Multiphysics Field Distribution Characteristics within the One-Cell Solid Oxide Fuel Cell Stack with Typical Interdigitated Flow Channels
Abstract
:1. Introduction
2. Structure, Theory, and Numerical Model
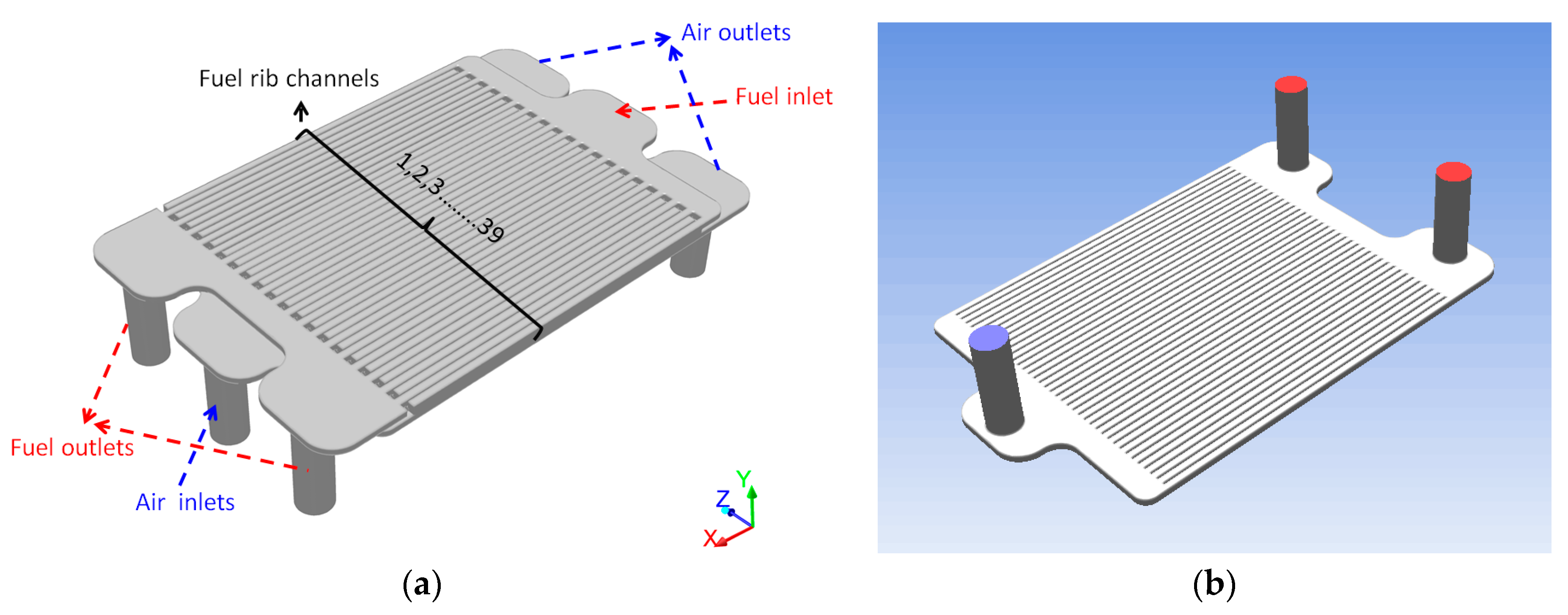
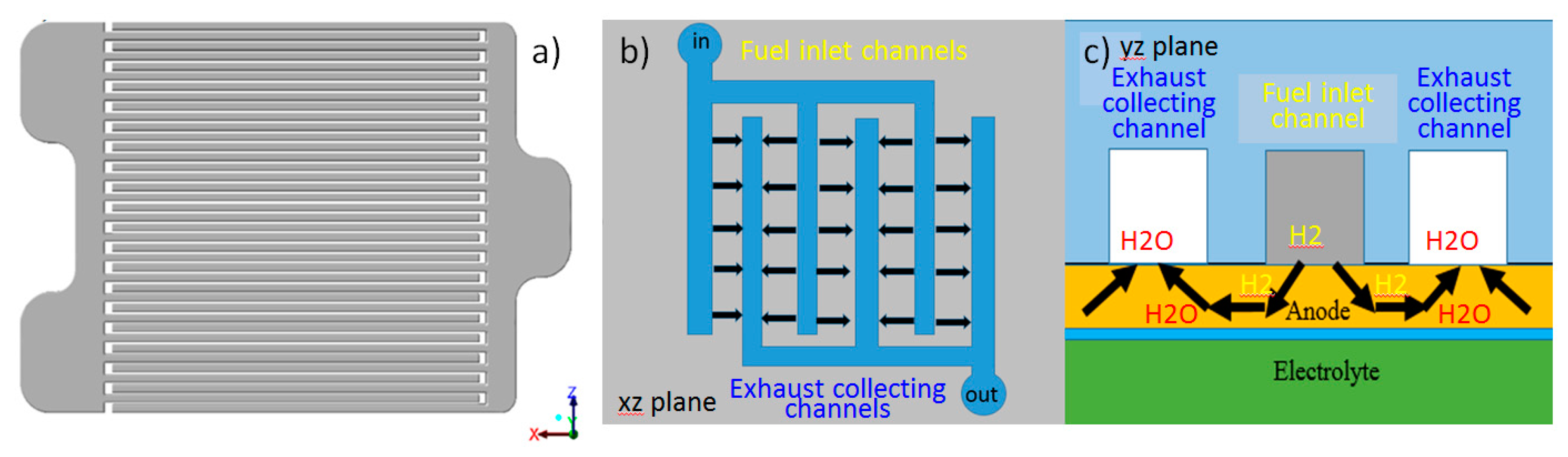
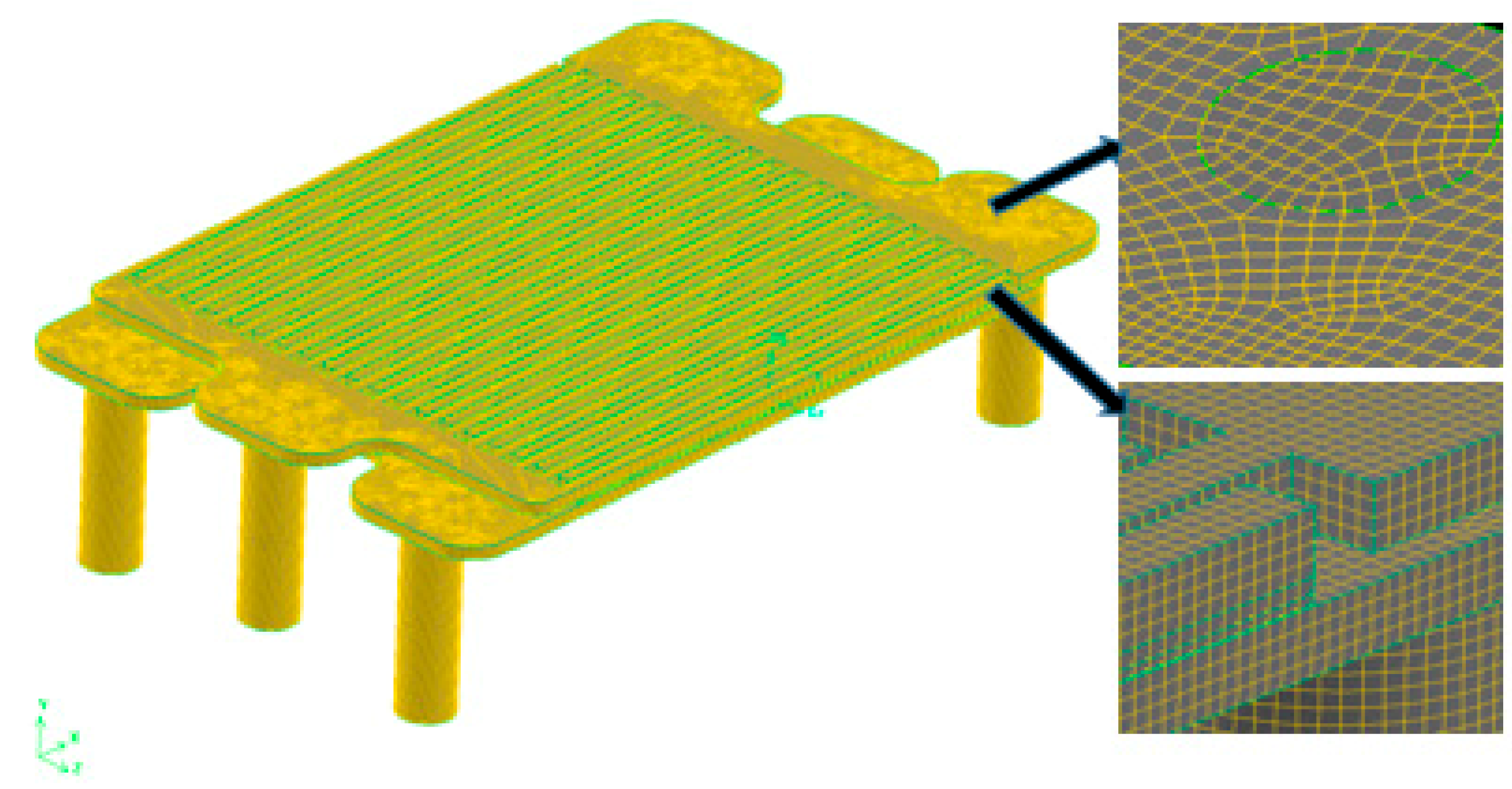
2.1. Model Structure and Mesh
2.2. Governing Equations
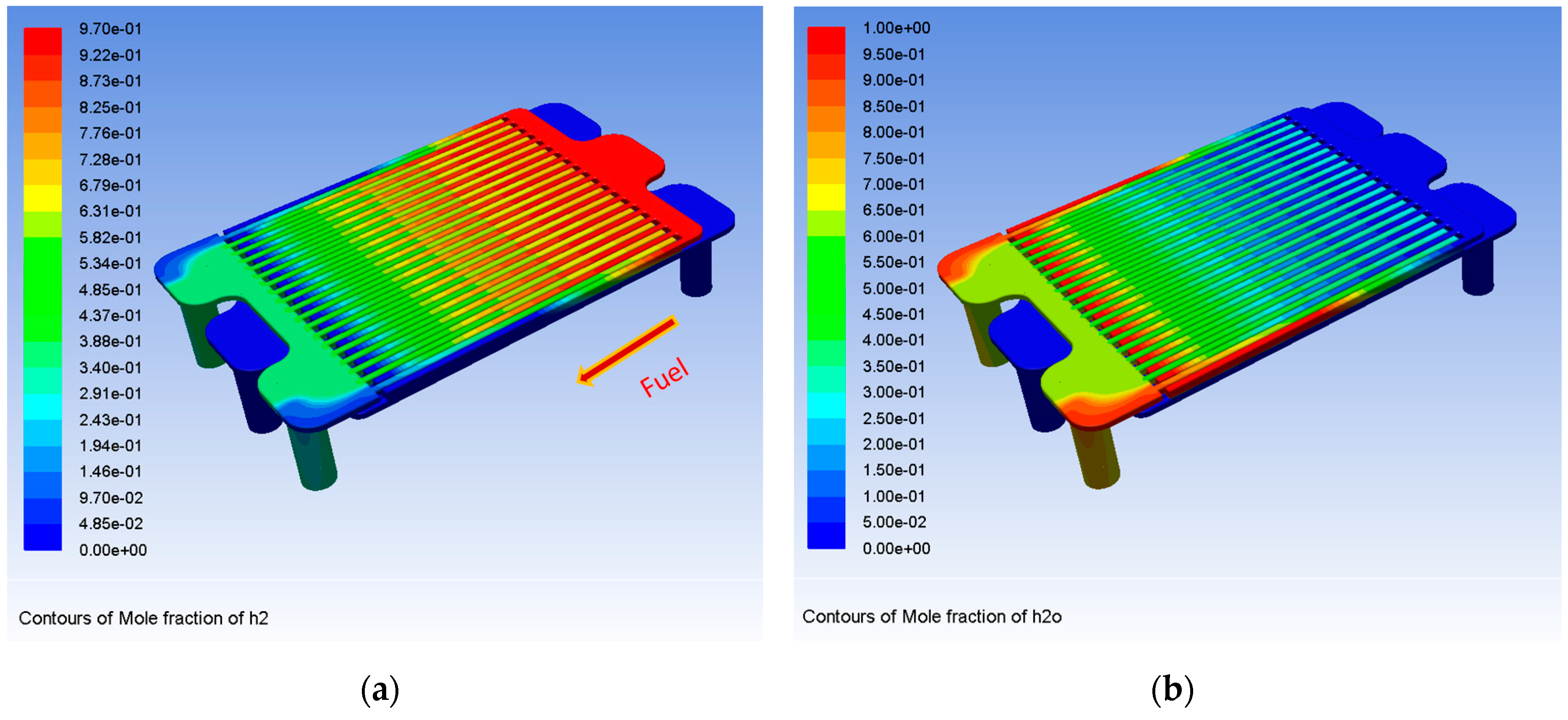
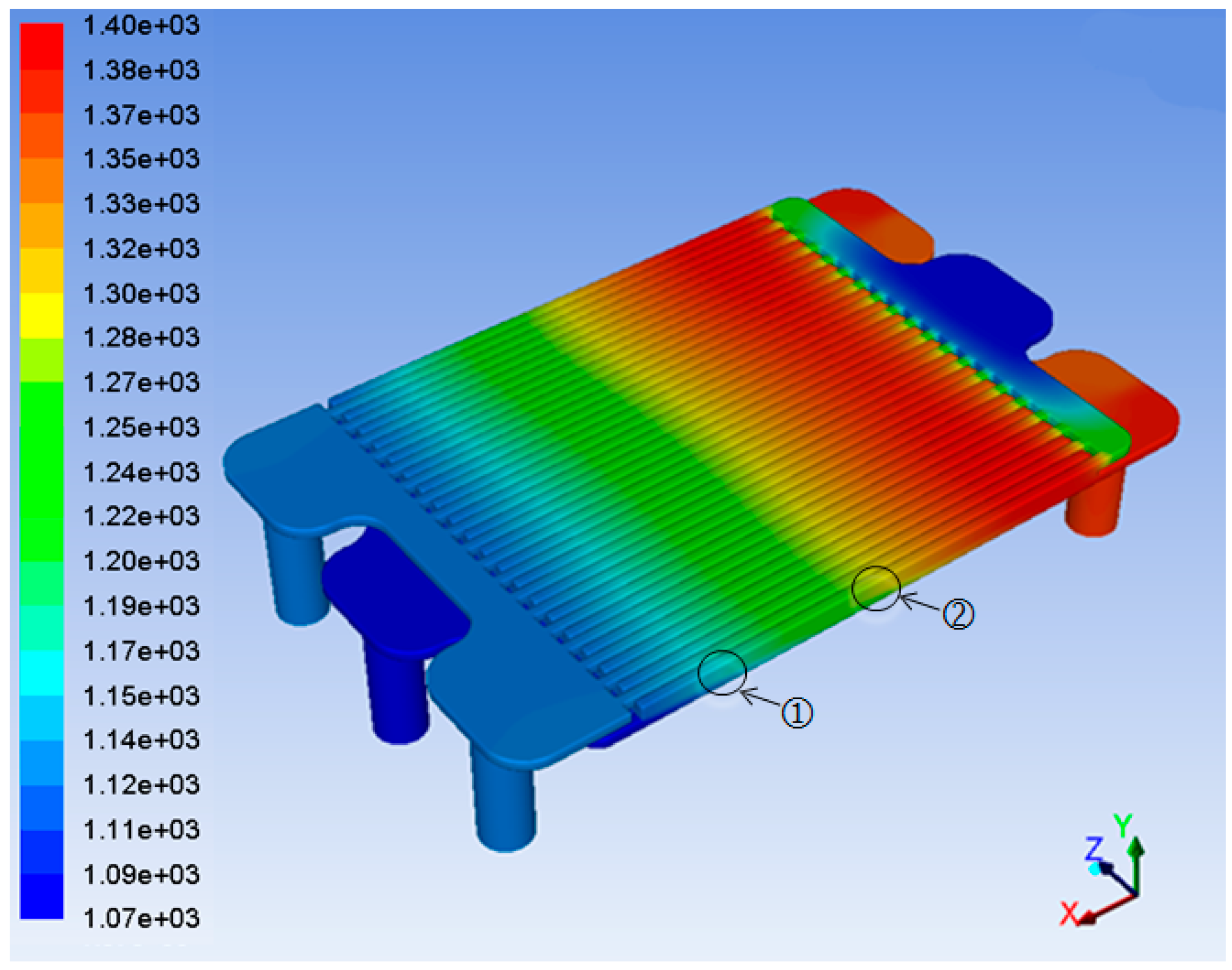
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; O’Hayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Hu, B.; Ding, K.; Yan, C.; Lu, L. The Geometry Effect of Cathode/Anode Areas Ratio on Electrochemical Performance of Button Fuel Cell Using Mixed Conducting Materials. Energies 2018, 11, 1875. [Google Scholar] [CrossRef]
- Ni, M.; Shao, Z.; Chan, K. Modeling of Proton-Conducting Solid Oxide Fuel Cells Fueled with Syngas. Energies 2014, 7, 4381. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Y.; Hu, B.; Yan, C.; Lu, L. Investigation of proper external air flow path for tubular fuel cell stacks with an anode support feature. Energy Convers. Manag. 2018, 171, 807–814. [Google Scholar] [CrossRef]
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar] [CrossRef]
- Seymour, I.D.; Tarancón, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ion. 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Lu, L.; Zeng, W.; Hu, S.; Chen, D.; Lei, J.; Ren, N. Polarization-dependent fluorescence of CdSe/ZnS quantum dots coupling to a single gold-silver alloy nanotube. J. Alloy. Compd. 2018, 731 (Suppl. C), 753–759. [Google Scholar] [CrossRef]
- Xiao, B.B.; Jiang, X.B.; Jiang, Q. Density functional theory study of oxygen reduction reaction on Pt/Pd3Al(111) alloy electrocatalyst. Phys. Chem. Chem. Phys. 2016, 18, 14234–14243. [Google Scholar] [CrossRef]
- Qu, Z.; Shi, M.; Wu, H.; Liu, Y.; Jiang, J.; Yan, C. An efficient binder-free electrode with multiple carbonized channels wrapped by NiCo2O4 nanosheets for high-performance capacitive energy storage. J. Power Sources 2009, 410–411, 179–187. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Q.; Lu, L.; Periasamy, V.; Tade, M.O.; Shao, Z. Multi scale and physics models for intermediate and low temperatures H+-solid oxide fuel cells with H+/e(−)/O2− mixed conducting properties: Part A, generalized percolation theory for LSCF-SDC-BZCY 3-component cathodes. J. Power Sources 2016, 303, 305–316. [Google Scholar] [CrossRef]
- Kong, W.; Zhang, M.; Han, Z.; Zhang, Q. A Theoretical Model for the Triple Phase Boundary of Solid Oxide Fuel Cell Electrospun Electrodes. Appl. Sci. 2019, 9, 493. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Kaleem Ullah, M.; Usman, A. A Brief Description of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- Chiabrera, F.; Garbayo, I.; López-Conesa, L.; Martín, G.; Ruiz-Caridad, A.; Walls, M.; Ruiz-González, L.; Kordatos, A.; Núñez, M.; Morata, A.; et al. Grain Boundaries: Engineering Transport in Manganites by Tuning Local Nonstoichiometry in Grain Boundaries. Adv. Mater. 2019, 31, 1970026. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625 A molecular dynamics study. Phys. Rev. B 2009, 79, 172102. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Campanari, S.; Brouwer, J. Solid Oxide Fuel Cell short stack performance testing—Part B: Operation in carbon capture applications and degradation issues. J. Power Sources 2017, 371, 238–248. [Google Scholar] [CrossRef]
- Nishida, R.T.; Beale, S.B.; Pharoah, J.G. Comprehensive computational fluid dynamics model of solid oxide fuel cell stacks. Int. J. Hydrogen Energy 2016, 41, 20592–20605. [Google Scholar] [CrossRef]
- Lindermeir, A.; Immisch, C.; Szepanski, C.; Hamje, J.; Bentaleb, A.; Dörrer, L. New SOFC-Stack Design with Parallel-Connected Cells—Basic Concept and Joining Aspects. Fuel Cells 2015, 15, 703–710. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Y.; Tade, M.O.; Shao, Z. General Regulation of Air Flow Distribution Characteristics within Planar Solid Oxide Fuel Cell Stacks. ACS Energy Lett. 2017, 2, 319–326. [Google Scholar] [CrossRef]
- Chen, D.; Ding, K.; Chen, Z.; Wei, T.; Liu, K. Physics field distributions within fuel cell stacks with manifolds penetrating through the plane zone and open outlet features. Energy Convers. Manag. 2018, 178, 190–199. [Google Scholar] [CrossRef]
- Hwang, J.J.; Liu, S.J. Comparison of temperature distributions inside a PEM fuel cell with parallel and interdigitated gas distributors. J. Power Sources 2006, 162, 1203–1212. [Google Scholar] [CrossRef]
- Grujicic, M.; Zhao, C.L.; Chittajallu, K.M.; Ochterbeck, J.M. Cathode and interdigitated air distributor geometry optimization in polymer electrolyte membrane (PEM) fuel cells. Mater. Sci. Eng. B 2004, 108, 241–252. [Google Scholar] [CrossRef]
- Hwang, J.J.; Chen, C.K.; Lai, D.Y. Computational analysis of species transport and electrochemical characteristics of a MOLB-type SOFC. J. Power Sources 2005, 140, 235–242. [Google Scholar] [CrossRef]
- Su, S.; He, H.; Chen, D.; Zhu, W.; Wu, Y.; Kong, W.; Wang, B.; Lu, L. Flow distribution analyzing for the solid oxide fuel cell short stacks with rectangular and discrete cylindrical rib configurations. Int. J. Hydrogen Energy 2015, 40, 577–592. [Google Scholar] [CrossRef]
- Chen, D.; Zeng, Q.; Su, S.; Bi, W.; Ren, Z. Geometric optimization of a 10-cell modular planar solid oxide fuel cell stack manifold. Appl. Energy 2013, 112, 1100–1107. [Google Scholar] [CrossRef]
- Wen, H.; Ordonez, J.C.; Vargas, J.V.C. Optimization of single SOFC structural design for maximum power. Appl. Therm. Eng. 2013, 50, 12–25. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Zhang, S.; Tade, M.O.; Shao, Z.; Chen, H. Multiscale model for solid oxide fuel cell with electrode containing mixed conducting material. AIChE J. 2015, 61, 3786–3803. [Google Scholar] [CrossRef]
- Ni, M. The effect of electrolyte type on performance of solid oxide fuel cells running on hydrocarbon fuels. Int. J. Hydrogen Energy 2013, 38, 2846–2858. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Kukolin, A.; Chen, D.; Yang, W. Multiphysics Field Distribution Characteristics within the One-Cell Solid Oxide Fuel Cell Stack with Typical Interdigitated Flow Channels. Appl. Sci. 2019, 9, 1190. https://doi.org/10.3390/app9061190
Xu Y, Kukolin A, Chen D, Yang W. Multiphysics Field Distribution Characteristics within the One-Cell Solid Oxide Fuel Cell Stack with Typical Interdigitated Flow Channels. Applied Sciences. 2019; 9(6):1190. https://doi.org/10.3390/app9061190
Chicago/Turabian StyleXu, Yu, Anton Kukolin, Daifen Chen, and Wei Yang. 2019. "Multiphysics Field Distribution Characteristics within the One-Cell Solid Oxide Fuel Cell Stack with Typical Interdigitated Flow Channels" Applied Sciences 9, no. 6: 1190. https://doi.org/10.3390/app9061190



