Optimizing the Preparation of Semi-Crystalline Paraffin/Poly(Urea-Formaldehyde) Microcapsules for Thermal Energy Storage
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
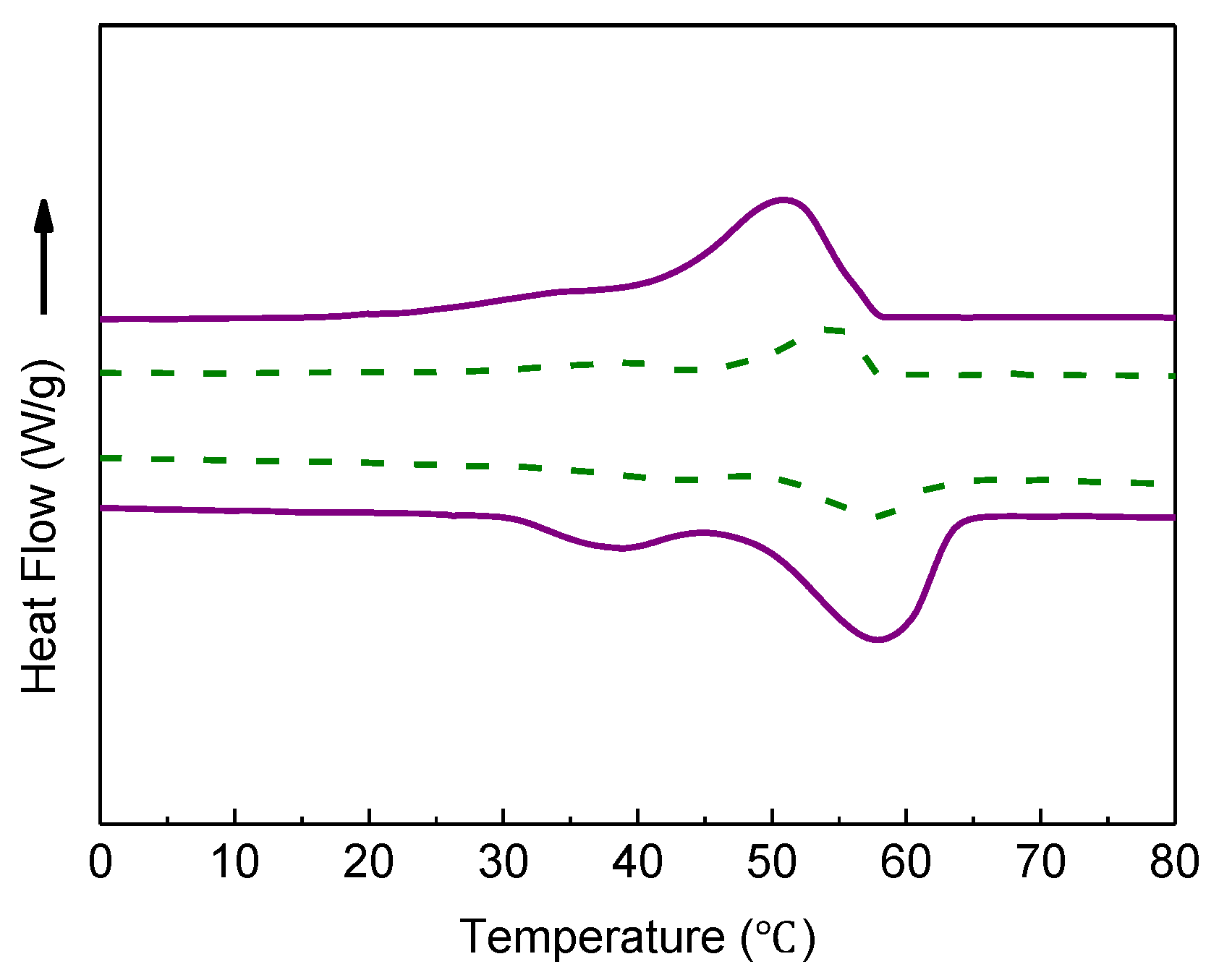
2.3. Characterization
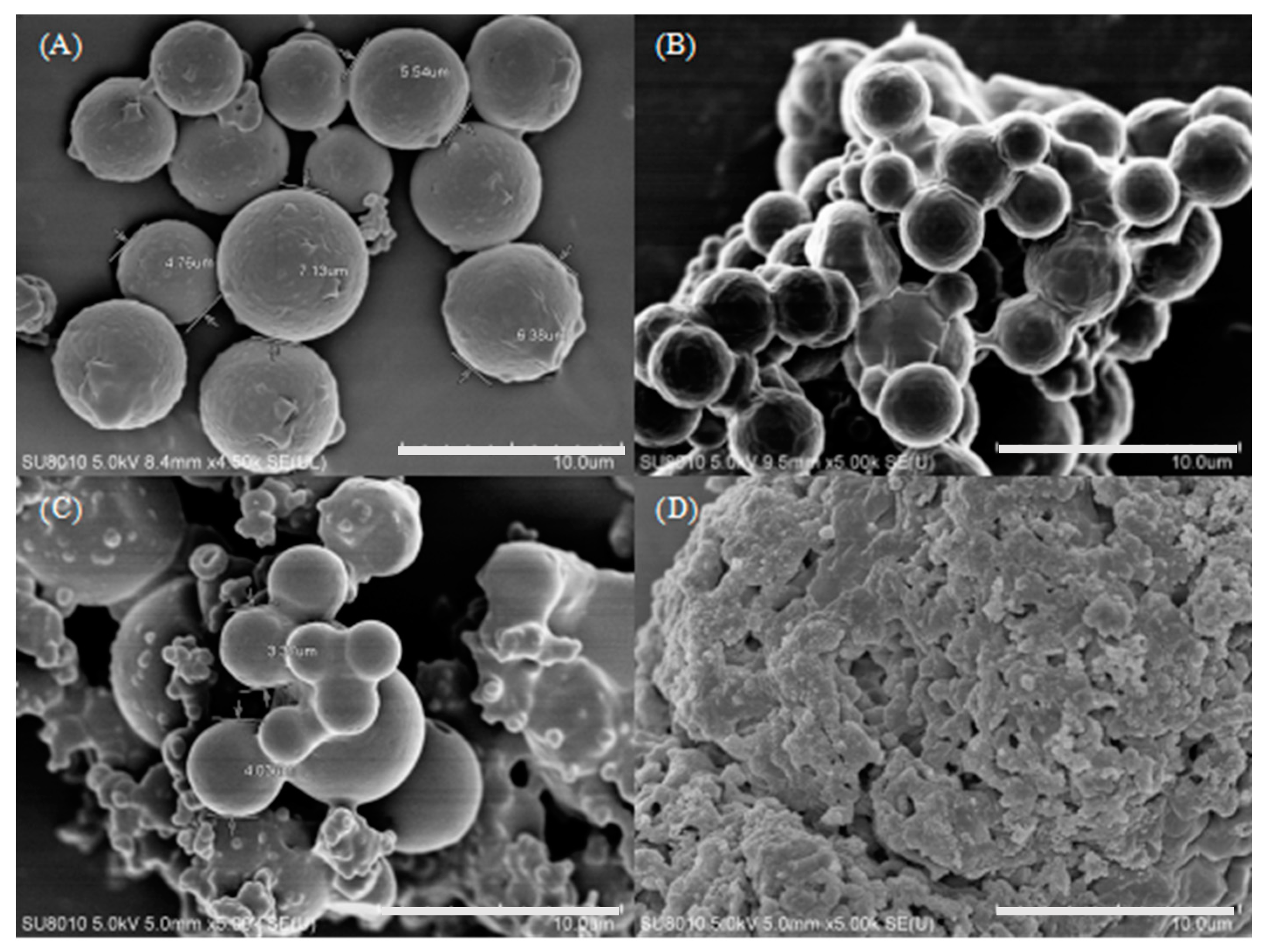
3. Results and Discussion
3.1. Performance of Microcapsules without Sodium Chloride and Crosslinker
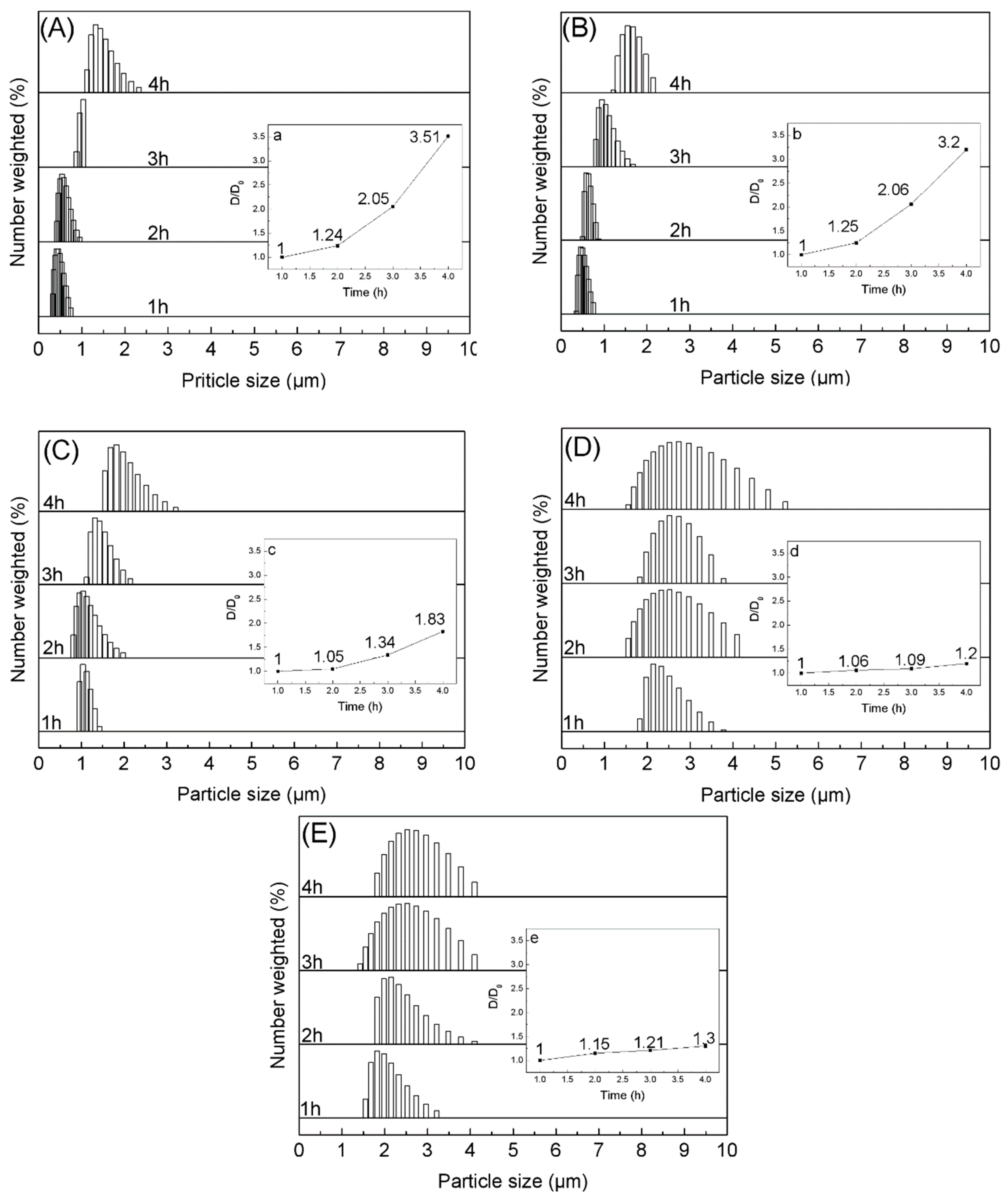
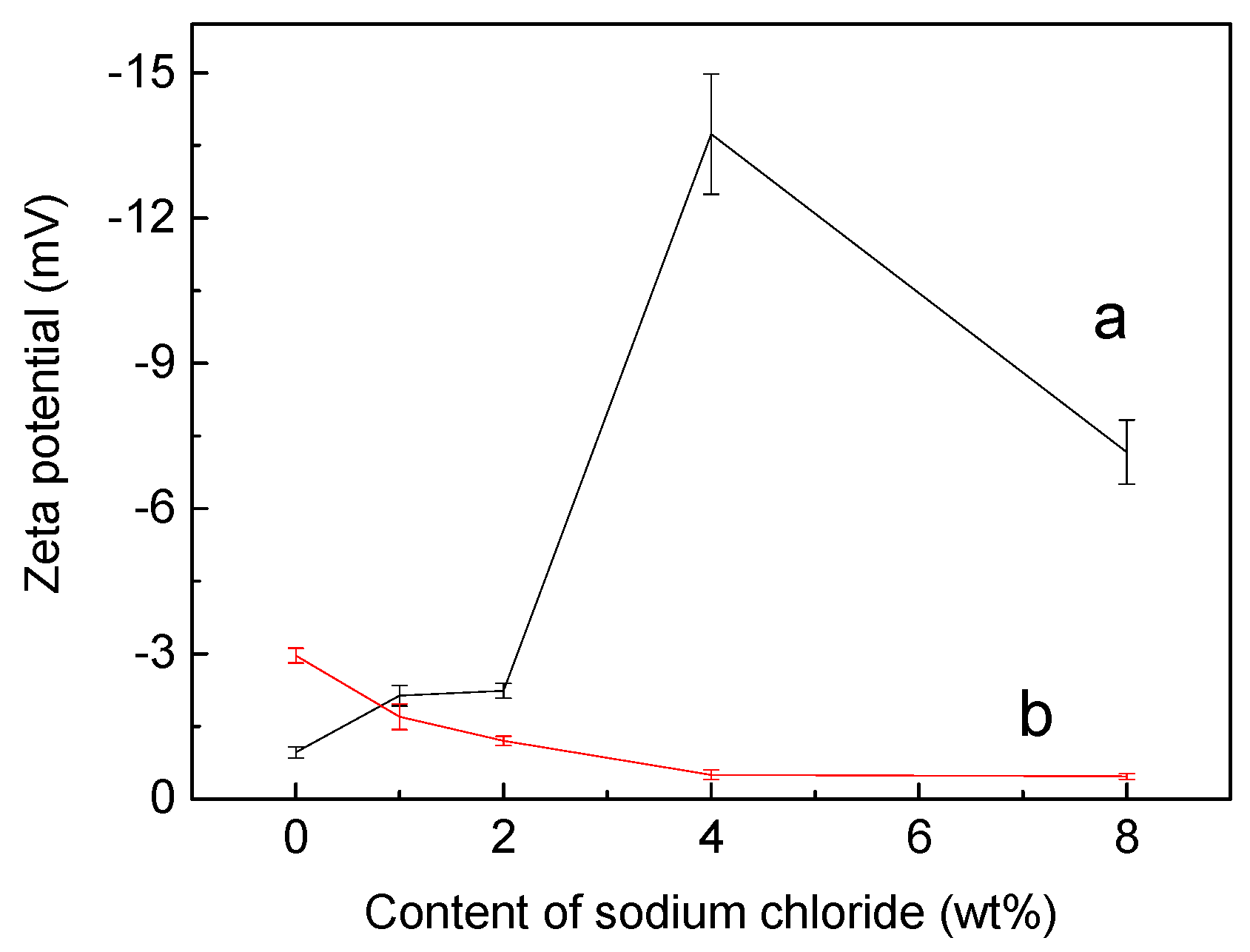
3.2. Optimizing Microcapsule Performance by Adding Sodium Chloride
3.3. Role of Sodium Chloride
3.4. Improve the Microcapsule Performance by Crosslinker
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zecca, A.; Chiari, L. Fossil-fuel constraints on global warming. Energy Policy 2010, 38, 1–3. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar energy for future world: A review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Agyenim, F.; Hewitt, N.; Eames, P.; Smyth, M. A review of materials, heat tranfer and phase change problem formulation for latent heat thermal energy storage systems. Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Dheep, G.R.; Sreekumar, A. Influence of nanomaterials on properties of latent heat solar thermal energy storage materials-A review. Energy Convers. Manag. 2014, 83, 133–148. [Google Scholar] [CrossRef]
- Li, G. Energy and exergy performance assessments for latent heat thermal energy storage systems. Renew. Sustain. Energy Rev. 2015, 51, 926–954. [Google Scholar] [CrossRef]
- de Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Ascione, A.; Bianco, N.; de Masi, R.F.; de Rossi, F.; Vanoli, G.P. Energy refurbishment of existing builds through the use of phase change materials: Energy saving and indoor comfort in the cooling season. Appl. Energy 2014, 113, 990–1007. [Google Scholar] [CrossRef]
- Gil, A.; Orό, E.; Mirό, L.; Peirό, G.; Ruiz, A.; Salmerόn, J.M.; Cabeza, L.F. Experimental analysis of hydroquinone used as phase change material to be applied in solar cooling refrigeration. Int. J. Refrig. 2014, 39, 95–103. [Google Scholar] [CrossRef]
- Liu, M.; Saman, W.; Bruno, F. Development of a novel refrigeration system for refrigerated trucks incorporating phase change material. Appl. Energy 2012, 92, 336–342. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Mohamed, A.L.; Wang, H.; Popescu, C.; Moller, M. Metal salts rented in silica microcapsules as inorganic phase change materials for textile uesage. Inorg. Chem. 2015, 10, 59–65. [Google Scholar]
- Shon, J.; Kim, H.; Lee, K. Improved heat storage rate for an automobile coolant waste heat recovery system using phase-change material in a fin-tube heat exchanger. Appl. Energy 2014, 113, 680–689. [Google Scholar] [CrossRef]
- Tu, Y.; Zhu, W.; Lu, T.; Deng, Y. A novel thermoelectric harvester based on high-performance phase change material for space application. Appl. Energy 2017, 206, 1194–1202. [Google Scholar] [CrossRef]
- Lu, W.; Tassou, S.A. Characterization and experiment investigation of phase change materials for chilled food refrigerated cabinet applications. Appl. Energy 2013, 112, 1376–1382. [Google Scholar] [CrossRef]
- Sharif, M.K.A.; Al-Abidi, A.A.; Mat, S.; Sopian, K.; Ruslan, M.H.; Sulaiman, M.Y.; Rosli, M.A.M. Review of the application of phase change material for heating and domestic hot water systems. Renew. Sustain. Energy Rev. 2015, 42, 557–568. [Google Scholar] [CrossRef]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques, and applications-A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Belessiotis, G.V.; Papadokostaki, K.G.; Favvas, E.P.; Efthimiadou, E.K.; Karellas, S. Preparation and investigation of distinct and shape stable paraffin/SiO2 composite PCM nanospheres. Energy Convers. Manag. 2018, 168, 382–394. [Google Scholar] [CrossRef]
- Li, B.; Liu, T.; Hu, L.; Wang, Y.; Gao, L. Fabrication and Propertied of microencapsulated paraffin@SiO2 Phase Change Composite for Thermal Energy Storage. ACS Sustain. Chem. Eng. 2013, 1, 374–380. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, S.; Chen, L.; Hou, W. Preparation and characterization of polyurea microencapsulated phase change material by interfacial polycondensation method. Powder Technol. 2016, 292, 217–222. [Google Scholar] [CrossRef]
- Rocha, G.A.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of lycopene by spray drying: Charcterization, stablity and application of microcapsules. Food Bioprod. Process. 2012, 90, 37–42. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Li, C.; Kou, K. Synthesis of Cyanate Ester Microcapsules via Solvent Evaporation Technique and Its Application in Epoxy Resins as a Healing Agent. Ind. Eng. Chem. Res. 2016, 55, 10941–10946. [Google Scholar] [CrossRef]
- Jacob, R.; Bruno, F. Review on shell materials used in the encapsulation of phase change materials for high temperature thermal energy storage. Renew. Sustain. Energy Rev. 2015, 48, 79–87. [Google Scholar] [CrossRef]
- Gaitzsch, J.; Huang, X.; Voit, B. Engineering Functional Polymer Capsules toward Smart Nanoreactors. Chem. Rev. 2016, 116, 1053–1093. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, F.; Ceglie, A.; De Leonadis, A.; Lopez, F. Polymer Capsules for Enzymatic Catalysis in Confined Environments. Catalysts 2019, 9, 1. [Google Scholar] [CrossRef]
- Giro-Paloma, J.; Martinez, M.; Cabeza, L.F.; Inés Fernández, A. Types, methods, techniques, and applications for miroencapsulated phase change materials: A review. Renew. Sustain. Energy Rev. 2016, 53, 1059–1075. [Google Scholar] [CrossRef]
- Sánchez-Silva, L.; Lopez, V.; Cuenca, N.; Valverde, J.L. Poly(urea-formaldehyde) microcapsules containing commercial paraffin: In situ polymerization study. Colloid Polym. Sci. 2018, 296, 1449–1457. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, Y.; Liu, J.; Yang, Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer 2008, 49, 2903–2910. [Google Scholar] [CrossRef]
- Xin, C.; Tian, Y.; Wang, Y.; Huang, X. Effect of curing temperature on the performance of microencapsulated low melting point paraffin using urea-formaldehyde resin as a shell. Text. Res. J. 2014, 84, 831–839. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, J.; Zhang, H. Effects of NaCl and dispersants on preparation of microencapsulated phase change paraffin. New Chem. Mater. 2009, 37, 56–59. [Google Scholar]
- Zhan, J.; Zou, D.; Li, L.; Ma, X.; Zhu, Y. Preparation and characterization of high temperature phase change paraffin microcapsules with urea-formaldehyde resin shell materials. Acta Mater. Compos. Sin. 2017, 34, 284–290. [Google Scholar]
- Fang, G.; Li, H.; Yang, F.; Liu, X.; Wu, S. Preparation and characterization of nano-encapsulated n-tetradecane as phase change material for thermal energy storage. Chem. Eng. J. 2009, 153, 217–221. [Google Scholar] [CrossRef]
- Fan, C.; Tang, J.; Zhou, X. Role of ammonium chloride in preparing poly(urea-formaldehyde) microcapsules using one-step method. J. Appl. Polym. Sci. 2013, 129, 2846–2856. [Google Scholar] [CrossRef]
- Wang, H.; Hu, S.; Cai, S.; Yu, F. Preparation and properties of bisphenol A epoxy resin microcapsules coated with melamine-formaldehyde resin. Polym. Bull. 2014, 71, 2407–2419. [Google Scholar] [CrossRef]
- Jiang, K.; Su, Y.; Xie, B.; Jiang, S.; Zhao, Y.; Wang, D. Effect of Geometrical Confinement on the Nucleation and Crystallization Behavior of n-Alkane Mixtures. J. Phys. Chem. B 2008, 112, 16485–16489. [Google Scholar] [CrossRef] [PubMed]
- Sandomirski, K.; Walta, S.; Dubbert, J.; Allahyarov, E.; Schofield, A.B.; Löwen, H.; Richtering, W.; Egelhaaf, S.U. Heterogenrous crystallization of hard and soft spheres near flat and curved walls. Eur. Phys. J. Spec. Top. 2014, 223, 439–454. [Google Scholar] [CrossRef]
- Steiger, M. Crystal growth in porous materials-II: Influence of crystal size on the crystallization pressure. J. Cryst. Growth 2005, 282, 470–481. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Almaadeed, M.A.; Jones, A. Preparation and characterization of urea-formaldehyde microcapsules filled with paraffin oil. Polym. Bull. 2016, 73, 631–646. [Google Scholar] [CrossRef]
- Qiao, Z.; Mao, J. Enhanced thermal properties with graphene oxide in the urea-formaldehyde microcapsules containing paraffin PCMs. J. Microencapsul. 2017, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yu, X.; Li, W.; Liu, S. Preparation of urea-formaldehyde paraffin microcapsules modified by carboxymethyl cellulose as a potential phase change material. J. For. Res. 2015, 26, 253–260. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid-liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Carpio, E.D.; Rodrígurz, S.; Rondόges, M.; Borges, B. Stability of water-Boscan crude oil emulsions: Effect of salts, alcohols and glycols. J. Pet. Sci. Eng. 2014, 122, 542–550. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. Studies on interfacial behavior and wettability change phenomena by ionic and nonionic surfactants in presence of alkalis and salt for enhanced oil recovery. Appl. Surf. Sci. 2016, 372, 42–51. [Google Scholar] [CrossRef]
- Li, C.; Mei, Z.; Liu, Q.; Wang, J.; Xu, J.; Sun, D. Formation and properties of paraffin wax submicron emulsions prepared by the emulsion inversion point method. Colloid Surf. A-Physicochem. Eng. Asp. 2010, 356, 1–3. [Google Scholar] [CrossRef]
- Capek, I. Degradation of kinetically-stable o/w emulsions. Adv. Colloid Interface Sci. 2004, 107, 2–3. [Google Scholar] [CrossRef]
- Cosco, S.; Ambrogi, V.; Musto, P.; Carfagna, C. Urea-Formaldehyde Microcapsules Containing an Epoxy Resin: Influence of Reaction Parameters on the Encapsulation Yield. Macromol. Symp. 2006, 234, 184–192. [Google Scholar] [CrossRef]
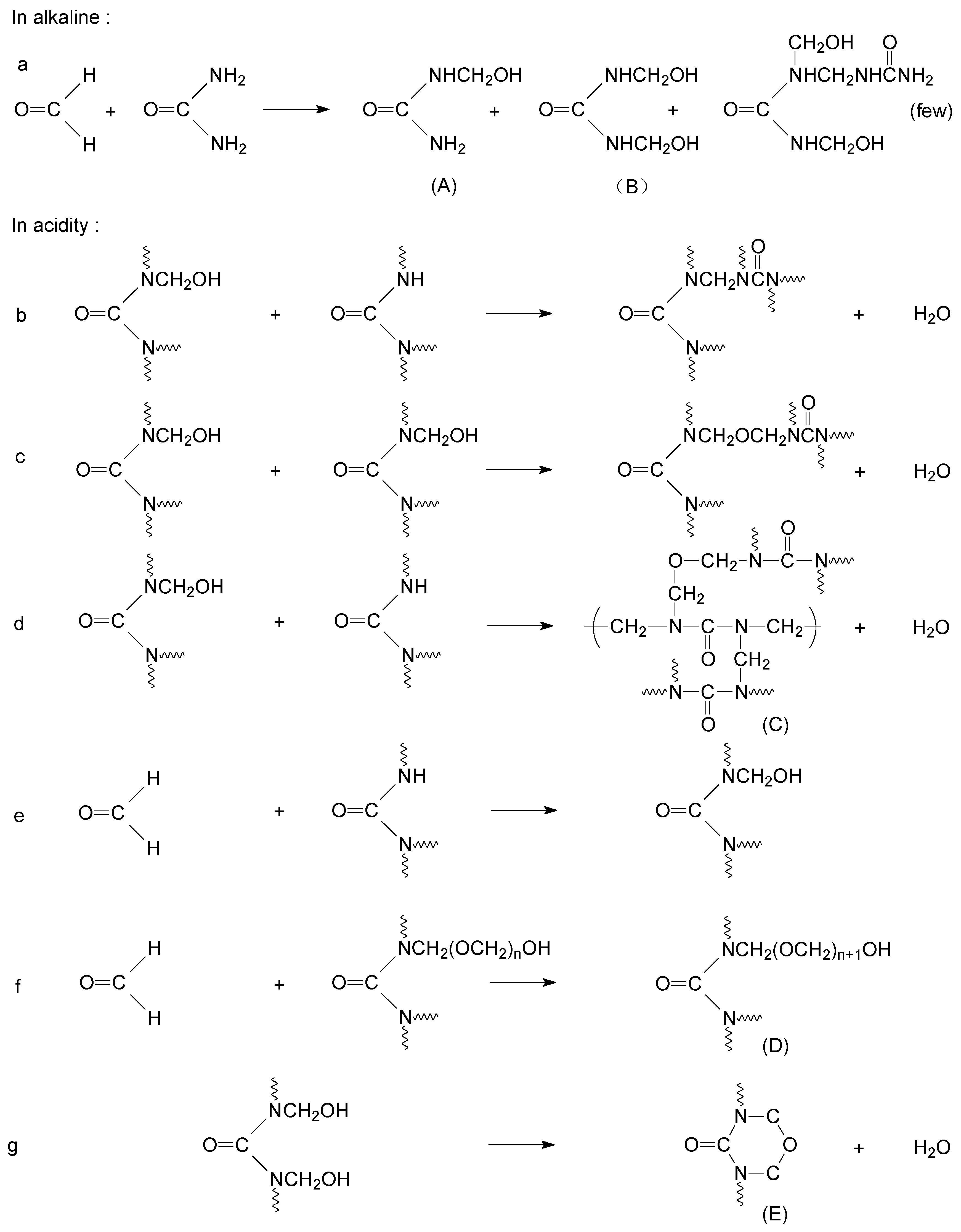







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhou, Q.; Ye, H.-M. Optimizing the Preparation of Semi-Crystalline Paraffin/Poly(Urea-Formaldehyde) Microcapsules for Thermal Energy Storage. Appl. Sci. 2019, 9, 599. https://doi.org/10.3390/app9030599
Zhang K, Zhou Q, Ye H-M. Optimizing the Preparation of Semi-Crystalline Paraffin/Poly(Urea-Formaldehyde) Microcapsules for Thermal Energy Storage. Applied Sciences. 2019; 9(3):599. https://doi.org/10.3390/app9030599
Chicago/Turabian StyleZhang, Kai, Qian Zhou, and Hai-Mu Ye. 2019. "Optimizing the Preparation of Semi-Crystalline Paraffin/Poly(Urea-Formaldehyde) Microcapsules for Thermal Energy Storage" Applied Sciences 9, no. 3: 599. https://doi.org/10.3390/app9030599
APA StyleZhang, K., Zhou, Q., & Ye, H.-M. (2019). Optimizing the Preparation of Semi-Crystalline Paraffin/Poly(Urea-Formaldehyde) Microcapsules for Thermal Energy Storage. Applied Sciences, 9(3), 599. https://doi.org/10.3390/app9030599




