Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction
Abstract
Featured Application
Abstract
1. Introduction
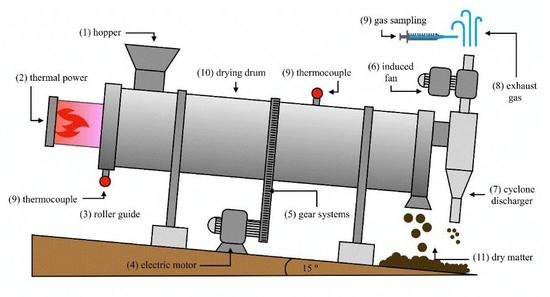
2. Materials and Methods
2.1. Identification of the Samples
2.2. Environmental Conditions
2.3. Sampling of VOCs and Conditions of Thermal Desorption-Gas Chromatography/Mass Spectrometry (TD-GC/MS)
2.4. Reagents
3. Results and Discussion
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Oil. Available online: http://www.internationaloliveoil.org/store/index/48-olivae-publications (accessed on 20 March 2018).
- Chile Oliva. Available online: https://www.chileoliva.cl/es/principal/ (accessed on 25 March 2017).
- Hernández, D.; Astudillo, L.; Gutiérrez, M.; Tenreiro, C.; Retamal, C.; Rojas, C. Biodiesel production from an industrial residue: Alperujo. Ind. Crops Prod. 2014, 52, 495–498. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; González, J.; García, D.; Cegarra, J. Agrochemical characterization of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Christoforou, E.; Kylili, A.; Fokaides, P. Technical and economical evaluation of olive mills solid waste pellets. Renew. Energy 2016, 96, 33–41. [Google Scholar] [CrossRef]
- Rincón, B.; Bujalance, L.; Fermoso, F.G.; Martín, A.; Borja, R. Effect of Ultrasonic Pretreatment on Biomethane Potential of Two-Phase Olive Mill Solid Waste: Kinetic Approach and Process Performance. Sci. World J. 2014, 2014, 648624. [Google Scholar] [CrossRef] [PubMed]
- Arjona, R.; García, A.; Ollero, P. Drying of alpeorujo, a waste product of the olive oil mill industry. J. Food Energy 1999, 41, 229–234. [Google Scholar] [CrossRef]
- Gómez de la Cruza, F.; Casanova-Peláez, P.; Palomar-Carnicero, J.; Cruz-Peragóna, F. Characterization and analysis of the drying real process in an industrial olive-oil mill waste rotary dryer: A case of study in Andalusia. Appl. Therm. Eng. 2017, 116, 1–10. [Google Scholar] [CrossRef]
- Fagernäs, L.; Brammer, J.; Wilén, C.; Lauer, M.; Verhoeff, F. Drying of biomass for second generation synfuel production. Biomass Bioenergy 2010, 34, 1267–1277. [Google Scholar] [CrossRef]
- Hernández, D.; Astudillo, C.A.; Fernández-Palacios, E.; Cataldo, F.; Tenreiro, C.; Gabriel, D. Evolution of physical-chemical parameters, microbial diversity and VOC emissions of olive oil mill waste exposed to ambient conditions in open reservoirs. Waste Manag. 2018, 79, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, J.; Martin-Gullon, I.; Conesa, J.; Font, R. Emissions from pyrolysis and combustion of olive oil solid waste. J. Anal. Appl. Pyrolysis 2005, 74, 512–517. [Google Scholar] [CrossRef]
- DIN. Available online: http://www.din.de/de (accessed on 25 February 2017).
- Official Methods of Analysis of AOAC INTERNATIONAL, 20th ed. 2016. Available online: http://www.aoac.org/aoac_prod_imis/AOAC/Publications/Official_Methods_of_Analysis/AOAC_Member/Publications/OMA/AOAC_Official_Methods_of_Analysis.aspx (accessed on 16 February 2017).
- NIST Chemistry Webbook. 2010. Available online: http://webbook.nist.gov/ chemistry (accessed on 10 June 2018).
- Dorado, A.D.; Husni, S.; Pascual, G.; Puigdellivol, C.; Gabriel, D. Inventory and treatment of compost maturation emissions in a municipal solid waste treatment facility. Waste Manag. 2014, 34, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.-C. Volatile constituents detected in smoke condensates from the combination of the smoking ingredients sucrose, black tea leaves, and bread flour. J. Food Drug Anal. 2013, 21, 292–300. [Google Scholar] [CrossRef]
- Caprino, F.; Moretti, V.; Bellagamba, F.; Turchini, G.; Busetto, M.; Giani, I.; Paleari, M.; Pazzaglia, M. Fatty acid composition and volatile compounds of caviar from farmed white sturgeon (Acipenser transmontanus). Anal. Chim. Acta 2008, 617, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Buttery, B.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Nagata, Y. Odor Measurement Review, Measurement of Odor Threshold by Triangle Odor Bag Method; Ministery of Environmental Goverment of Japan: Tokyo, Japan, 2003; pp. 122–123.
- John, H.; Montgomery, B. Groundwater Chemicals Desk Reference, 3rd ed.; Taylor & Francis: Abingdon, UK, 1995. [Google Scholar]
- Odour Threshold Shop. Available online: http://www.odourthreshold.com/ (accessed on 3 August 2018).
- Dalai, A.; Schoenau, G.; Das, D.; Adapa, P. Volatile Organic Compounds emitted during High-temperature Alfalfa Drying. Biosyst. Eng. 2006, 94, 57–66. [Google Scholar] [CrossRef]
- Rodríguez, G.; Rodriguez, R.; Jimenez, A.; Guillén, R.; Fernández-Bolaños, J. Effect of steam treatment of alperujo on the composition, enzymatic saccharification, and in vitro digestibility of alperujo. J. Agric. Food Chem. 2007, 55, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Castells, X. Tratamiento y valorización energética de residuos; Fundación Universitaria Iberoamericana, Ed.; Ediciones Díaz de Santos: Madrid, Spain, 2005. [Google Scholar]
- Dexter, J.; Siemers, D.; Head, J.; Miller, D. Multi-Zonemethodfor Controlling Wocand Nox Emissions in a Flatline Conveyorwafer Drying System. U.S. Patent 5,749,160, 12 May 1998. [Google Scholar]
- DIPPR: The Design Institute for Physical Properties. DIPPR 801 Thermophysical Property Database and DIADEM Predictive, Software; Brigham Young University: Provo, UT, USA, 2007.
- Vichi, S.; Pizzale, L.; Conte, L.S.; Buxaderas, S.; Lopez-Tamames, E. Solid-phase microextraction in the analysis of virgin olive oil volatile fraction: Modifications induced by oxidation and suitable markers of oxidative status. J. Agric. Food Chem. 2003, 51, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R., Jr.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 10, 273–286. [Google Scholar] [CrossRef]
- Morales, M.T.; Rios, J.J.; Aparicio, R. Changes in the Volatile Composition of Virgin Olive Oil during Oxidation: Flavors and Off-Flavors. J. Agric. Food Chem. 1997, 45, 2666–2673. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.; Chu, Z.; Qin, L. Effect of Oven Drying, Microwave Drying, and Silica Gel Drying Methods on the Volatile Components of Ginger (Zingiber officinale Roscoe) by HS-SPME-GC-MS. Dry. Technol. 2012, 30, 248–255. [Google Scholar] [CrossRef]



| Month | Moisture Content (% wb) ** Oven Method—AOAC 945.15 | Ashes (% wb) * Muffle Method—AOAC 940.26 | Measuring Heating Value (MJ kg−1) * Norm DIN Serie 51.900 | |||
|---|---|---|---|---|---|---|
| Alperujo | Orujo | Alperujo | Orujo | Alperujo | Orujo | |
| Jul | 77.7 ± 0.1 | 79.5 ± 0.2 | 2.4 ± 0.1 | 3.2 ± 0.1 | 22.3 ± 0.1 | 22.4 ± 0.2 |
| Aug | 77.9 ± 0.5 | 80.2 ± 0.3 | 2.2 ± 0.1 | 3.1 ± 0.1 | 22.1 ± 0.2 | 22.4 ± 0.1 |
| Sept | 80.0 ± 0.5 | 81.8 ± 0.4 | 1.9 ± 0.2 | 2.9 ± 0.1 | 22.4 ± 0.1 | 22.5 ± 0.1 |
| Oct | 73.1 ± 0.2 | 77.4 ± 0.2 | 1.6 ± 0.1 | 2.7 ± 0.1 | 22.2 ± 0.1 | 22.4 ± 0.2 |
| Nov | 60.5 ± 0.4 | 63.4 ± 0.2 | 1.6 ± 0.1 | 2.2 ± 0.1 | 22.1 ± 0.1 | 22.6 ± 0.2 |
| Dec | 53.0 ± 1.0 | 55.4 ± 0.4 | 1.2 ± 0.2 | 2.0 ± 0.1 | 22.3 ± 0.1 | 22.7 ± 0.2 |
| Based Compound | Sample | Standard 1 | Standard 2 | Standard 3 |
|---|---|---|---|---|
| Methanol | n-Hexane | 0.5 ppmv | 1.5 ppmv | 3.0 ppmv |
| Methanol | n-Octane | 0.5 ppmv | 1.5 ppmv | 3.0 ppmv |
| Methanol | n-Nonane | 0.5 ppmv | 2.0 ppmv | 5.0 ppmv |
| Methanol | D3710-95 | 0.5 ppmv | 1.0 ppmv | 2.5 ppmv |
| Family | Name | Months (1–6) | Odor Threshold | Reference | |
|---|---|---|---|---|---|
| Alperujo | Orujo | ppmv | |||
| Aldehydes | 2-Furancarboxaldehyde, 5-methyl- | 1,3,4 | 1 | 3.0 | Sung et al. [16] |
| 3-Cyclopentene-1-acetaldehyde, 2-oxo- | 1,3 | 1–3 | |||
| 9-Octadecenal | 3,4 | >1.0 | Caprino et al. [17] | ||
| Benzaldehyde | 1–6 | 1–6 | 0.35–3.5 | Buttery et al. [18] | |
| Furfural | 1–6 | 1–6 | 3.0–23.0 | Buttery et al. [18] | |
| Hexanal | 1–6 | 1–6 | 0.28 | Nagata [19] | |
| Methyl glyoxal | 3,4 | 3–5 | |||
| Nonanal | 1–6 | 0.34 | Nagata [19] | ||
| Octanal | 1–6 | 0.01 | Nagata [19] | ||
| Amides | Propanamide, 2-hydroxy- | 2–6 | 1–6 | ||
| Amines | Pyridine, 3-ethyl- | 2–3 | 2–3 | ||
| Phenolic alcohols | |||||
| 2-Methoxy-4-vinylphenol | 2–3 | 1–6 | 0.003 | Nagata [19] | |
| 3-tert-Butyl-4-hydroxyanisole | 2–6 | 2–3 | |||
| Phenol | 1–6 | 1–6 | 0.0056 | Nagata [19] | |
| Phenol, 2-methoxy-4-(1-propenyl)- | 2–5 | 1–6 | |||
| Phenol, 2,6-dimethoxy- | 1–2 | 1 | |||
| Phenol, 4-ethyl-2-methoxy- | 1–5 | 1–6 | |||
| 5-tert-Butylpyrogallol | 2–3 | 2-3 | |||
| Aliphatic alcohols | 1-Dodecanol, 3,7,11-trimethyl | 1–3 | |||
| 1-Dodecanol, 3,7,11-trimethyl- | 2–5 | 2–5 | |||
| 1-Hexadecanol, 2-methyl- | 1 | 1–2 | |||
| 3-Nonen-1-ol, (E)- | 2–3 | ||||
| 2-Furanmethanol | 1–6 | 1-6 | 8.0 | Montgomery [20] | |
| Aromatic HCs | Benzene, 1-azido-3-methyl- | 2–3 | 2 | ||
| Benzene, 1,3-dimethyl- | 2–5 | 2 | |||
| Toluene | 1–6 | 1–6 | 0.33 | Nagata [19] | |
| Esters | 10-Octadecenoic acid, methyl ester | 2–3 | |||
| Hexanoic acid, 2-phenylethyl ester | 2–6 | ||||
| Carboxylic acids | Acetic acid | 1–6 | 1–6 | 0.0060 | Nagata [19] |
| Propanoic acid | 4–5 | 0.0057 | Nagata [19] | ||
| 9-Hexadecenoic acid | 1–6 | ||||
| Aliphatic HCs | 1-Decene | 3–6 | 5–6 | ||
| 2-Octene | 4–5 | 99.808 | http://www.odourthreshold.com [21] | ||
| 8-Heptadecene | 5 | 3–6 | |||
| Heptane | 1–6 | ||||
| Hexane | 1–6 | 1–6 | 1.5 | Nagata [19] | |
| Nonane | 1–6 | 1–6 | 2.2 | Nagata [19] | |
| Octane | 1–6 | 1–6 | 1.7 | Nagata [19] | |
| Tetradecane, 2,6,10-trimethyl- | 3–6 | 1–6 | |||
| 1,4-Pentadiene | 2–3 | 1–2 | |||
| Ketones | 1,2-Cyclopentanedione, 3-methyl- | 5–3 | 2 | ||
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 1–6 | 4–5 | |||
| 2-Cyclopenten-1-one, 2-methyl- | 3–6 | 1–6 | |||
| 2-Propanone, 1-(acetyloxy)- | 4–5 | 1–6 | |||
| 2-Propanone, 1-hydroxy- | 2–6 | 5 | |||
| Ethers | Octane, 1-methoxy- | 1–2 | 6 | ||
| Family | Name | Boiling Temperature | Vapor Pressure | Jul | Aug | Sep | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|
| ºC | bar | 1 | 2 | 3 | 4 | 5 | 6 | average | ||
| Alcohols | 2-Furanmethanol | 170.10 * | 0.45 | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.5 ± 0.1 | 4.0 ± 0.1 | 4.2 ± 0.1 | 5.2 ± 0.2 | 3.8 ± 0.5 |
| Phenol | 181.93 * | 0.40 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.9 ± 0.2 | 1.0 ± 0.1 | 0.5 ± 0.4 | |
| Aldehydes | Benzaldehyde | 178.66 * | 0.46 | 3.5 ± 0.0 | 4.0 ± 0.1 | 4.2 ± 0.1 | 4.8 ± 0.2 | 4.9 ± 0.1 | 5.1 ± 0.1 | 4.4 ± 0.6 |
| Furfural | 161.55 ** | 0.73 | 6.3 ± 0.1 | 7.5 ± 0.1 | 7.8 ± 0.1 | 8.3 ± 0.1 | 8.9 ± 0.0 | 10.5 ± 0.0 | 8.2 ± 1.4 | |
| Hexanal | 128.14 * | 1.80 | 0.1 ± 0.1 | 0.5 ± 0.0 | 0.8 ±0.0 | 1.2 ± 0.0 | 1.3 ± 0.1 | 1.5 ± 0.0 | 0.9 ± 0.5 | |
| Nonanal | 194.93 * | 0.29 | 0.3 ± 0.0 | 0.6 ± 0.1 | 0.9 ± 0.1 | 1.5 ± 0.0 | 1.8 ± 0.1 | 2.1 ± 0.0 | 1.2 ± 0.7 | |
| Octanal | 174.20 * | 0.53 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.5 ± 0.1 | 0.9 ± 0.0 | 1.1 ± 0.2 | 1.2 ± 0.1 | 0.7 ± 0.5 | |
| Aromatic HCs | Toluene | 110.68 * | 2.75 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.9 ± 0.1 | 1.2 ± 0.1 | 0.8 ± 0.2 |
| Carboxylic acids | Acetic acid | 118.01 * | 2.49 | 2.8 ± 0.1 | 3.5 ± 0.1 | 2.9 ± 0.1 | 3.5 ± 0.1 | 4.2 ± 0.0 | 4.9 ± 0.1 | 3.6 ± 0.8 |
| Aliphatic HCs | Hexane | 68.73 * | 7.41 | 0.9 ± 0.0 | 1.9 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.0 | 2.5 ± 0.1 | 1.5 ± 0.2 | 2.1 ± 0.8 |
| Octane | 125.69 * | 1.90 | 3.2 ± 0.0 | 3.2 ± 0.1 | 3.5 ± 0.2 | 2.1 ± 0.0 | 1.0 ± 0.1 | 0.5 ± 0.1 | 2.3 ± 1.3 | |
| Nonane | 150.66 * | 0.99 | 2.1 ± 0.1 | 2.9 ± 0.1 | 3.0 ± 0.1 | 1.5 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 2.2 ± 0.6 |
| Family | Name | Boiling Temperature | Vapor Pressure | Jul | Aug | Sep | Oct | Nov | Dec | |
|---|---|---|---|---|---|---|---|---|---|---|
| °C | bar | 1 | 2 | 3 | 4 | 5 | 6 | average | ||
| Alcohols | 2-Furanmethanol | 170.10 * | 0.45 | 3.1 ± 0.1 | 3.9 ± 0.2 | 4.2 ± 0.2 | 4.2 ± 0.1 | 4.9 ± 0.0 | 5.7 ± 0.2 | 4.3 ± 0.9 |
| Phenol | 181.93 * | 0.40 | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.2 | 0.8 ± 0.7 | |
| Aldehydes | Benzaldehyde | 178.66 * | 0.46 | 3.1 ± 0.2 | 3.2 ± 0.1 | 3.8 ± 0.1 | 4.3 ± 0.1 | 4.9 ± 0.0 | 4.9 ± 0.1 | 4.0 ± 0.8 |
| Furfural | 161.55 ** | 0.73 | 3.9 ± 0.1 | 4.3 ± 0.1 | 5.8 ± 0.1 | 5.5 ± 0.2 | 6.9 ± 0.1 | 8.6 ± 0.0 | 5.8 ± 1.7 | |
| Hexanal | 128.14 * | 1.80 | 1.7 ± 0.0 | 1.1 ± 0.1 | 1.3 ± 0.1 | 2.1 ± 0.2 | 2.2 ± 0.1 | 2.3 ± 0.0 | 1.8 ± 0.5 | |
| Nonanal | 194.93 * | 0.29 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.0 | 1.5 ± 0.0 | 0.9 ± 0.5 | |
| Octanal | 174.20 * | 0.53 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.8 ± 0.1 | 1.2 ± 0.2 | 1.8 ± 0.2 | 2.1 ± 0.1 | 1.1 ± 0.8 | |
| Aromatic HCs | Toluene | 110.68 * | 2.75 | 0.8 ± 0.1 | 0.8 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.1 | 1.7 ± 0.2 | 2.1 ± 0.1 | 1.3 ± 0.5 |
| Carboxylic acids | Acetic acid | 118.01 * | 2.49 | 2.4 ± 0.1 | 3.2 ± 0.1 | 3.8 ± 0.1 | 4.1 ± 0.0 | 4.5 ± 0.1 | 5.2 ± 0.1 | 3.9 ± 1.0 |
| Aliphatic HCs | Hexane | 68.73 * | 7.41 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 1.9 ± 0.1 | 1.2 ± 0.0 | 1.0 ± 0.2 | 1.7 ± 0.5 |
| Octane | 125.69 * | 1.90 | 1.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.3 | 1.9 ± 0.1 | 0.5 ± 0.2 | 0.2 ± 0.0 | 1.4 ± 0.9 | |
| Nonane | 150.66 * | 0.99 | 2.4 ± 0.1 | 3.1 ± 0.1 | 3.4 ± 0.1 | 3.8 ± 0.0 | 4.2 ± 0.1 | 4.1 ± 0.1 | 3.5 ± 0.7 |
| VOC | Compound | Odor Impact Value | |
|---|---|---|---|
| Family | Alperujo | Orujo | |
| Phenolic alcohols | Phenol | 89.29 | 142.86 |
| Aliphatic alcohols | 2-Furanmethanol | 0.48 | 0.54 |
| Aldehyde | Benzaldehyde | 1.26 | 1.14 |
| Furfural | 0.36 | 0.25 | |
| Hexanal | 3.21 | 6.43 | |
| Nonanal | 3.53 | 2.65 | |
| Octanal | 70.00 | 110.00 | |
| Aromatic | Toluene | 2.42 | 3.94 |
| Acetic acid | 3.60 | 3.90 | |
| Aliphatic HC | Hexane | 1.40 | 1.13 |
| Nonane | 1.00 | 1.59 | |
| Octane | 1.35 | 0.82 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández, D.; Quinteros-Lama, H.; Tenreiro, C.; Gabriel, D. Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction. Appl. Sci. 2019, 9, 519. https://doi.org/10.3390/app9030519
Hernández D, Quinteros-Lama H, Tenreiro C, Gabriel D. Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction. Applied Sciences. 2019; 9(3):519. https://doi.org/10.3390/app9030519
Chicago/Turabian StyleHernández, Diógenes, Héctor Quinteros-Lama, Claudio Tenreiro, and David Gabriel. 2019. "Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction" Applied Sciences 9, no. 3: 519. https://doi.org/10.3390/app9030519
APA StyleHernández, D., Quinteros-Lama, H., Tenreiro, C., & Gabriel, D. (2019). Assessing Concentration Changes of Odorant Compounds in the Thermal-Mechanical Drying Phase of Sediment-Like Wastes from Olive Oil Extraction. Applied Sciences, 9(3), 519. https://doi.org/10.3390/app9030519