New Insights into the Symbiotic Relationship between Orchids and Fungi
Abstract
:1. Introduction
2. Orchid Mycorrhiza and Its Role in Orchid Seed Germination
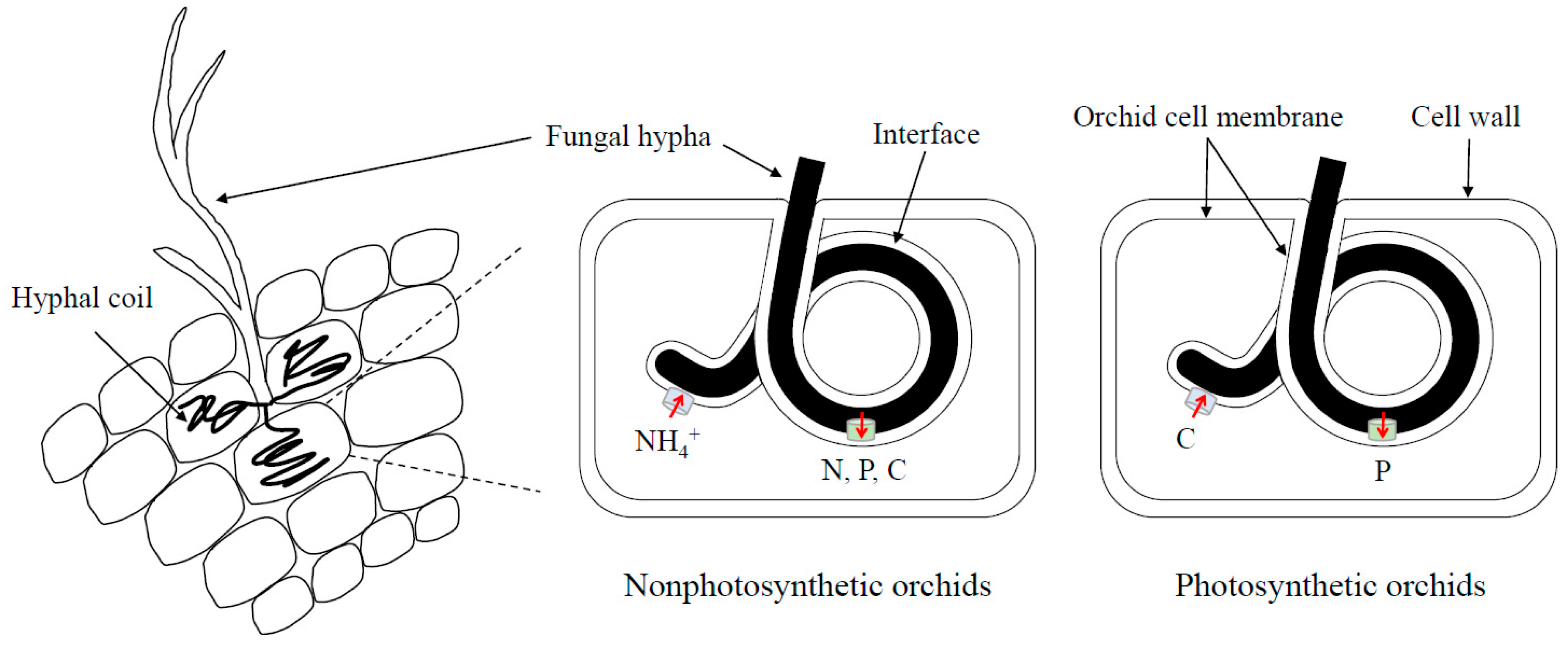
3. Nutrient Exchange between Orchids and Mycorrhizal Fungi
4. Molecular Studies on Orchid Mycorrhizal Symbiosis by Next-Generation Sequencing and Proteomics
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mohammadi, K.; Khalesro, S.; Sohrabi, Y.; Heidari, G. A Review: Beneficial Effects of the Mycorrhizal Fungi for Plant Growth. J. Appl. Environ. Biol. Sci. 2011, 1, 310–319. [Google Scholar]
- Pérez-Brocal, V.; Latorre, A.; Moya, A. Symbionts and Pathogens: What is the Difference? Curr. Top. Microbiol. Immunol. 2013, 358, 215–243. [Google Scholar] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unravelling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Molecular basis of plant-symbiotic fungi interaction: An overview. SCI WORLD J. 2007, 5, 115–131. [Google Scholar] [CrossRef]
- Behie, S.W.; Bidochka, M.J. Nutrient transfer in plant-fungal symbioses. Trends Plant Sci. 2014, 19, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Massicotte, H.B.; Melville, L.H. Mycorrhizas: Anatomy and Cell Biology; National Research Council Research Press: Ottawa, ON, Canada, 2004. [Google Scholar]
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.A.; Pieterse, C.M.J. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M. An orchid-fungus marriage-physical promiscuity, conflict and cheating. New Phytol. 2003, 154, 46. [Google Scholar] [CrossRef]
- Herrera, H.; Valadares, R.; Contreras, D.; Bashan, Y.; Arriagada, C. Mycorrhizal compatibility and symbiotic seed germination of orchids from the Coastal Range and Andes in south central Chile. Mycorrhiza 2017, 27, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Arditti, J. Factors affecting the germination of orchid seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef] [Green Version]
- Kauth, P.J.; Dutra, D.; Johnson, T.R.; Stewart, S.L.; Kane, M.E.; Vendram, W. Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 5, Global Science Books, Techniques and Applications of In Vitro Orchid Seed Germination; Global Science Books, Ltd.: Ikenobe, Japan, 2008. [Google Scholar]
- Yamazaki, J.; Miyoshi, K. In vitro asymbiotic germination of immature seed and formation of protocorm by Cephalanthera falcata (Orchidaceae). Ann. Bot. 2006, 98, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, J.D.W. Further advances in orchid mycorrhizal research. Mycorrhiza 2007, 17, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, H.N.; Rasmussen, F.N. Climactic and seasonal regulation of seed plant establishment in Dactylorhiza majalis inferred from symbiotic experiments in vitro. Lindleyana 1991, 6, 221–227. [Google Scholar]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 33, 1–14. [Google Scholar] [CrossRef]
- Peterson, R.L.; Farquhar, M.L. Mycorrhizas: Integrated development between roots and fungi. Mycologia 1994, 86, 311–326. [Google Scholar]
- Rasmussen, H.N. Cell differentiation and mycorrhizal infection in Dactylorhiza majalis (Rchb. F.) Hunt & Summerh. (Orchidaceae) during germination in vitro. New Phytol. 1990, 116, 137–143. [Google Scholar]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bosch, R.; van der Hejiden, M.; Sieverding, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Xu, X.; Chen, C.; Zhang, Z.; Sun, S.; Chen, Y.; Jiang, J.; Shen, Z. The influence of environmental factors on communities of arbuscular mycorrhizal fungi associated with Chenopodium ambrosioides revealed by MiSeq sequencing investigation. Sci. Rep. 2017, 7, 45134. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Bücking, H.; Kafle, A. Role of Arbuscular mycorrhizal fungi in the nitrogen uptake of plants: Current knowledge and research gaps. Agronomy 2015, 5, 587–612. [Google Scholar] [CrossRef]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in arbuscular mycorrhizal symbiosis. Mol. Plant. 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Brys, R.; Waud, M.; Busschaert, P.; Lievens, B. Mycorrhizal networks and coexistence in species-rich orchid communities. New Phytol. 2015, 206, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Keymer, A.; Pimprikar, P.; Wewer, V.; Huber, C.; Brands, M.; Bucerius, S.L.; Delaux, P.M.; Klingl, V.; Röpenack-Lahaye, E.V.; Wang, T.L.; et al. Lipid transfer from plants to arbuscular mycorrhiza fungi. Elife 2017, 20, 6. [Google Scholar] [CrossRef]
- Keymer, A.; Gutjahr, C. Cross-kingdom lipid transfer in arbuscular mycorrhiza symbiosis and beyond. Curr. Opin. Plant Biol. 2018, 44, 137–144. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Cameron, D.D. Nitrogen transport in the orchid mycorrhizal symbiosis—Further evidence for a mutualistic association. New Phytol. 2017, 213, 10–12. [Google Scholar] [CrossRef]
- Chen, A.; Gu, M.; Wang, S.; Chen, J.; Xu, G. Transport properties and regulatory roles of nitrogen in arbuscular mycorrhizal symbiosis. Semin. Cell Dev. Biol. 2018, 74, 80–88. [Google Scholar] [CrossRef]
- Selosse, M.A.; Martos, F. Do chlorophyllous orchids heterotrophically use mycorrhizal fungal carbon? Trends Plant Sci. 2014, 19, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Leake, J.R. Plants parasitic on fungi: Unearthing the fungi in myco-heterotrophs and debunking the ‘saprophytic’ plant myth. Mycologist 2005, 19, 113–122. [Google Scholar] [CrossRef]
- Julou, T.; Burghardt, B.; Gebauer, G.; Berveiller, D.; Damesin, C.; Selosse, M.-A. Mixotrophy in orchids: Insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalanthera damasonium. New Phytol. 2005, 166, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, G.; Preiss, K.; Gebauer, A.C. Partial mycoheterotrophy is more widespread among orchids than previously assumed. New Phytol. 2016, 211, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girlanda, M.; Selosse, M.-A.; Cafasso, D.; Brilli, F.; Delfine, S.; Fabian, R.; Ghigone, P.; Pinelli, P.; Segreto, R.; Loreto, F.; et al. Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae. Mol. Ecol. 2006, 15, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Lallemand, F.; Figura, T.; Damesin, C.; Fresneau, C.; Griveau, C.; Fontaine, N.; Zeller, B.; Selosse, M.A. Mixotrophic orchids do not use photosynthates for perennial underground organs. New Phytol. 2019, 221, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.W.; Girlanda, M.; Grigoriev, I.V.; Martin, F.; et al. Fungal and plant gene expression in the Tulasnella calospora-Serapias vomeracea symbiosis provides clues about N pathways in orchid mycorrhizas. New Phytol. 2017, 213, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Richardson, K.A.; Peterson, R.L.; Currah, R.S. Seed reserves and early symbiotic protocorm development of Platanthera hyperborea (Orchidaceae). Can. J. Bot. 1992, 70, 291–300. [Google Scholar] [CrossRef]
- Yeung, E.C.; Zee, S.Y.; Ye, X.L. Embryology of Cymbidium sinense: Embryo development. Ann. Bot. 1996, 78, 105–110. [Google Scholar] [CrossRef]
- Cameron, D.D.; Leake, J.R.; Read, D.J. Mutualistic mycorrhiza in orchids: Evidence from plant-fungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens. New Phytol. 2006, 171, 405–416. [Google Scholar] [CrossRef]
- Kuga, U.; Sakamoto, N.; Yurimoto, H. Stable isotope imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 2014, 202, 594–605. [Google Scholar] [CrossRef] [PubMed]
- McKendrick, S.L.; Leake, J.R.; Taylor, D.L.; Read, D.J. Symbiotic germination and development of myco-heterotrophic plants in nature: Ontogeny of Corallorhiza trifida and characterisation of its mycorrhizal fungi. New Phytol. 2000, 145, 523–537. [Google Scholar] [CrossRef]
- McKendrick, S.L.; Leake, J.R.; Read, D.J. Symbiotic germination and development of myco-heterotrophic plants in nature: Transfer of carbon from ectomycorrhizal Salix repens and Betula pendula to the orchid Corallorhiza trifida through shared hyphal connections. New Phytol. 2000, 145, 539–548. [Google Scholar] [CrossRef]
- Campbell, E.O. Morphology of the fungal association in three species of Corallorhiza in Michigan. Mich. Bot. 1970, 9, 108–113. [Google Scholar]
- Zelmer, C.D.; Currah, R.S. Evidence for a fungal liaison between Corallorhiza trifida (Orchidaceae) and Pinus contorta (Pinaceae). Can. J. Bot. 1995, 73, 862–866. [Google Scholar] [CrossRef]
- Taylor, D.L.; Bruns, T.D. Independent, specialized invasions of ectomycorrhizal mutualism by two nonphotosynthetic orchids. Proc. Natl. Acad. Sci. USA 1997, 94, 4510–4515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougoure, J.J.; Brundrett, M.C.; Grierson, P.F. Carbon and nitrogen supply to the underground orchid, Rhizanthella gardneri. New Phytol. 2010, 186, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Bidartondo, M.I.; Burghardt, B.; Gebauer, G.; Bruns, T.D.; Read, D.J. Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. Lond. B 2004, 271, 1799–1806. [Google Scholar] [CrossRef]
- Weiss, M.; Selosse, M.A.; Rexer, K.H.; Urban, A.; Oberwinkler, F. Sebacinales: A hitherto overlooked cosm of heterobasidiomycetes with broad mycorrhizal potential. Mycol. Res. 2004, 108, 1003–1010. [Google Scholar] [CrossRef]
- Waterman, R.J.; Bidartondo, M.I. Deception above, deception below: Linking pollination and mycorrhizal biology of orchids. J. Exp. Bot. 2008, 59, 1085–1096. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C.; et al. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, X.; Liu, H.; Tian, Y.; Lian, J.; Yang, R.; Hao, S.; Wang, X.; Yang, S.; Li, Q.; et al. The genome of Dendrobium officinale illuminates the biology of the important traditional Chinese orchid herb. Mol. Plant 2015, 8, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F.; et al. The Dendrobium catenatum lindl. Genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Liu, K.W.; Li, Z.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J.; et al. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, X.; Liu, J.; Zhao, X.; Zhou, J.; Wang, X.; Wang, D.; Lai, C.; Xu, W.; Huang, J.; et al. The Gastrodia elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.T.; Chen, W.C.; Chen, C.Y.; Ho, H.Y.; Yeh, C.H.; Kuo, Y.T.; Su, C.L.; Yen, S.H.; Hsueh, H.Y.; Yeh, J.H.; et al. Chromosome-level assembly, genetic and physical mapping of Phalaenopsis aphrodite genome provides new insights into species adaptation and resources for orchid breeding. Plant Biotechnol. J. 2018, 16, 2027–2041. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Chen, C.; Yang, J.; Zhu, H.; Liu, M.; Lv, F. Deep sequencing-based comparative transcriptional profiles of Cymbidium hybridum roots in response to mycorrhizal and non-mycorrhizal beneficial fungi. BMC Genom. 2014, 15, 747. [Google Scholar] [CrossRef] [PubMed]
- Perotto, S.; Rodda, M.; Benetti, A.; Sillo, F.; Ercole, E.; Rodda, M.; Girlanda, M.; Murat, C.; Balestrini, R. Gene expression in mycorrhizal orchid protocorms suggests a friendly plant-fungus relationship. Planta 2014, 239, 1337–1349. [Google Scholar] [CrossRef]
- Liu, S.S.; Chen, J.; Li, S.C.; Zeng, X.; Meng, Z.X.; Guo, S.X. Comparative transcriptome analysis of genes involved in GA-GID1-DELLA regulatory module in symbiotic and asymbiotic seed germination of Anoectochilus roxburghii (Wall.) Lindl. (Orchidaceae). Int. J. Mol. Sci. 2015, 16, 30190–30203. [Google Scholar] [CrossRef]
- Tsai, C.C.; Wu, K.M.; Chiang, T.Y.; Huang, C.Y.; Chou, C.H.; Li, S.J.; Chiang, Y.C. Comparative transcriptome analysis of Gastrodia elata (Orchidaceae) in response to fungus symbiosis to identify gastrodin biosynthesis-related genes. BMC Genom. 2016, 17, 212. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.S.; Kohler, A.; Yan, B.; Luo, H.M.; Chen, X.M.; Guo, S.X. iTRAQ and RNA-Seq analyses provide new insights into regulation mechanism of symbiotic germination of Dendrobium officinale seeds (Orchidaceae). J. Proteome Res. 2017, 16, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Suetsugu, K.; Yamato, M.; Miura, C.; Yamaguchi, K.; Takahashi, K.; Ida, Y.; Shigenobu, S.; Kaminaka, H. Comparison of green and albino individuals of the partially mycoheterotrophic orchid Epipactis helleborine on molecular identities of mycorrhizal fungi, nutritional modes and gene expression in mycorrhizal roots. Mol. Ecol. 2017, 26, 1652–1669. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Yamaguchi, K.; Miyahara, R.; Yamamoto, T.; Fuji, M.; Yagame, T.; Imaizumi-Anraku, H.; Yamato, M.; Shigenobu, S.; Kaminaka, H. The mycoheterotrophic symbiosis between orchids and mycorrhizal fungi possesses major components shared with mutualistic plant-mycorrhizal symbioses. Mol. Plant Microbe Interact. 2018, 31, 1032–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Valadares, R.B.; Perotto, S.; Santos, E.C.; Lambais, M.R. Proteome changes in Oncidium sphacelatum (Orchidaceae) at different trophic stages of symbiotic germination. Mycorrhiza 2014, 24, 349–360. [Google Scholar] [CrossRef] [PubMed]
- López-Chávez, M.Y.; Guillén-Navarro, K.; Bertolini, V.; Encarnación, S.; Hernández-Ortiz, M.; Sánchez-Moreno, I.; Damon, A. Proteomic and morphometric study of the in vitro interaction between Oncidium sphacelatum Lindl. (Orchidaceae) and Thanatephorus sp. Rg26 (Ceratobasidiaceae). Mycorrhiza 2016, 26, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yang, J.Z.; Liu, S.; Chen, C.L.; Zhu, H.Y.; Cao, J.X. The colonization patterns of different fungi on roots of Cymbidium hybridum plantlets and their respective inoculation effects on growth and nutrient uptake of orchid plantlets. World J. Microbiol. Biotechnol. 2014, 30, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Blakeman, J.P.; Mokahel, M.A.; Hadley, G. Effect of mycorrhizal infection on respiration and activity of some oxidase enzymes of orchid protocorms. New Phytol. 1976, 77, 697–704. [Google Scholar] [CrossRef]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar]
- Marini, A.M.; Soussi-Boudekou, S.; Vissers, S.; Andre, B. A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell Biol. 1997, 17, 4282–4293. [Google Scholar] [CrossRef]
- Selosse, M.A. The latest news from biological interactions in orchids: In love, head to toe. New Phytol. 2014, 202, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Stöckel, M.; Těšitelová, T.; Jersáková, J.; Bidartondo, M.I.; Gebauer, G. Carbon and nitrogen gain during the growth of orchid seedlings in nature. New Phytol. 2014, 202, 606–615. [Google Scholar] [Green Version]
| Orchid Species/Lifestyle | Fungi Strain | Platform | Differentially Expressed Genes/proteins | Ref. |
|---|---|---|---|---|
| Cymbidium hybridium (terrestrial) | Epulorhiza repens ML01 (mycorrhizal); Umbelopsis nana ZH3A-3 (non-mycorrhizal) | Illumina HiSeq 2000 (transcriptomics) | Up-regulation under all fungal treatments (ML01, ZH3A-3, ML01+ZH3A-3): phosphate transport, root morphogenesis, cell wall modification, ROS detoxification, secondary metabolism and hormone biosynthesis and signaling. Up-regulation under ML01 treatment only: signaling (LysM domain receptor-like kinase 3-like), cell wall degradation and reinforcement, protein metabolism and processing, defense (chitinase and mannose-specific lectin), N and Fe transport and auxin response. | [57] |
| Serapias vomeraceae (terrestrial) | Tulasnella calospora | 454-pyrosequencing (transcriptomics) | Up-regulation of marker genes of mutualism:SvNod1, SvNod9, SvLect3, SvLect5. No significant induction of pathogenesis and wound/stress-related genes. | [58] |
| Anoectochilus roxburghii (terrestrial) | Isolate Ar-34 (unpublished) | Illumina HiSeq 4000 (transcriptomics) | Up-regulation of the GA-GID1-DELLA module: 2 GA biosynthesis genes (GA20-oxidase: c99861_g1, c90765_g1), 2 GA catabolism genes (GA2-oxidase: c99070_g1, c106997_g1), 2 GA signaling genes (DELLA family protein SLR1: c85242_g1, c93049_g1). | [59] |
| Gastrodia elata (terrestrial) | Armillaria mella | Illumina HiSeq 2000 (transcriptomics) | Identification of key genes involved in the gastrodin biosynthesis pathway in response to fungus symbiosis: the putative monooxygenase (unigene TRINITY_DN54282_c0_g1) and glycosyltransferase (unigene TRINITY_DN50323_c0_g1) genes. | [60] |
| Serapias vomeracea (terrestrial) | Tulasnella calospora | Illumina HiSeq 2000 (transcriptomics) | Up-regulation in the orchid:SvAMT1, SvAMT2 (ammonium transporter); SvAAP1, SvAAP2 (amino acid transporter); SvLHT (oligo-peptide transporter); SvGS (GS/GOGAT pathway). Up-regulation in the mycorrhizal fungus: TcAMT2 (ammonium transporter). No nitrate transporter and nitrate/nitrite reductase genes were found. | [37] |
| Epipactis helleborine (terrestrial) | Wilcoxina | Illumina HiSeq 1500 (transcriptomics) | Up-regulation in the orchid: antioxidant metabolism (such as thioredoxin, glutathione peroxidase and catalase-peroxidase thioredoxin genes), nutrient transport (such as putative SWEET gene), related to arbuscular mycorrhizal colonization (RAM2, subtilisin-like protease, gibberellin 20 oxidase, cytokinin dehydrogenase precursor, auxin efflux carrier component genes). Up-regulation in the mycorrhizal fungus: for sugars, nucleosides, N or P transport (such as the major facilitator superfamily genes). | [62] |
| Bletilla striata (terrestrial) | Tulasnella sp. HR1-1 | Illumina HiSeq 1500 (transcriptomics) | Identification of a set of common symbiotic genes (CSG) in the orchid:BsCCaMK (highly conserved with LjCCaMK), 8 BsAM genes (highly similar to the AM marker genes in rice), 17 putative nitrogen and sugar transporter genes (amino acid transporter genes, bidirectional sugar transporter SWEET family genes), oligopeptide transporter genes, hormone transporter genes. | [63] |
| Oncidium sphacelatum (epiphytic) | Ceratobasidium sp. | iTRAQ-2D-LC-MS/MS (proteomics) | Identification of 88 differentially expressed proteins in mycorrhizal protocorms: mainly involved in molecular signaling, cell rescue and defense, energy and secondary metabolism. | [65] |
| Oncidium sphacelatum (epiphytic) | Thanateporus sp. RG26 | 2D-MALDI-TOF MS (proteomics) | Identification of 11 differentially expressed proteins in mycorrhizal protocorms: cell cycle proteins (β-tubulin), energy metabolism (photosystem I assembly protein Ycf4, RubisCo activase B, chloplastic), purine recycling (xanthine dehydrogenase), ribosome biogenesis (nucleolar GTP-binding, DEAD-box ATP RNA helicase domains) and vesicle secretion (dymeclin domain). Identification of 12 differentially expressed proteins in the mycorrhizal fungus: related to protein-protein interaction (TPR-2 domain), stress response (stress-activated MAP kinase-interacting protein 1), enzyme activation (molybdenum cofactor sulfurase domain) and saccharides biosynthesis (UDP-3-O-[3-hydroxymyristoyl] glucosamine N-acyltransferase domain). | [66] |
| Dendrobium officinale (epiphytic) | Tulasnella sp. S6 | Illumina HiSeq 2000/iTRAQ (transcriptomics/proteomics) | Identification of 32 differentially expressed genes/proteins during symbiotic germination: symbiotic signal transduction (calreticulin), defense reaction (epidermis-specific secreted glycoprotein EP1), storage protein utilization (subtilisin-like proteases, basic 7S globulin), metabolisms of N, carbohydrates and lipids (glutamine synthetase nodule isozyme, probable 6-phosphogluconolactonase 4, acyl-CoA-binding protein). | [61] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-M.; Chung, K.; Liang, C.-K.; Tsai, W.-C. New Insights into the Symbiotic Relationship between Orchids and Fungi. Appl. Sci. 2019, 9, 585. https://doi.org/10.3390/app9030585
Yeh C-M, Chung K, Liang C-K, Tsai W-C. New Insights into the Symbiotic Relationship between Orchids and Fungi. Applied Sciences. 2019; 9(3):585. https://doi.org/10.3390/app9030585
Chicago/Turabian StyleYeh, Chuan-Ming, KwiMi Chung, Chieh-Kai Liang, and Wen-Chieh Tsai. 2019. "New Insights into the Symbiotic Relationship between Orchids and Fungi" Applied Sciences 9, no. 3: 585. https://doi.org/10.3390/app9030585
APA StyleYeh, C.-M., Chung, K., Liang, C.-K., & Tsai, W.-C. (2019). New Insights into the Symbiotic Relationship between Orchids and Fungi. Applied Sciences, 9(3), 585. https://doi.org/10.3390/app9030585






