A Form Stable Composite Phase Change Material for Thermal Energy Storage Applications over 700 °C
Abstract
:1. Introduction
- Most studies are on PCM use in low to medium temperature applications and eutectic nitrate salts are the most studied;
- Very few studies have been done on PCM use in high temperature applications (>500 °C);
- Carbon materials, particularly graphite, have been the main materials used to enhance PCM thermal conductivities, which are less likely to be applicable at high temperatures due to oxidation [5];
- Little work has been done on the thermal conductivity enhancement of salt-based composite PCMs for applications above ~500 °C;
- Different materials have been used as skeleton materials but many of them cannot be used at high temperatures;
2. Materials and Methodology
2.1. Preparation of Composite PCM
- The mixed powders were first compressed at 40 MPa to give tablets with a diameter of 15 mm;
- The raw tablets were heated up to 750 °C at a heating rate of 5 °C/min and maintained at 750 °C for 30 min;
- The composite PCM tablets were then allowed to cool to room temperature naturally.
2.2. Sample Characterization
3. Results and Discussion
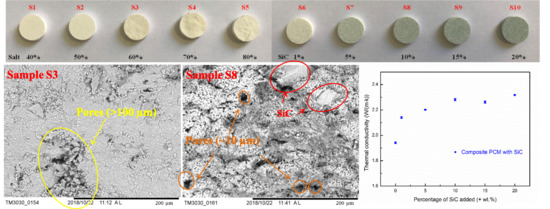
3.1. Visual Observation of the Composite PCM
3.2. Thermal Conductivity
3.3. Microstructural Observations
3.4. Chemical Compatibility
3.4.1. Chemical Compatibility between Different Components
3.4.2. Chemical Compatibility after 100 Thermal Cycles
3.5. Thermal Properties of the Composite PCM and Energy Density
- Temperature range between 550 °C and 710 °C (where Cp is in J·g−1 °C −1 and T is in °C):
- Temperature range between 710 °C and 750 °C:
3.6. Thermal Stability of the Composite PCM
4. Conclusions
Symbols
| DSC | Differential scanning calorimetry |
| SEM | Scanning electron microscopy |
| LFA | Laser flash analysis |
| TEM | Transmission electron microscopy |
| TG | Thermogravimetric analysis |
| EDS | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| XRD | X-ray diffraction |
| PCM | Phase change material |
| TES | Thermal energy storage |
| EG | Expanded graphite |
| STA | Simultaneous thermal analysis |
| XRF | X-ray fluorescence spectrometer |
| BET | BET surface area analyzer |
| DTA | Differential thermal analysis |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Energy and CO2 Status Report; International Energy Agency: Paris, France, 2017.
- Gielen, D. Tracking Industrial Energy Efficiency and CO2 Emissions. Int. Energy Agency 2007, 34, 1–2. [Google Scholar]
- Balcilar, M.; Ozdemir, Z.A.; Ozdemir, H.; Shahbaz, M. The renewable energy consumption and growth in the G-7 countries: Evidence from historical decomposition method. Renew. Energy 2018, 126, 594–604. [Google Scholar] [CrossRef] [Green Version]
- Hasan, R.; Mekhilef, S.; Seyedmahmoudian, M.; Horan, B. Grid-connected isolated PV microinverters: A review. Renew. Sustain. Energy Rev. 2017, 67, 1065–1080. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energy Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Lefebvre, D.; Tezel, F.H. A review of energy storage technologies with a focus on adsorption thermal energy storage processes for heating applications. Renew. Sustain. Energy Rev. 2017, 67, 116–125. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Saktisahdan, T.J.; Jannifar, A.; Hasan, M.H.; Matseelar, H.S.C. A review of available methods and development on energy storage: Technology update. Renew. Sustain. Energy Rev. 2014, 33, 532–545. [Google Scholar] [CrossRef]
- Ge, Z.; Li, Y.; Li, D.; Sun, Z.; Jin, Y.; Liu, C.; Li, C.; Leng, G. Thermal energy storage: Challenges and the role of particle technology. Particuology 2014, 15, 2–8. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Huang, Y.; Ye, F.; Wang, X.; Li, D.; Wang, C.; Ding, Y. Principles and new development of thermal storage technology. Energy Storage Sci. Technol. 2013, 2, 69–72. [Google Scholar]
- Nomura, T.; Okinaka, N.; Akiyama, T. Technology of latent heat storage for high temperature application: A review. ISIJ Int. 2010, 50, 1229–1239. [Google Scholar] [CrossRef]
- Michels, H.; Pitz-Paal, R. Cascaded latent heat storage for parabolic trough solar power plants. Sol. Energy 2007, 81, 829–837. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Jiang, Z.; Leng, G.; Ye, F.; Ge, Z.; Liu, C.; Wang, L.; Huang, Y.; Ding, Y. Form-stable LiNO3–NaNO3–KNO3–Ca(NO3)2/calcium silicate composite phase change material (PCM) for mid-low temperature thermal energy storage. Energy Convers. Manag. 2015, 106, 165–172. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Luan, Y.; Wang, G.; Feng, Y.; Feng, D. Preparation and thermal properties of porous heterogeneous composite phase change materials based on molten salts/expanded graphite. Solar Energy 2014, 107, 63–73. [Google Scholar] [CrossRef]
- Huang, Z.; Gao, X.; Xu, T.; Fang, Y.; Zhang, Z. Thermal property measurement and heat storage analysis of LiNO3/KCl—Expanded graphite composite phase change material. Appl. Energy 2014, 115, 265–271. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Min, X.; Deng, Y.; Guan, W.; Ning, L. Diatomite: A promising natural candidate as carrier material for low, middle and high temperature phase change material. Energy Convers. Manag. 2015, 98, 34–45. [Google Scholar] [CrossRef]
- Pincemin, S.; Olives, R.; Py, X.; Christ, M. Highly conductive composites made of phase change materials and graphite for thermal storage. Sol. Energy Mater. Sol. Cells 2008, 92, 603–613. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Paraffin/diatomite composite phase change material incorporated cement-based composite for thermal energy storage. Appl. Energy 2013, 105, 229–237. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, J.; Zhu, P.; Wang, C.; Li, X. Preparation, characterization and thermal properties of binary nitrate salts/expanded graphite as composite phase change material. Thermochim. Acta 2014, 587, 52–58. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Huang, G.; Kubo, S.; Shu, P. Characterization and thermal performance of nitrate mixture/SiC ceramic honeycomb composite phase change materials for thermal energy storage. Appl. Therm. Eng. 2015, 81, 193–197. [Google Scholar] [CrossRef]
- Acem, Z.; Lopez, J.; Palomo Del Barrio, E. KNO3/NaNO3–Graphite materials for thermal energy storage at high temperature: Part I.—Elaboration methods and thermal properties. Appl. Therm. Eng. 2010, 30, 1580–1585. [Google Scholar] [CrossRef]
- Xu, G.; Leng, G.; Yang, C.; Qin, Y.; Wu, Y.; Chen, H.; Cong, L.; Ding, Y. Sodium nitrate—Diatomite composite materials for thermal energy storage. Sol. Energy 2017, 146, 494–502. [Google Scholar] [CrossRef]
- Li, R.; Zhu, J.; Zhou, W.; Cheng, X.; Li, Y. Thermal properties of sodium nitrate-expanded vermiculite form-stable composite phase change materials. Mater. Des. 2016, 104, 190–196. [Google Scholar] [CrossRef]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Preparation and thermal properties characterization of carbonate salt/carbon nanomaterial composite phase change material. Energy Convers. Manag. 2015, 97, 103–110. [Google Scholar] [CrossRef]
- Ge, Z.; Ye, F.; Cao, H.; Leng, G.; Qin, Y. Carbonate-salt-based composite materials for medium- and high-temperature thermal energy storage. Particuology 2014, 15, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Ye, F.; Ding, Y. Composite Materials for Thermal Energy Storage: Enhancing Performance through Microstructures. ChemSusChem 2014, 7, 1318–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Wang, W.; Ding, J.; Wei, X.; Huang, C. Preparation of binary eutectic chloride/expanded graphite as high-temperature thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2016, 149, 187–194. [Google Scholar] [CrossRef]
- Qin, Y.; Leng, G.; Yu, X.; Cao, H.; Qiao, G.; Dai, Y.; Zhang, Y.; Ding, Y. Sodium sulfate–diatomite composite materials for high temperature thermal energy storage. Powder Technol. 2015, 282, 37–42. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Xu, X.; Yang, L.; Wu, J.; Zhao, F.; Li, C. Molten salts/ceramic-foam matrix composites by melt infiltration method as energy storage material. J. Wuhan Univ. Technol. Sci. Ed. 2009, 24, 651–653. [Google Scholar] [CrossRef]
- Ronchi, C.; Sheindlin, M. Melting point of MgO. J. Appl. Phys. 2001, 90, 3325–3331. [Google Scholar] [CrossRef]
- Prochazka, S. Dense Silicon Carbide Ceramic and Method of Making Same. U.S. Patent 3,852,099, 1972. [Google Scholar]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Watari, K.; Nakano, H.; Sato, K.; Urabe, K.; Ishizaki, K.; Cao, S.; Mori, K. Effect of Grain Boundaries on Thermal Conductivity of Silicon Carbide Ceramic at 5 to 1300 K. J. Am. Ceram. Soc. 2003, 86, 1812–1814. [Google Scholar] [CrossRef]
- Gubernat, A.; Pichór, W.; Lach, R.; Zientara, D.; Sitarz, M.; Springwald, M. Low-temperature synthesis of silicon carbide powder using shungite. Boletín la Soc. Española Cerámica y Vidr. 2017, 56, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Slifka, A.J.; Filla, B.J.; Phelps, J.M. Thermal conductivity of magnesium oxide from absolute, steady-state measurements. J. Res. Natl. Inst. Stand. Technol. 1998, 103, 357. [Google Scholar] [CrossRef] [PubMed]
- Roest, D.L.; Ballone, P.; Bedeaux, D.; Kjelstrup, S. High-Temperature Metal/Molten Alkali Carbonate Interfaces: A Molecular Dynamics Study. 2017. [Google Scholar]
- Navarro, M.E.; Andreu, P.; Qiao, G.; Ding, Y. Thermal properties of a novel medium temperature thermal energy storage composite based on sodium nitrate as phase change material. In Proceedings of the 12th International Conference on Heat Transfer, Fluid Mechanics and Thermodynamics, Costa de Sol, Spain, 11–13 July 2016. [Google Scholar]
- Do, K.S.; Aktay, C.; Tamme, R.; Müller-Steinhagen, H. Thermal Conductivity of High-Temperature Multicomponent Materials with Phase Change. Int. J. Thermophys. 2008, 29, 678–692. [Google Scholar]
- Pezoldt, J.; Stottko, B.; Kupris, G.; Ecke, G. Sputtering effects in hexagonal silicon carbide. Mater. Sci. Eng. 1995, 29, 94–98. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, T. Influence of SiO2 pore structure on phase change enthalpy of shape-stabilized polyethylene glycol/silica composites. J. Mater. Sci. 2013, 48, 3716–3721. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Zhao, M. Synthesis and characterization of PEG/ZSM-5 composite phase change materials for latent heat storage. Renew. Energy 2018, 121, 45–52. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, S.; James, R. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures; US Government Printing Office: Washington, WA, USA, 1995.
- Munro, R.G. Material Properties of a Sintered α-SiC. J. Phys. Chem. Ref. Data 1997, 26, 1195–1203. [Google Scholar] [CrossRef]
- Yin, H.; Lu, B.; Xu, Y.; Tang, D.; Mao, X.; Xiao, W.; Wang, D.; Alshawabkeh, A.N. Harvesting Capacitive Carbon by Carbonization of Waste Biomass in Molten Salts. Environ. Sci. Technol. 2014, 48, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Matsunami, J.; Hosokawa, Y.; Yokota, O.; Tamaura, Y.; Kitamura, M. Coal/CO2 Gasification System Using Molten Carbonate Salt for Solar/Fossil Energy Hybridization. Energy Fuels 1999, 13, 961–964. [Google Scholar] [CrossRef]
- Ren, N.; Wu, Y.; Ma, C.; Sang, L. Preparation and thermal properties of quaternary mixed nitrate with low melting point. Sol. Energy Mater. Sol. Cells 2014, 127, 6–13. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, H.-G. Thermal and Carbothermic Decomposition of Na2 CO3 and Li2 CO3. Metall. Mater. Trans. B 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Lehman, R.L.; Gentry, J.S.; Glumac, N.G. Thermal stability of potassium carbonate near its melting point. Thermochim. Acta 1998, 316, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. Thermal stability of the eutectic composition in LiNO3–NaNO3–KNO3 ternary system used for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 100, 162–168. [Google Scholar] [CrossRef]










| No. | Melting Temperature (°C) | PCM | Skeleton Materials | Thermal Conductivity Enhancer | Characterization | Ref. |
|---|---|---|---|---|---|---|
| 1 | 103.5 | LiNO3-NaNO3-KNO3-Ca(NO3)2 | Calcium silicate | Graphite | SEM, XRD, STA, Hot Plate, 1000 thermal cycles | [14] |
| 2 | 168.5–229.7 | LiNO3-KCl, LiNO3-NaNO3, LiNO3-NaCl | EGa | EG | SEM, XRD, STA, Hot Disc | [15] |
| 3 | 200 | LiNO3-KCl | EG | EG | DSC, Hot Disc | [16] |
| 4 | 240-255 | LiNO3 | Diatomite | - | SEM, XRD, DSC, XRF, BET, FTIR, TEM | [17] |
| 5 | 220 | NaNO3-KNO3 | Graphite | Graphite | DTA, Hot Plate | [18] |
| 6 | 220-350 | NaNO3-KNO3, NaNO3, KNO3 | EG | EG | DSC, Hotwire | [19] |
| 7 | 220 | NaNO3-KNO3 | EG | EG | SEM, XRD, DSC, EDS, TEM | [20] |
| 8 | 220 | NaNO3-KNO3 | SiC | SEM, DSC | [21] | |
| 9 | 220 | NaNO3-KNO3 | Graphite | Graphite | Hot Plate | [22] |
| 10 | 300 | NaNO3 | Diatomite | Graphite | SEM, XRD, STA, XRF, BET, 300 thermal cycles | [23] |
| 11 | 300 | NaNO3 | Expanded vermiculite | SiC | SEM, XRD, DSC, FTIR, Hot Disc | [24] |
| 12 | 486 | Li2CO3-K2CO3 | Graphite | Graphite | DSC, LFA | [25] |
| 13 | 500 | Li2CO3-Na2CO3 | MgO | Graphite | SEM, XMT, LFA, TG-DSC | [26,27] |
| 14 | 506 | NaCl-CaCl2 | EG | EG | SEM, XRD, DSC, Hot Disc | [28] |
| 15 | 880 | Na2SO4 | Diatomite | - | SEM, XRD, STA, XRF, BET | [29] |
| 16 | 880 | Na2SO4 | SiC | - | XRD, SEM, TG-DSC | [30] |
| Sample | K2CO3-Na2CO3 | MgO | SiC |
|---|---|---|---|
| S1 | 40 | 60 | - |
| S2 | 50 | 50 | - |
| S3 | 60 | 40 | - |
| S4 | 70 | 30 | - |
| S5 | 80 | 20 | - |
| S6 | 60 | 40 | 1 |
| S7 | 60 | 40 | 5 |
| S8 | 60 | 40 | 10 |
| S9 | 60 | 40 | 15 |
| S10 | 60 | 40 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Jiang, F.; Li, C.; Leng, G.; Zhao, X.; Li, Y.; Zhang, T.; Xu, G.; Jin, Y.; Yang, C.; et al. A Form Stable Composite Phase Change Material for Thermal Energy Storage Applications over 700 °C. Appl. Sci. 2019, 9, 814. https://doi.org/10.3390/app9050814
Jiang Z, Jiang F, Li C, Leng G, Zhao X, Li Y, Zhang T, Xu G, Jin Y, Yang C, et al. A Form Stable Composite Phase Change Material for Thermal Energy Storage Applications over 700 °C. Applied Sciences. 2019; 9(5):814. https://doi.org/10.3390/app9050814
Chicago/Turabian StyleJiang, Zhu, Feng Jiang, Chuan Li, Guanghui Leng, Xuemin Zhao, Yunren Li, Tongtong Zhang, Guizhi Xu, Yi Jin, Cenyu Yang, and et al. 2019. "A Form Stable Composite Phase Change Material for Thermal Energy Storage Applications over 700 °C" Applied Sciences 9, no. 5: 814. https://doi.org/10.3390/app9050814
APA StyleJiang, Z., Jiang, F., Li, C., Leng, G., Zhao, X., Li, Y., Zhang, T., Xu, G., Jin, Y., Yang, C., & Ding, Y. (2019). A Form Stable Composite Phase Change Material for Thermal Energy Storage Applications over 700 °C. Applied Sciences, 9(5), 814. https://doi.org/10.3390/app9050814