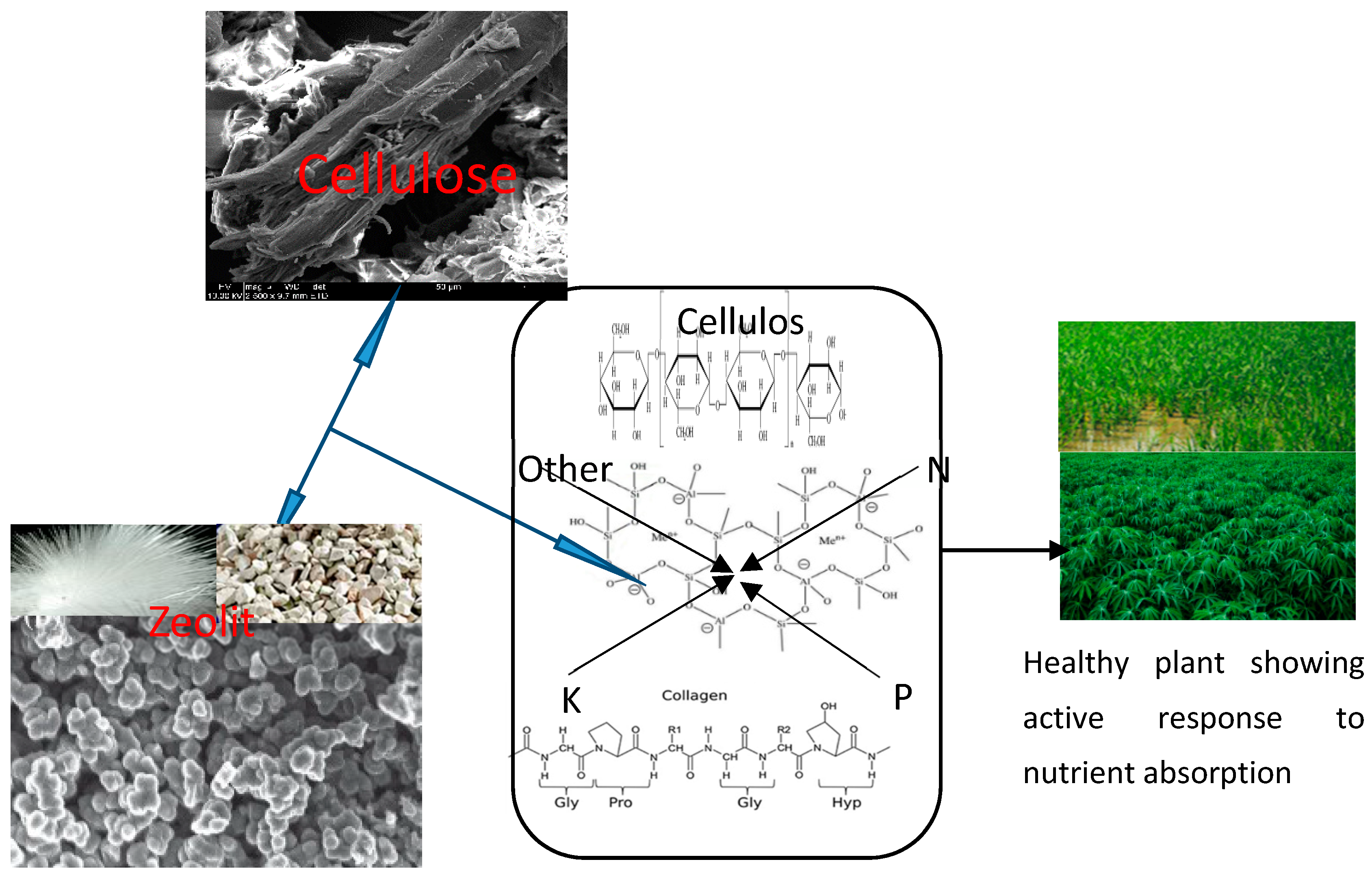
6.1. Zeolite-Based Nanofertilizer System for Sustainable Agriculture
6.2. Synthesis and Role of Zinc/Zinc Oxide Nanoparticles in Fertilizers
6.3. Iron Oxide Nanoparticles and Their Role in Plant Nutrient Fortification
6.4. Copper and Copper Oxide Nanoparticles (CuO NPs)
6.5. Titanium Dioxide Nanoparticles (TiO2 NPs)
6.6. Cerium Oxide Nanoparticles (CeO2NPs)
6.7. Noble Metal Nanoparticles
6.8. Selenium Nanoparticles (SeNPs)
6.9. Carbon-Based Nanomaterials in Plant Fortification
6.10. Nanosilicon Dioxide
7. General Synthesis of Nanomaterials
8. Future of Nanotechnology in Plant Improvement
9. Biosafety of Nanomaterials in Sustainable Agriculture
10. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- United Nation Department of Economic and Social Affairs. World Population Projected to Reach 9.6 Billion by 2050; United Nation Department of Economic and Social Affairs: New York, NY, USA, 2015. [Google Scholar]
- Subramanian, K.S.; Tarafdar, J.C. Prospects of nanotechnology in Indian farming. Indian J. Agric. Sci. 2011, 81, 887–893. [Google Scholar]
- Xiong, T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Shahid, M.; Pierart, A.; Sobanska, S. Copper Oxide Nanoparticle Foliar Uptake, Phytotoxicity, and Consequences for Sustainable Urban Agriculture. Environ. Sci. Technol. 2017, 51, 5242–5251. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, T.; Kumar, R. Bio-Nanotechnology and its Role in Agriculture and Food Industry. J. Mol. Genet. Med. 2018, 12, 1–5. [Google Scholar]
- Kabiri, S.; Degryse, F.; Tran, D.N.H.; Da-Silva, R.C.; Mclaughlin, M.J.; Losic, D. Graphene oxide; A new carrier for slow release of plant micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325. [Google Scholar] [CrossRef] [PubMed]
- Harper, T. The Year of the Trillion Dollar Nanotechnology Market? AZoNetwork UK Ltd.: Manchester, UK, 2015. [Google Scholar]
- Hossain, S.M.; Mohiuddin, A.K.M. Study on Biofortification of Rice by Targeted Genetic Engineering. Int. J. Agric. Res. Innov. Technol. 2012, 2, 25–35. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Food Fortification Programs to Alleviate Micronutrient Deficiencies. J. Food Process Technol. 2013, 4, 257–267. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Kumar, R.; Bhutta, Z.A. Micronutrient fortification of food and its impact on woman and child health: A systematic review. Syst. Rev. 2013, 2, 67. [Google Scholar] [CrossRef]
- Hwalla, N.; Al Dhaheri, A.S.; Radwan, H.; Alfawaz, H.A.; Fouda, M.A.; Al-Daghri, N.M.; Zaghloul, S.; Blumberg, J.B. The prevalence of micronutrient deficiencies and inadequacies in the middle east and approaches to interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Burchi, F.; Fanzo, J.; Frison, E. The Role of Food and Nutrition System Approaches in Tackling Hidden Hunger. Int. J. Environ. Res. Public Health 2011, 8, 358–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, S. Zn and Fe biofortification: The right chemical environment for human bioavailability. Plant Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Datta, M.; Vitolins, M.Z. Food Fortification and Supplement Use-Are There Health Implications? Crit. Rev. Food Sci. Nutr. 2016, 56, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Aggarwal, P.; Kaur, A. Biofortification: A new approach to eradicate hidden hunger. Food Rev. Int. 2017, 33, 1–21. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Nestel, P.; Meenakshi, J.V.; Qaim, M.; Sachdev, H.P.S.; Bhutta, Z.A. Plant breeding to control zinc deficiency in India: How cost-effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar] [CrossRef]
- WHO. Nutrition for Health and Development; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Smith, I.F. Micronutrient interventions: Options for Africa. Food Nutr. Bull. 2000, 21, 532–537. [Google Scholar] [CrossRef]
- Bouis, H. An Overview of the landscape and approach for Biofortification in Africa. Afr. J. Food Agric. Nutr. Dev. 2017. [Google Scholar] [CrossRef]
- Gibson, R.S.; Anderson, V.P. A review of interventions based on dietary diversification or modification strategies with the potential to enhance intakes of total and absorbable zinc. Food Nutr. Bull. 2009, 30, 108–143. [Google Scholar] [CrossRef]
- Arimond, M.; Ruel, M.T. Dietary Diversity Is Associated with Child Nutritional Status: Evidence from 11 Demographic and Health Surveys. J. Nutr. 2004, 134, 2579–2585. [Google Scholar] [CrossRef]
- Joy, E.J.M.; Kumssa, D.B.; Broadley, M.R.; Watts, M.J.; Young, S.D.; Chilimba, A.D.C.; Ander, E.L. Dietary mineral supplies in Malawi: Spatial and socioeconomic assessment. BMC Nutr. 2015, 1, 42. [Google Scholar] [CrossRef]
- Semba, R.D. The vitamin A story: Lifting the shadow of death. Vitamin A Story Lift Shad Death 2012, 104, 1–207. [Google Scholar]
- Murgia, I.; Arosio, P.; Tarantino, D.; Soave, C. Biofortification for combating ‘hidden hunger’ for iron. Trends Plant Sci. 2012, 17, 47–55. [Google Scholar] [CrossRef]
- Rawat, R.; Nguyen, P.H.; Ali, D.; Saha, K.; Alayon, S.; Kim, S.S.; Ruel, M.; Menon, P. Learning How Programs Achieve their Impact: Embedding Theory-Driven Process Evaluation and Other Program Learning Mechanisms in Alive & Thrive. Food Nutr. Bull. 2013, 34, S212–S225. [Google Scholar]
- Gilligan, D.O. Biofortification, agricultural technology adoption, and nutrition policy: Some lessons and emerging challenges. CESifo Econ Stud. 2012, 58, 405–421. [Google Scholar] [CrossRef]
- Gibson, R.S.; Hotz, C. Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br. J. Nutr. 2001, 85, S159. [Google Scholar] [CrossRef]
- Pandav, C.S.; Yadav, K.; Srivastava, R.; Pandav, R.; Karmarkar, M.G. Iodine deficiency disorders (IDD) control in India. Indian J. Med. Res. 2013, 138, 418–433. [Google Scholar]
- Tan-Torres, E.T.; Aikins, M.; Black, R.; Wolfson, L.; Hutubessy, R.; Evans, D.B. Cost effectiveness analysis of strategies for child health in developing countries. BMJ 2005, 331, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, D.K. Vitamin a supplementation for preventing death and illness in children 6 months to 5 years of age. In Cochrane Database of Systematic Reviews; Tovey, D., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Dubock, A. An overview of agriculture, nutrition and fortification, supplementation and biofortification: Golden Rice as an example for enhancing micronutrient intake. Agric. Food Secur. 2017, 6, 59. [Google Scholar] [CrossRef]
- Mayer, J.E.; Pfeiffer, W.H.; Beyer, P. Biofortified crops to alleviate micronutrient malnutrition. Curr. Opin. Plant Biol. 2008, 11, 166–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucca, P.; Hurrell, R.; Potrykus, I. Fighting Iron Deficiency Anemia with Iron-Rich Rice. J. Am. Coll. Nutr. 2002, 21, 184S–190S. [Google Scholar] [CrossRef]
- Pandya-Lorch, S.F.R. About IFPRI and the 2020 Vision Initiative; International Food Policy Research Institute: Washington, DC, USA, 2012. [Google Scholar]
- Bilski, J.; Jacob, D.; Soumaila, F.; Kraft, C.; Farnsworth, A. Agronomic Biofortification of Cereal Crop Plants with Fe, Zn, and Se, by the Utilization of Coal Fly Ash as Plant Growth Media. Adv. Biores. 2012, 3, 130–136. [Google Scholar]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Melash, A.A.; Mengistu, D.K.; Aberra, D.A. Linking Agriculture with Health through Genetic and Agronomic Biofortification. Agric. Sci. 2016, 07, 295–307. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic Biofortification of Cereal Grains with Iron and Zinc. In Advances in Agronomy; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 55–91. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R.P. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D.; Welch, R.M.; Saunders, D.A.; Ortiz-Monasterio, I.; Bouis, H.E.; Bonierbale, M.; de Haan, S.; Burgos, G.; Thiele, G.; Liria, R.; et al. Nutritious Subsistence Food Systems. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; pp. 1–74. [Google Scholar]
- Duffner, A.; Weng, L.; Hoffland, E.; van der Zee, S.E.A.T.M. Multi-surface Modeling to Predict Free Zinc Ion Concentrations in Low-Zinc Soils. Environ. Sci. Technol. 2014, 48, 5700–5708. [Google Scholar] [CrossRef] [PubMed]
- Ros, G.H.; Van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Jones, K.M.; de Brauw, A. Using Agriculture to Improve Child Health: Promoting Orange Sweet Potatoes Reduces Diarrhea. World Dev. 2015, 74, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Dimkpa, C.O.; Bindraban, P.S. Fortification of micronutrients for efficient agronomic production: A review. Agron. Sustain. Dev. 2016, 36, 7. [Google Scholar] [CrossRef]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef] [Green Version]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Raj, T.G.; Khan, N.A. Designer nanoparticle: Nanobiotechnology tool for cell biology. Nano Converg. 2016, 3, 22. [Google Scholar]
- Bindraban, P.S.; Dimkpa, C.; Nagarajan, L.; Roy, A.; Rabbinge, R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biol. Fertil. Soils 2015, 51, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–3. [Google Scholar]
- McKenzie, R. Crop Nutrition and Fertilizer Requirements Essential Plant Nutrients. Agri-Facts 1998, 540, 1–7. [Google Scholar]
- Monreal, C.M.; Derosa, M.; Mallubhotla, S.C.; Bindraban, P.S.; Dimkpa, C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. [Google Scholar] [CrossRef]
- Rech, I.; Polidoro, J.C.; Pavinato, P.S. Additives incorporated into urea to reduce nitrogen losses after application to the soil. Pesqui Agropecu Bras 2017, 52, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Fang, Y.; Sun, D.; Shi, Y. Efficiency of two nitrification inhibitors (dicyandiamide and 3, 4-dimethypyrazole phosphate) on soil nitrogen transformations and plant productivity: A meta-analysis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Monostori, T. Crop Production; University of Szeged, Faculty of Agriculture Andrássy út 15: Hódmezővásárhely, Hungary, 2014; pp. 15–20. [Google Scholar]
- Diatta, J.; Borowiak, K.; Szczepaniak, W. Evaluation of fertilizers solubility and phosphate release in slightly acidic arable soil. Arch. Agron. Soil Sci. 2018, 64, 1131–1141. [Google Scholar] [CrossRef]
- Hagin, J.; Tucker, B. Fertilization of Dryland and Irrigated Soils; Advanced Series in Agricultural Sciences; Springer: Berlin/Heidelberg, Germany, 1982; pp. 120–140. [Google Scholar]
- Olson-Rutz, K.; Jones, C. Soil Nutrient Management for Forages; Department of Land Resources and Environmental Sciences, Montana State University: Bozeman, MT, USA, 2015; pp. 1–10. [Google Scholar]
- Manikandan, A.; Subramanian, K. Evaluation of Zeolite Based Nitrogen Nano-fertilizers on Maize Growth, Yield and Quality on Inceptisols and Alfisols. Int. J. Plant Soil Sci. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Rai, A.; Rai, S.; Rakshit, A. Mycorrhiza-mediated phosphorus use efficiency in plants. Environ. Exp. Biol. 2013, 11, 107–117. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front Microbiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suppan, S. Nanomaterials in Soil; Institute for Agriculture and Trade Policy: Washington, DC, USA, 2013. [Google Scholar]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2017, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shan, C.; Zhang, Y.; Cai, J.; Zhang, W.; Pan, B. Arsenate Adsorption by Hydrous Ferric Oxide Nanoparticles Embedded in Cross-linked Anion Exchanger: Effect of the Host Pore Structure. ACS Appl. Mater. Interfaces 2016, 8, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Kamran, A.; Haroon, Z.K.; Muhammad, Z.; Imdad, H.; Zeeshan, A. Nano-zinc oxide as a future fertilizer. Weekly Technology Times, 27 April 2016. [Google Scholar]
- Azam, F. Added nitrogen interaction in the soil-plant system—A review. Pakistan J. Agron. 2002, 1, 54–59. [Google Scholar]
- Morales-Díaz, A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef] [Green Version]
- Suppan, S. Applying Nanotechnology to Fertilizer the Institute for Agriculture and Trade Policy Works Locally and Globally at the Intersection of Policy and Practice to Ensure Fair and Sustainable Food, Farm and Trade Systems. October 2017. Available online: iatp.org (accessed on 23 January 2019).
- Eroglu, N.; Emekci, M.; Athanassiou, C.G. Applications of natural zeolites on agriculture and food production. J. Sci. Food Agric. 2017, 97, 3487–3499. [Google Scholar] [CrossRef] [PubMed]
- Polat, E.; Karaca, M.; Demir, H.; Onus, N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Guo, J. Synchrotron radiation, soft-X-ray spectroscopy and nanomaterials. Int. J. Nanotechnol. 2004, 1, 193–225. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K.S. Development of slow release Zn fertilizer using nano-zeolite as carrier. J. Plant Nutr. 2018, 41, 311–320. [Google Scholar] [CrossRef]
- Suarato, G.; Rosalia Bertorelli, R.; Athanassiou, A. Borrowing from Nature: Biopolymers and Biocomposites as Smart Wound Care Materials. Front. Bioeng. Biotechnol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
- Tarmizi, E.Z.M.; Baqiah, H.; Talib, Z.A.; Kamari, H.M. Preparation and physical properties of polypyrrole/zeolite composites. Results Phys. 2018, 11, 793–800. [Google Scholar] [CrossRef]
- Singh, N.B.; Amist, N.; Yadav, K.; Singh, D.; Pandey, J.K.; Singh, S.C. Zinc Oxide Nanoparticles as Fertilizer for the Germination, Growth and Metabolism of Vegetable Crops. J. Nanoeng. Nanomanuf. 2013, 3, 353–364. [Google Scholar] [CrossRef]
- Biesalski, H.K. Hidden Hunger; Springer: Berlin, Germany, 2013. [Google Scholar]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 905–927. [Google Scholar] [CrossRef]
- Guilbert, J.J. The world health report 2002—Reducing risks, promoting healthy life. Educ. Health 2003, 16, 230. [Google Scholar]
- Mortvedt, J.J.; Giordano, P.M. Crop Response to Zinc Oxide Applied in Liquid and Granular Fertilizers. J. Agric. Food Chem. 1967, 15, 118–122. [Google Scholar] [CrossRef]
- Milani, N.; Hettiarachchi, G.M.; Kirby, J.K.; Beak, D.G.; Stacey, S.P.; McLaughlin, M.J. Fate of zinc oxide nanoparticles coated onto macronutrient fertilizers in an alkaline calcareous soil. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Phull, A.R.; Ali, J.S. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49. [Google Scholar]
- Hu, J.; Guo, H.; Li, J.; Wang, Y.; Xiao, L.; Xing, B. Interaction of γ-Fe2O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. Nanobiotechnol. 2017, 15, 1–12. [Google Scholar] [CrossRef]
- Corredor, E.; Testillano, P.S.; Coronado, M.J.; González-Melendi, P.; Fernández-Pacheco, R.; Marquina, C.; Ibarra, M.R.; De La Fuente, J.M.; Rubiales, D.; Pérez-De-Luque, A.; et al. Nanoparticle penetration and transport in living pumpkin plants: In situ subcellular identification. BMC Plant Biol. 2009, 9, 1–11. [Google Scholar] [CrossRef]
- Liu, J.; Dhungana, B.; Cobb, G.P. Environmental behavior, potential phytotoxicity, and accumulation of copper oxide nanoparticles and arsenic in rice plants. Environ. Toxicol. Chem. 2018, 37, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.T.; Dumat, C.; Dappe, V.; Vezin, H.; Schreck, E.; Sahid, M.; Pierart, A.; Sobanksa, S. Potential Contamination of Copper Oxide Nanoparticles and Possible Consequences on Urban Agriculture. Environ. Sci. Technol. 2017, 78, 5774–5782. [Google Scholar]
- Pestovsky, Y.S.; Martínez-Antonio, A. The Use of Nanoparticles and Nanoformulations in Agriculture. J. Nanosci. Nanotechnol. 2017, 17, 8699–8730. [Google Scholar] [CrossRef]
- Zheng, L.; Hong, F.; Lu, S.; Liu, C. Effect of Nano-TiO2 on Strength of Naturally Aged Seeds and Growth of Spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Gui, X.; Zhang, Z.; Liu, S.; Ma, Y.; Zhang, P.; He, X.; Li, Y.; Zhang, J.; Li, H.; Rui, Y.; et al. Fate and phytotoxicity of CeO2 nanoparticles on lettuce cultured in the potting soil environment. PLoS ONE 2015, 10, 1–10. [Google Scholar] [CrossRef]
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (: Glycine max (L.) Merr.). Environ. Sci. Nano 2017, 4, 1086–1094. [Google Scholar] [CrossRef]
- Saurabh, S.; Singh, B.K.; Yadav, S.M.; Gupta, A.K. Applications of Nanotechnology in Agricultural and their Role in Disease Management. Res. J. Nanosci. Nanotechnol. 2015, 5, 15. [Google Scholar]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masrahi, A.; VandeVoort, A.R.; Arai, Y. Effects of silver nanoparticle on soil-nitrification processes. Arch. Environ. Contam. Toxicol. 2014, 66, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Guleria, P.; Kumar, V.; Yadav, S.K. Gold nanoparticle exposure induces growth and yield enhancement in Arabidopsis thaliana. Sci. Total Environ. 2013, 461, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green synthesis of nanoparticles: Their advantages and disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar]
- Mahakham, W.; Theerakulpisut, P.; Maensiri, S.; Phumying, S.; Sarmah, A.K. Environmentally benign synthesis of phytochemicals-capped gold nanoparticles as nanopriming agent for promoting maize seed germination. Sci. Total Environ. 2016, 573, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of gold nanomaterials to plants: Importance of particle size and surface coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Astafurova, T.; Zotikova, A.; Morgalev, Y.; Verkhoturova, G.; Postovalova, V.; Kulizhskiy, S.; Mikhailova, S. Effect of platinum nanoparticles on morphological parameters of spring wheat seedlings in a substrate-plant system. IOP Conf. Ser. Mater. Sci. Eng. 2015, 98, 012004. [Google Scholar] [CrossRef] [Green Version]
- Asztemborska, M.; Steborowski, R.; Kowalska, J.; Bystrzejewska-Piotrowska, G. Accumulation of platinum nanoparticles by Sinapis alba and Lepidium sativum plants. Water Air Soil Pollut. 2015, 226, 126. [Google Scholar] [CrossRef]
- Ngo, Q.B.; Dao, T.H.; Nguyen, H.C.; Tran, X.T.; Van Nguyen, T.; Khuu, T.D.; Huynh, T.H. Effects of nanocrystalline powders (Fe, Co and Cu) on the germination, growth, crop yield and product quality of soybean (Vietnamese species DT-51). Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 015016. [Google Scholar] [CrossRef] [Green Version]
- Skalickova, S.; Milosavljevic, V.; Cihalova, K.; Horky, P.; Richtera, L.; Adam, V. Selenium nanoparticles as a nutritional supplement. Nutrition 2017, 33, 83–90. [Google Scholar] [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected MajorRisks; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- Uttam, S.; Abioye, F.L.S. Selenium in the Soil-Plant Environment: A Review. Int. J. Appl. Agric. Sci. 2017, 3, 1–18. [Google Scholar]
- Meetu, G.; Shikha, G. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar]
- Carvalho, K.M.; Gallardo-Williams, M.T.; Benson, R.F.; Martin, D.F. Effects of selenium supplementation on four agricultural crops. J. Agric. Food Chem. 2003, 51, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Dall’acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.M.A. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanumlycopersicon L.). Aquat. Toxicol. 2012. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T. Chemoprotection against cancer by isothio-cyanates: A focus on the animal models and the protective mechanisms. Top. Curr. Chem. 2013, 329, 179–201. [Google Scholar] [PubMed]
- Lahiani, M.H.; Chen, J.; Irin, F.; Puretzky, A.A.; Green, M.J.; Khodakovskaya, M.V. Interaction of carbon nanohorns with plants: Uptake and biological effects. Carbon 2015, 81, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Hyung, H.; Fortner, J.D.; Hughes, J.B.; Kim, J.-H. Natural Organic Matter Stabilizes Carbon Nanotubes in the Aqueous Phase. Environ. Sci. Technol. 2007, 41, 179–184. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef]
- Chen, R.; Ratnikova, T.A.; Stone, M.B.; Lin, S.; Lard, M.; Huang, G.; Hudson, J.A.S.; Ke, P.C. Differential uptake of carbon nanoparticles by plant and mammalian cells. Small 2010, 6, 612–617. [Google Scholar] [CrossRef]
- De La Torre-Roche, R.; Hawthorne, J.; Deng, Y.; Xing, B.; Cai, W.; Newman, L.A.; Wang, C.; Ma, X.; White, J.C. Fullerene-enhanced accumulation of p, p’-DDE in agricultural crop species. Environ. Sci. Technol. 2012, 46, 9315–9323. [Google Scholar] [CrossRef] [PubMed]
- Khodakovskaya, M.V.; De Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon nanotubes induce growth enhancement of tobacco cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-M.; Lin, C.; Fugetsu, B. Studies on toxicity of multi-walled carbon nanotubes on suspension rice cells. Carbon 2009, 47, 3479–3487. [Google Scholar] [CrossRef] [Green Version]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2017, 66, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Avestan, S.; Naseri, L.A.; Hassanzade, A.; Sokri, S.M.; Barker, A.V. Effects of nanosilicon dioxide application on in vitro proliferation of apple rootstock. J. Plant Nutr. 2016, 39, 850–855. [Google Scholar] [CrossRef]
- Yeo, A.R.; Flowers, S.A.; Rao, G.; Welfare, K.; Senanayake, N.; Flowers, T.J. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 1999, 22, 559–565. [Google Scholar] [CrossRef]
- Faryadi, S.; Sheikhahmadi, A. Effect of nanosilicon dioxide on growth performance, egg quality, liver histopathology and concentration of calcium, phosphorus and silicon in egg, liver and bone in laying quails. Appl. Nanosci. 2017, 7, 765–772. [Google Scholar] [CrossRef] [Green Version]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front Plant Sci. 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front Chem. 2017, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; White, J.C.; Xing, B. Interactions between engineered nanomaterials and agricultural crops: Implications for food safety. J. Zhejiang Univ. Sci. A 2014, 15, 552–572. [Google Scholar] [CrossRef]
- Raddy, R. Efficacy of Nano Zinc Particle on Growth. Master’s Thesis, University of Agricultural Sciences, Bangalore, India, 2014. [Google Scholar]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a Beneficial Element for Crop Production. Front. Plant Sci. 2017, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Le, V.N.; Rui, Y.; Gui, X.; Li, X.; Liu, S.; Han, Y. Uptake, transport, distribution and Bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Zarafshar, M.; Akbarinia, M.; Askari, H.; Hosseini, S.M.; Rahaie, M.; Struve, D. Toxicity Assessment of SiO2 Nanoparticles to Pear Seedlings. Int. J. Nanosci. Nanotechnol. 2015, 11, 13–22. [Google Scholar]
- Sidkey, N.M.; Ismail, A.A.; Arafa, R.A.; Fathy, R.M. Impact of Silver and Selenium Nanoparticles Synthesized by Gamma Irradiation and Their Physiological Response on Early Blight Disease of Potato. J. Chem. Pharm. Res. 2016, 8, 934–951. [Google Scholar]
- Aslani, F.; Bagheri, S.; Julkapli, N.M.; Juraimi, A.S.; Sadat, F.; Hashemi, G.; Baghdadi, A. Effects of Engineered Nanomaterials on Plants Growth: An Overview. Sci. World J. 2014, 1–28. [Google Scholar] [CrossRef]
- Sztrik, A. Production of Nano-Size Elemental Selenium Particles and Its Investigation in the Soil-Plant-Animal System. Ph.D. Thesis, School of Crop Production, Horticulture and Food Science, University of Debrecen, Debrecen, Hungary, 2016; pp. 1–26. [Google Scholar]
- Ragavan, P.; Ananth, A.; Rajan, M.R. Impact of Selenium Nanoparticles on Growth, Biochemical Characteristics and Yield of Cluster Bean Cyamopsis tetragonoloba. J. Environ. Agric. Biotechnol. 2017, 2, 2917–2926. [Google Scholar] [CrossRef]
- Shekhawat, M.S.; Kannan, N.; Manokari, M.; Ravidran, C.P. In vitro regeneration of shoots and ex-vitro rooting of an important medicinal plant Passiflora foetida L through segment cultures. J. Genetic Eng. Biotechnol. 2015, 13, 209–214. [Google Scholar] [CrossRef]
- Charoenkit, S.; Yieemwattana, S. Role of specific plant characteristics on thermal and carbon sequestration properties of living walls in tropical climate. Build. Environ. 2017, 115, 67–79. [Google Scholar] [CrossRef]
- Riaz, M.; Yan, L.; Wu, X.; Hussain, S.; Aziz, O.; Imran, M.; Rana, M.S.; Jiang, C. Boron reduces aluminum-induced growth inhibition, oxidative damage and alterations in the cell wall components in the roots of trifoliate orange. Ecotoxicol. Environ. Safety 2018, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, T.D.A.; Barcala-Jorge, A.S.; Andrade, J.M.O.; Ferreira, E.C.N.; Crespo, T.S.; Batista-Jorge, G.C.; Vieira, C.A.; Lelis, D.D.F.; Paraíso, A.F.; et al. Obesity and malnutrition similarly alter the renin–angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017, 48, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Arole, V.M.; Munde, S.V. Fabrication of nanomaterials by top-down and bottom-up approaches—An overview. J. Adv. Appl. Sci. Technol. 2014, 1, 89–93. [Google Scholar]
- Sharma, G.; Sharma, A.R.; Bhavesh, R.; Park, J.; Ganbold, B.; Nam, J.S.; Lee, S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried vitis vinifera (raisin) extract. Molecules 2014, 19, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, V. Biogenic Synthesis and Characterization of Selenium Nanoparticles Using the Flower of Bougainvillea spectabilis Willd. Int. J. Sci. Res. 2015, 4, 690–695. [Google Scholar]
- Surega, R. Green Synthesis of Bioactive Silver Nanoparticles Using Plant Extracts and Their Antinemic Properties. Ph.D. Thesis, Dept of Nematology, Centre for Plant Protection Studies, Tamil Nadu Agricultural University Coimbatore, Coimbatore, India, 2015. [Google Scholar]
- Zhang, J.-J.; Li, Z.; Zhao, S.; Lu, Y. Size-dependent modulation of graphene oxide–aptamer interactions for an amplified fluorescence-based detection of aflatoxin B1 with a tunable dynamic range. Analyst 2016, 141, 4029–4034. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Liu, Y.-G.; Jiang, L.-P.; Zhu, J.-J. Synthesis, characterization of silica-coated gold nanorods and its applications in electroanalysis of hemoglobin. Electrochem. Commun. 2008, 10, 355–358. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Gu, M.-M.; Zheng, T.-T.; Zhu, J.-J. Synthesis of Gelatin-Stabilized Gold Nanoparticles and Assembly of Carboxylic Single-Walled Carbon Nanotubes/Au Composites for Cytosensing and Drug Uptake. Anal. Chem. 2009, 81, 6641–6648. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P.; Dong, L.; Ruan, Y.; Lu, H. Highly conductive, mechanically strong graphene monolith assembled by three-dimensional printing of large graphene oxide. J.Col. Interf. Sci. 2019, 534, 12–19. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.-M.; Zhu, W.-Y.; Zhang, C.-H.; Tang, H.; Jiang, J.-H. Conjugated polymer nanoparticles-based fluorescent biosensor for ultrasensitive detection of hydroquinone. Anal. Chim. Acta 2018, 1012, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, S.; Sen, S. Rapid ultrasound assisted hydrothermal synthesis of highly pure nanozeolite X from fly ash for efficient treatment of industrial effluent. Chemosphere 2018, 210, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Neena, G.; Venugopal, B.; Honey, J.; Mathiazhagan, A.; Rani, J. Nanosilica decorated multiwalled carbon nanotubes (CS hybrids) in natural rubber latex. Polymer 2019, 161, 170–180. [Google Scholar]
- Barrena, R.; Casals, E.; Colón, J.; Font, X.; Sánchez, A.; Puntes, V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 2009, 75, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Fortner, J.D.; Sayes, C.M.; Colvin, V.L.; Hughe, J.B. Bacterial Cell Association and Antimicrobial Activity of a C 60 Water Suspension. Environ. Toxicol. Chem. 2005, 24, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- AG Chemgroup. Study Finds Potential Use for Zinc Oxide Nanoparticles for Fertilizer Feedstock; AG Chemgroup: Praha, Czech Republic, 2017. [Google Scholar]
- de Madrid, U.P. Will we be able to use zinc oxide nanoparticles as fertilizers. Science Daily, 11 October 2017. [Google Scholar]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Regulation of morphological, molecular and nutrient status in Arabidopsis thaliana seedlings in response to ZnO nanoparticles and Zn ion exposure. Sci. Total Environ. 2017, 575, 187–198. [Google Scholar] [CrossRef]
- Schützendübel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Reddy, P.V.L.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lessons learned: Are engineered nanomaterials toxic to terrestrial plants? Sci. Total Environ. 2016, 568, 470–479. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Hua, T.; Xiao, F.; Chen, C.; Gersberg, R.M.; Liu, Y.; Stuckey, D.; Ng, W.J.; Tan, S.K. Phytotoxicity and bioaccumulation of ZnO nanoparticles in Schoenoplectus tabernaemontani. Chemosphere 2015, 120, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kellermeier, F.; Armengaud, P.; Seditas, T.J.; Danku, J.; Salt, D.E.; Amtmann, A. Analysis of the Root System Architecture of Arabidopsis Provides a Quantitative Readout of Crosstalk between Nutritional Signals. Plant Cell 2014, 26, 1480–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, H.; Yao, J.; Sun, J.; Zhang, C.; Liu, W.; Zhu, M.; Ceccanti, B. The effect of metal oxide nanoparticles on functional bacteria and metabolic profiles in agricultural soil. Bull. Environ. Contam. Toxicol. 2015, 94, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Joan, E.; McLean, D.E.; Latta, E.M. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation and induction of oxidative stress in sand-grown wheat. Nanoparticle Res. 2012, 14, 1125–1140. [Google Scholar] [CrossRef]
- Tang, Y.; He, R.; Zhao, J.; Nie, G.; Xu, L.; Xing, B. Oxidative stress-induced toxicity of CuO nanoparticles and related toxicogenomic responses in Arabidopsis thaliana. Environ. Pollut 2016, 212, 605–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Wu, G.R.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 22, 168–172. [Google Scholar]
- Krishnaraj, C.; Ramachandran, R.; Mohan, K.; Kalaichelvan, P.T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 93, 95–99. [Google Scholar] [CrossRef]
- Yanga, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef]
- Rico, C.M.; Morales, M.I.; Barrios, A.C.; McCreary, R.; Hong, J.; Lee, W.Y.; Nunez, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J. Agric. Food Chem. 2013, 61, 11278–11285. [Google Scholar] [CrossRef]

| Fertilizers | N | P2O5 | K2O |
|---|---|---|---|
| Nitrogen fertilizers | |||
| Ammonium nitrate | 34 | 0 | 0 |
| Ammonium sulfate | 21 | 0 | 0 |
| Urea | 45–48 | 0 | 0 |
| Urea-ammonium nitrate | 28–33 | 0 | 0 |
| Anhydrous ammonia | 82 | 0 | 0 |
| Ammonium polyphosphate (a or b) | 10–11 | 34–37 | 0 |
| Phosphorus fertilizers | |||
| Ammoniated super-phosphate | 3–6 | 48–53 | 0 |
| Ammoniated super-phosphate | 3–6 | 48–53 | 0 |
| Di-ammonium phosphate | 11–18 | 48 | 0 |
| Mono-ammonium phosphate | 11 | 48–55 | 0 |
| Super-phosphate | 0 | 18–50 | 0 |
| Triple super phosphate | 0 | 46 | 0 |
| Ammonium polyphosphate | 10–15 | 34–37 | 0 |
| Urea ammonium phosphate | 28 | 27 | 0 |
| Potassium fertilizers | |||
| Potassium chloride (muriate of potash) | 0 | 0 | 60–63 |
| Protassium+® (sulfate of potash) | 0 | 0 | 50 |
| Potassium nitrate | 13 | 0 | 44 |
| Potassium-magnesium sulfate | 0 | 0 | 22 |
| Nanofertilizers | Constituents | Name of Manufacturer |
|---|---|---|
| Nano Ultra-Fertilizer (500) g | organic matter, 5.5%; Nitrogen, 10%; P2O5, 9%; K2O, 14%; P2O5, 8%; K2O, 14%; MgO, 3% | SMTET Eco-technologies Co., Ltd., Taiwan |
| Nano Calcium (Magic Green) (1) kg | CaCO3, 77.9%; MgCO3, 7.4%; SiO2, 7.47%; K, 0.2%; Na, 0.03%; P., 0.02%; Fe-7.4 ppm; Al2O3, 6.3 ppm; Sr, 804 ppm; sulfate, 278 ppm; Ba, 174 ppm; Mn, 172 ppm; Zn, 10 ppm | AC International Network Co., Ltd., Germany |
| Nano Capsule | N, 0.5%; P2O5, 0.7%; K2O, 3.9%; Ca, 2.0%; Mg, 0.2%; S, 0.8%; Fe, 2.0%; Mn, 0.004%; Cu, 0.007%; Zn, 0.004% | The Best International Network Co., Ltd., Thailand |
| Nano Micro Nutrient (EcoStar) (500) g | Zn, 6%; B, 2%; Cu, 1%; Fe, 6%+; EDTA Mo, 0.05%; Mn, 5%+; AMINOS, 5% | Shan Maw Myae Trading Co., Ltd., India |
| PPC Nano (120) mL | M protein, 19.6%; Na2O, 0.3%; K2O, 2.1%; (NH4)2SO4, 1.7%; diluent, 76% | WAI International Development Co., Ltd., Malaysia |
| Nano Max NPK Fertilizer | Multiple organic acids chelated with major nutrients, amino acids, organic carbon, organic micro nutrients/trace elements, vitamins, and probiotic | JU Agri Sciences Pvt. Ltd., Janakpuri, New Delhi, India |
| TAG NANO (NPK, PhoS, Zinc, Cal, etc.) fertilizers | Proteino-lacto-gluconate chelated with micronutrients, vitamins, probiotics, seaweed extracts, and humic acid | Tropical Agrosystem India (P) Ltd., India |
| Nano Green | Extracts of corn, grain, soybeans, potatoes, coconut, and palm | Nano Green Sciences, Inc., India |
| Biozar Nano-Fertilizer | Combination of organic materials, micronutrients, and macromolecules | Fanavar Nano-Pazhoohesh Markazi Company, Iran |
| Engineered Nanomaterials | Positive Effects on Crop Production | Negative Effects on Plants | Required Dosage for Plants | Biosafety Information | References |
|---|---|---|---|---|---|
| SWCNT | Improves the germination rates of crops | Higher conc. (100 mg/L) may lead to toxic effects such as necrosis and apoptosis | 10 mg/L for pepper (C. annuum) and 30 mg/L for salvia (S. macrosiphon) and tall fescue (F. arundinacea) | Responses depends on the type of plants or genotypic differences of the plants and seed size. small-seeded species, such as lettuce, onion, and tomato may be more sensitive and vulnerable | [124] |
| MWCNT | Absorption of nitrogen and phosphorus in waste water to deliver to crops | There could be increased ROS formation, reduced chlorophyll content and cell viability. There has been recorded DNA damage in onion roots. | In tobacco, 100 mg/ L | The dosage to be used depends on the plants. | [124] |
| Fullerene (C60) | They impede the uptake of pesticides by some plant species. | They inhibited chlorophyll accumulation in duckweed, photosynthesis and Mg uptake of phytoplankton. | [125] | ||
| Graphene | They can improve seed water content when applied moderately | At low or high concentrations, they can cause impaired antioxidative glutathione metabolism and increase the amount of ROS. It can also cause mechanical damages of cell wall and other organelles | 5–50 mg/L for growth stimulation and uptake into seedlings, 400 and 800 mg/L for Glutathione formation | The needed dosage may exceed the environmental requirement, so caution must be taken in the application. | [125] |
| Nanozeolite (building blocks of SiO4 and AlO4) | Improvement of soil quality, | [126] | |||
| FeO NPs, nano-zero-valent iron (nZVI) | root elongation, transforms and detoxifies chemicals in the soils | Reduce germination observed at 250 mg/L (Hordeum vulgare and Linum usitatissimum seeds) | [127] | ||
| ZnO NPs | It can lead to higher yield in plants | It can reduce the number of roots, length of rice seedlings and inhibit chlorophyll photosynthesis | ≤200 mg/kg Reduced germination observed at 2000 mg/L for Zea mays | [128,129] | |
| CeO NPs | Root elongation | May affect fruit flavor, nutrition levels and metabolites content | Reduced germination observed at 2000 mg/L for Medicago sativa, Zea mays and Cucumis sativus seeds | [127] | |
| TiO2 NPs | Enhances water and oxygen penetration into the capsules for quick germination and also improves seed stress resistance | Sometimes, their high quantity penetration could damage seed embryo and affect germination | The size and quantity administered determines the performance | [130] | |
| Cu/CuO NPs | Enhanced Plant growth | High doses lead to stunted growth, cell death and loss of leaf coloration | ≤10 mg/L | Toxicity depends on the plant species. High levels lead to liver and lung cell damage in human | [126] |
| Impaired photosynthesis | Above 1 mg/L | ≤0.25 mg/L | Complete inhibition of photosynthesis at higher doses | ||
| SiO2NPs | Used to deliver DNA, proteins, and other chemicals in plants | It can affect plant height, shoot and root biomasses; Cu, Mg, Na translocation can also be affected grossly It can support the uptake of K in leaves and reduce that of N and P. | ≤100 mg/L | [126,131,132] | |
| AuNPs | Improves root elongation | Damages cell division process, e.g. in onions. | [95] | ||
| AgNPs | It improves the chlorophyll content and can equally enhance catalase activity especially in potato | Sulfidation could occur (conversion of the nanoparticles to silver sulfides) which impedes root hair growth and thereby affects the absorption of nutrients. At high concentrations of about 3000–6000 μgmL−1, it can retard seed germination, and root and shoot growth, especially in rice Mung bean and Chinese cabbage | Approximately 150 ppm | They have to be applied in very low concentrations. It may be have more positive effects when applied with K2SO4 | [133,134] |
| SeNPs | Improves the root and shoot length. It equally enhances the chlorophyll and other plant metabolites | At high quantities, it can become pro-oxidant and cause damage to plants | 0.05–0.1 mg/kg | The quantity needed depends on the plant, size and method of preparation of the SeNPs. Higher concentration may hinder the production of the photosynthetic pigments | [135,136] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. https://doi.org/10.3390/app9030499
Elemike EE, Uzoh IM, Onwudiwe DC, Babalola OO. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Applied Sciences. 2019; 9(3):499. https://doi.org/10.3390/app9030499
Chicago/Turabian StyleElemike, Elias E., Ifeyinwa Monica Uzoh, Damian C. Onwudiwe, and Olubukola Oluranti Babalola. 2019. "The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production" Applied Sciences 9, no. 3: 499. https://doi.org/10.3390/app9030499
APA StyleElemike, E. E., Uzoh, I. M., Onwudiwe, D. C., & Babalola, O. O. (2019). The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Applied Sciences, 9(3), 499. https://doi.org/10.3390/app9030499








