Photocatalytic Lithography
Abstract
:1. Introduction
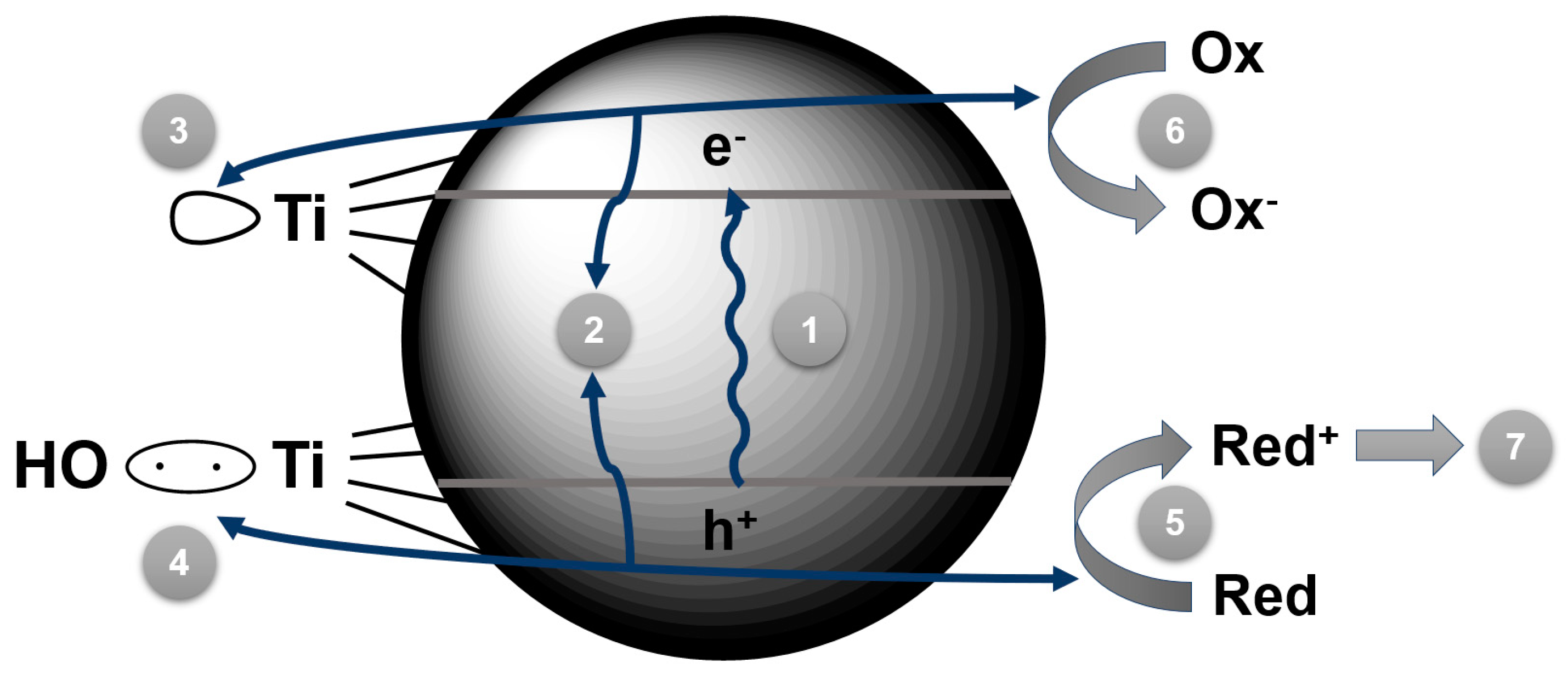
2. Titanium Dioxide: A Gold Standard for Photocatalysis
3. Photocatalytic Lithography
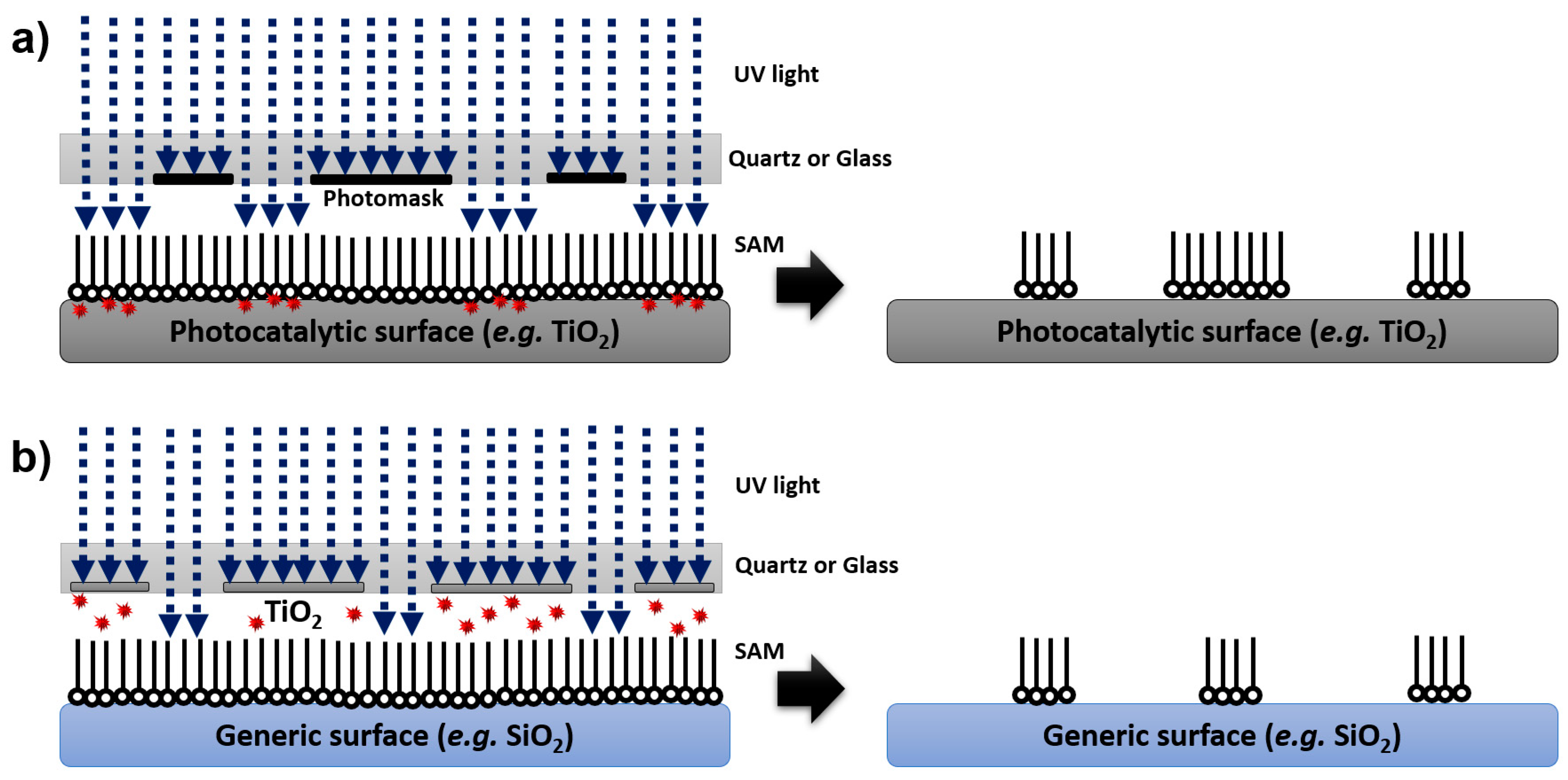
Direct and Remote Photocatalytic Lithography
4. Applications of Direct and Remote Photocatalytic Lithography
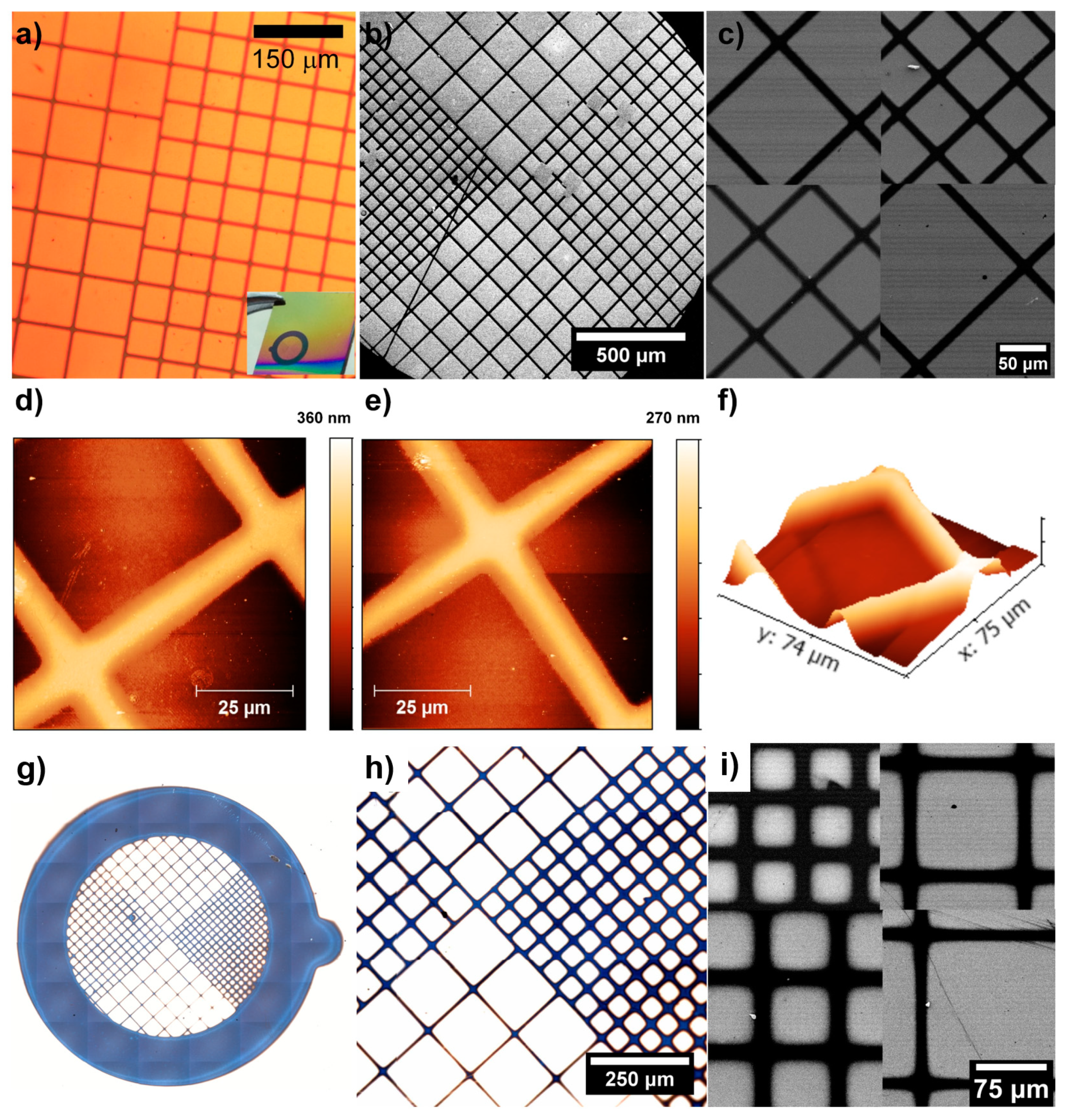
4.1. Patterning of Self-Assembled Monolayers and Generation of Superhydrophilic–Superhydrophobic Patterns
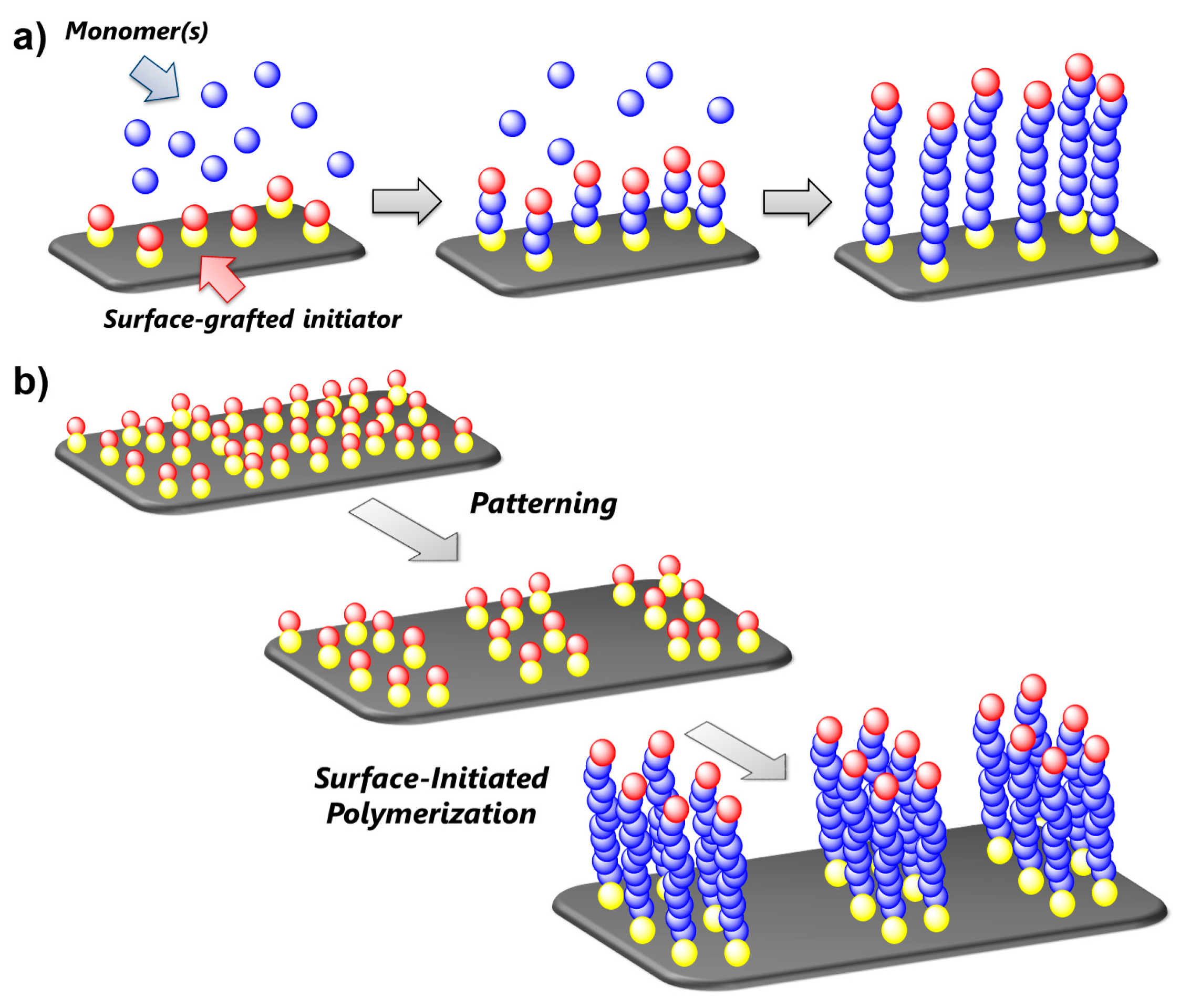
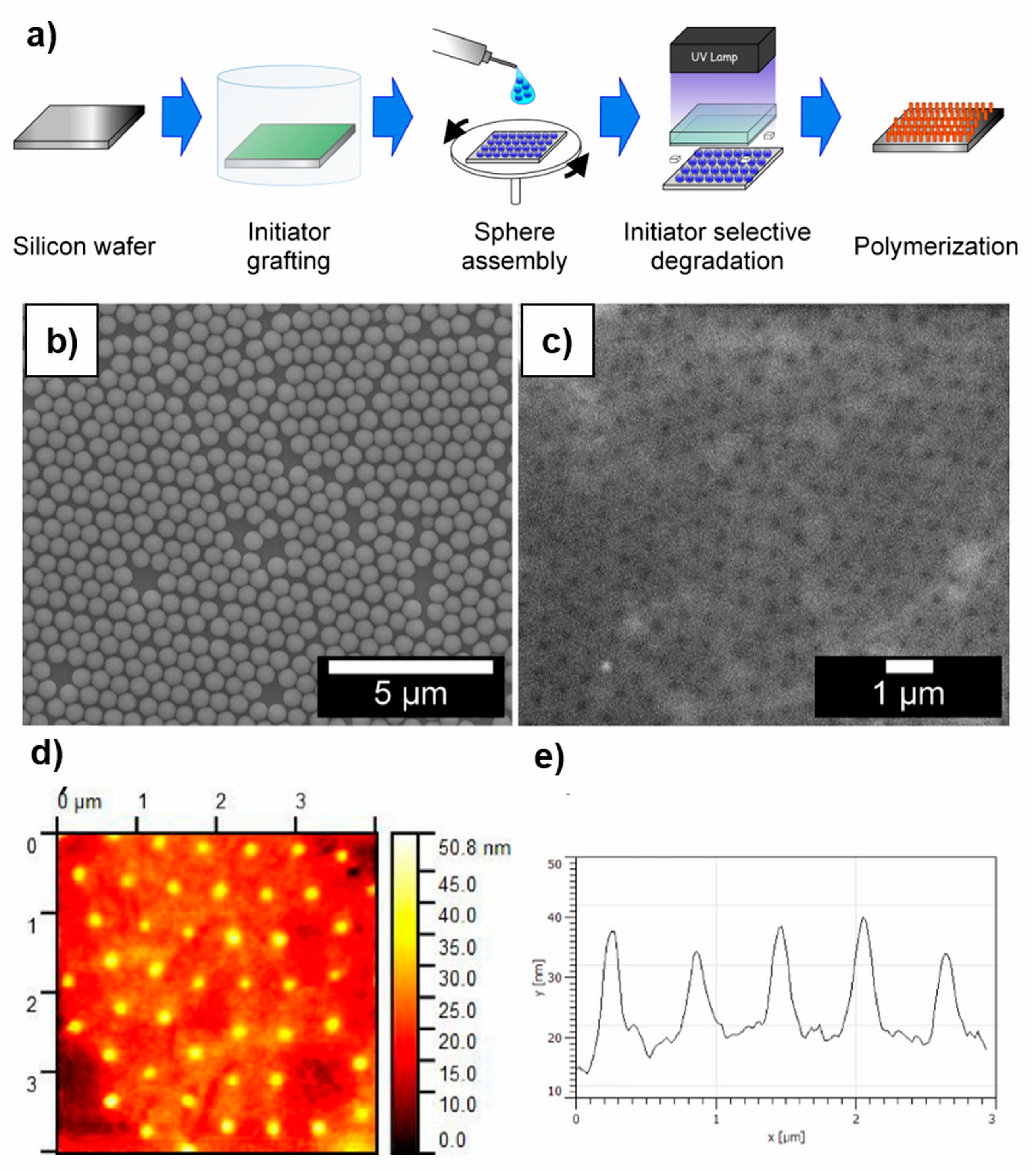
4.2. Patterning of Polymer Brushes
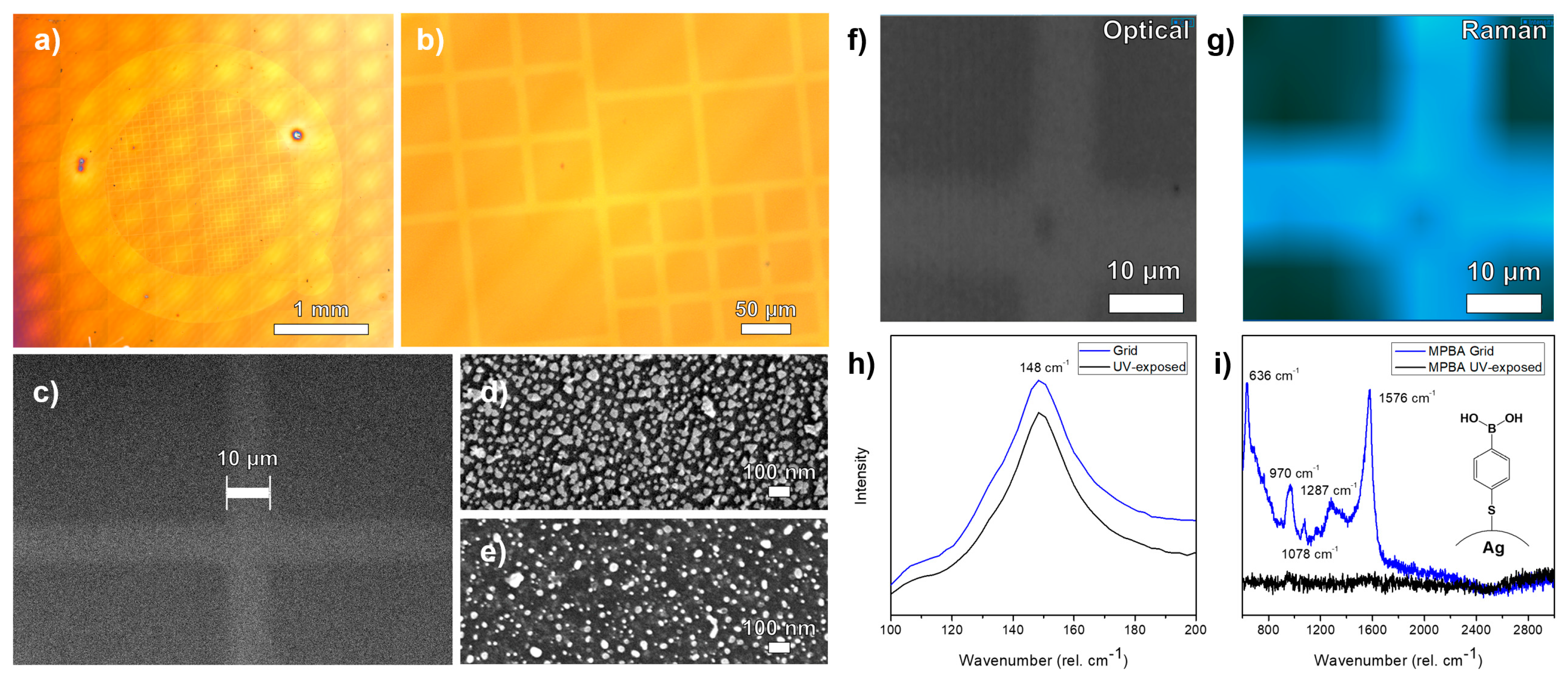
4.3. Patterning of Metal Surfaces and Sculpturing of Metal Nanoparticles
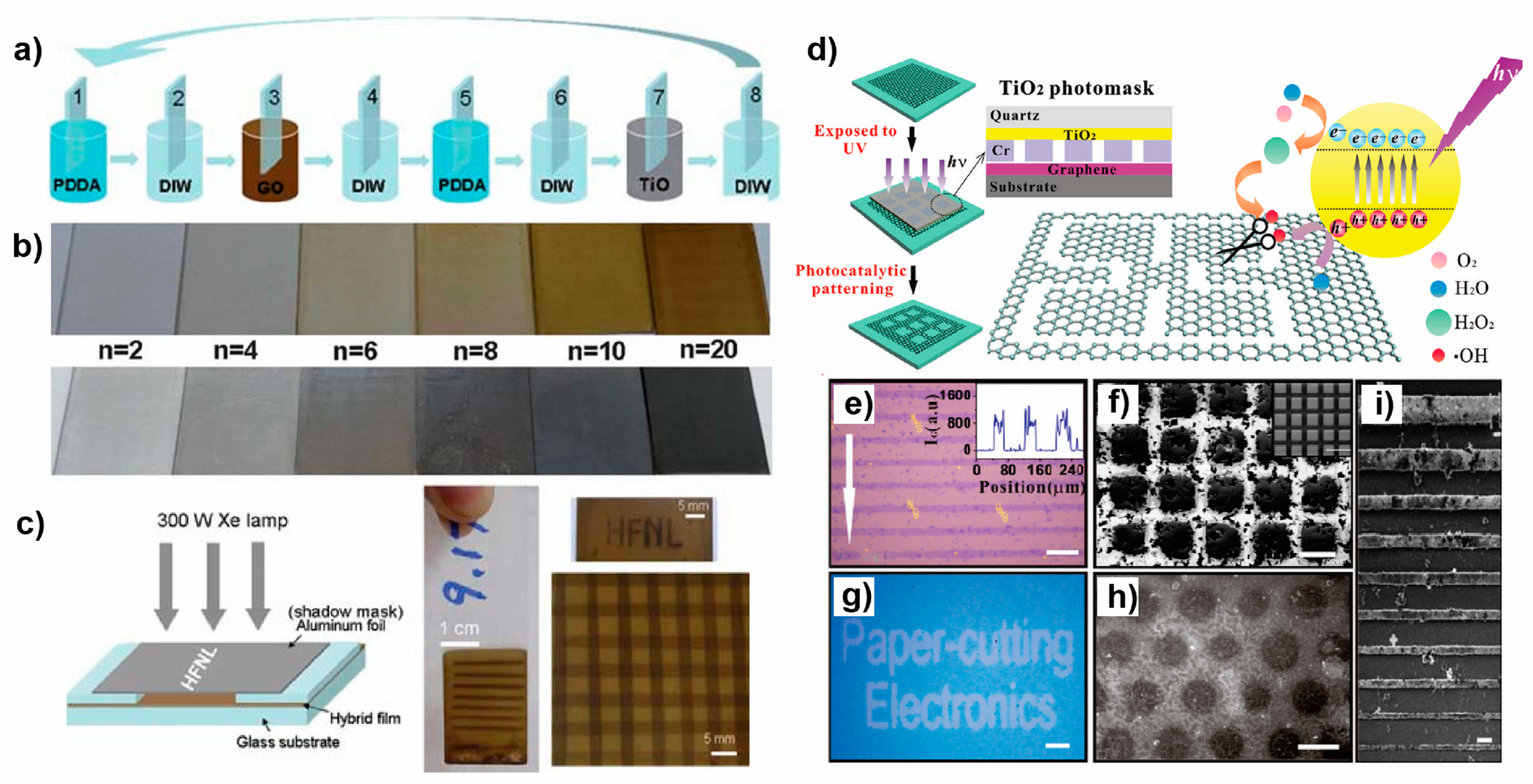
4.4. Patterning of Graphene and Graphene Oxide
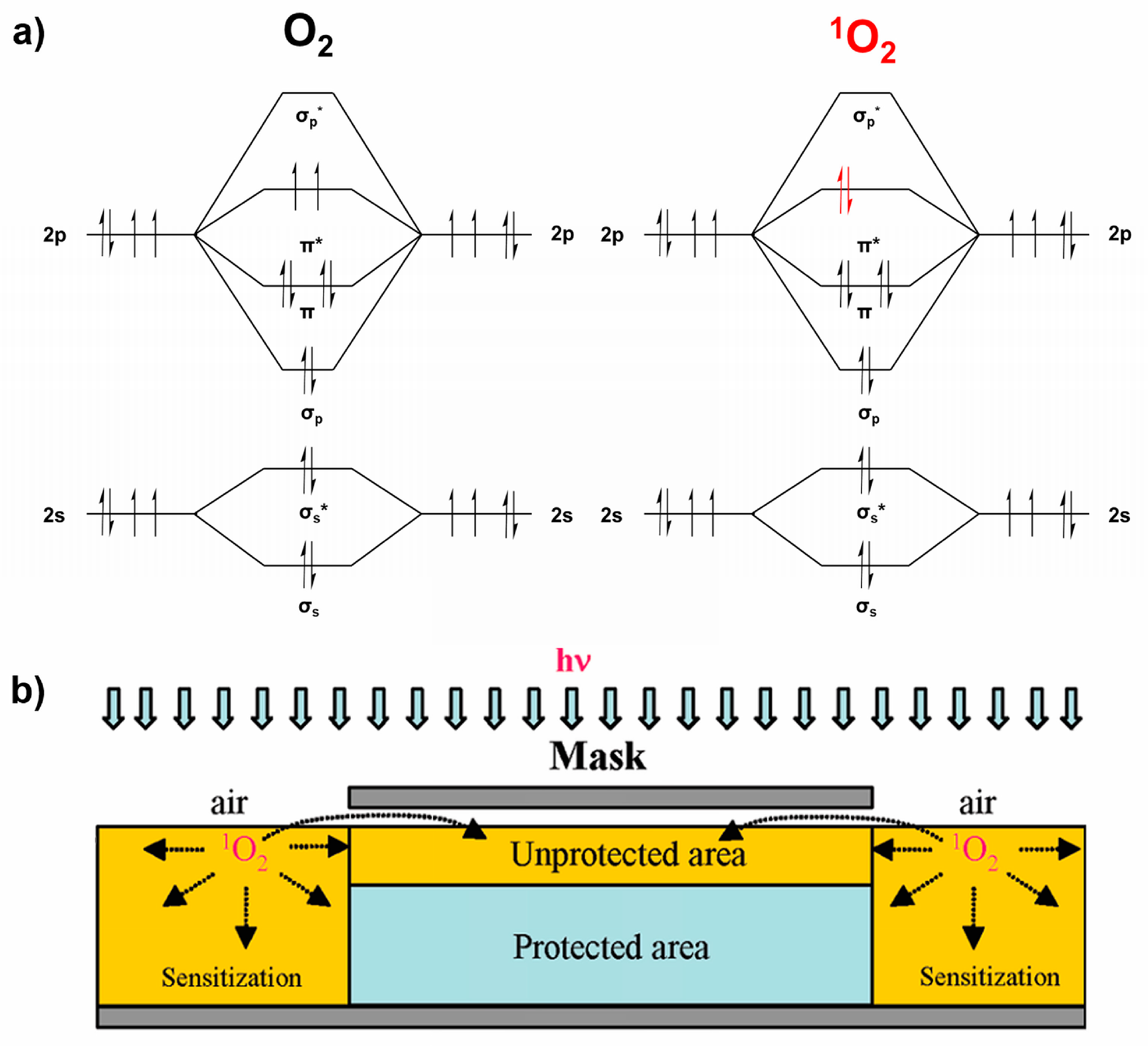
5. Singlet Oxygen-Based Photocatalytic Lithography
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Griffiths, A. Prints and Printmaking: An Introduction to the History and Techniques, 2nd ed.; University of California Press: Berkeley, CA, USA, 1996; ISBN 978-0-52020-714-1. [Google Scholar]
- Xia, Y.; Rogers, J.A.; Paul, K.E.; Whitesides, G.M. Unconventional Methods for Fabricating and Patterning Nanostructures. Chem. Rev. 1999, 99, 1823–1848. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium Dioxide (TiO2) Nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef] [PubMed]
- Kubo, W.; Tatsuma, T. Mechanisms of photocatalytic remote oxidation. J. Am. Chem. Soc. 2006, 128, 16034–16035. [Google Scholar] [CrossRef]
- Kubo, W.; Tatsuma, T. Conversion of a solid surface from super-hydrophobic to super-hydrophilic by photocatalytic remote oxidation and photocatalytic lithography. Appl. Surf. Sci. 2005, 243, 125–128. [Google Scholar] [CrossRef]
- Ganter, P.; Lotsch, B.V. Photocatalytic Nanosheet Lithography: Photolithography based on Organically Modified Photoactive 2D Nanosheets. Angew. Chem. Int. Ed. 2017, 56, 8389–8392. [Google Scholar] [CrossRef] [Green Version]
- Tatsuma, T.; Tachibana, S.; Miwa, T.; Tryk, D.A.; Fujishima, A. Remote Bleaching of Methylene Blue by UV-Irradiated TiO2 in the Gas Phase. J. Phys. Chem. B 1999, 103, 8033–8035. [Google Scholar] [CrossRef]
- Tatsuma, T.; Kubo, W.; Fujishima, A. Patterning of solid surfaces by photocatalytic lithography based on the remote oxidation effect of TiO2. Langmuir 2002, 18, 9632–9634. [Google Scholar] [CrossRef]
- Spori, D.M.; Venkataraman, N.V.; Tosatti, S.G.P.; Durmaz, F.; Spencer, N.D.; Zürcher, S. Influence of alkyl chain length on phosphate self-assembled monolayers. Langmuir 2007, 23, 8053–8060. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y. Self-assembled monolayers and titanium dioxide: From surface patterning to potential applications. Beilstein J. Nanotechnol. 2011, 2, 845–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurišić, A.B.; Chan, W.K. Photocatalytic activity of metal oxides-The role of holes and OH• radicals. Appl. Catal. B Environ. 2011, 107, 150–157. [Google Scholar] [CrossRef]
- Degawa, R.; Wang, P.; Tanaka, D.; Park, S.; Sakai, N.; Tatsuma, T.; Okamoto, K.; Tamada, K. Colorimetric Detection of an Airborne Remote Photocatalytic Reaction Using a Stratified Ag Nanoparticle Sheet. Langmuir 2016, 32, 8154–8162. [Google Scholar] [CrossRef]
- Kondrakov, A.O.; Ignatev, A.N.; Lunin, V.V.; Frimmel, F.H.; Bräse, S.; Horn, H. Roles of water and dissolved oxygen in photocatalytic generation of free OH radicals in aqueous TiO2 suspensions: An isotope labeling study. Appl. Catal. B Environ. 2016, 182, 424–430. [Google Scholar] [CrossRef]
- Kubo, W.; Tatsuma, T. Photocatalytic remote oxidation with various photocatalysts and enhancement of its activity. J. Mater. Chem. 2005, 15, 3104. [Google Scholar] [CrossRef]
- Smith, R.K.; Lewis, P.A.; Weiss, P.S. Patterning self-assembled monolayers. Prog. Surf. Sci. 2004, 75, 1–68. [Google Scholar] [CrossRef]
- Kubo, W.; Tatsuma, T.; Fujishima, A.; Kobayashi, H. Mechanisms and Resolution of Photocatalytic Lithography. J. Phys. Chem. B 2004, 108, 3005–3009. [Google Scholar] [CrossRef]
- Tatsuma, T.; Tachibana, S.; Fujishima, A. Remote oxidation of organic compounds by UV-irradiated TiO2 via the gas phase. J. Phys. Chem. 2001, 105, 6987–6992. [Google Scholar] [CrossRef]
- Tizazu, G.; El-Zubir, O.; Brueck, S.R.J.; Lidzey, D.G.; Leggett, G.J.; Lopez, G.P. Large area nanopatterning of alkylphosphonate self-assembled monolayers on titanium oxide surfaces by interferometric lithography. Nanoscale 2011, 3, 2511. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Nishimoto, S.; Kubo, A.; Tryk, D.; Ochiai, T.; Murakami, T.; Fujishima, A. Fabrication and application of TiO2-based superhydrophilic- superhydrophobic patterns on titanium substrates for offset printing. Chem. Asian J. 2009, 4, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Kubo, A.; Nohara, K.; Zhang, X.; Taneichi, N.; Okui, T.; Liu, Z.; Nakata, K.; Sakai, H.; Murakami, T.; et al. TiO2-based superhydrophobic-superhydrophilic patterns: Fabrication via an ink-jet technique and application in offset printing. Appl. Surf. Sci. 2009, 255, 6221–6225. [Google Scholar] [CrossRef]
- Blondiaux, N.; Zürcher, S.; Liley, M.; Spencer, N.D. Fabrication of multiscale surface-chemical gradients by means of photocatalytic lithography. Langmuir 2007, 23, 3489–3494. [Google Scholar] [CrossRef]
- Lee, J.P.; Sung, M.M. A New Patterning Method Using Photocatalytic Lithography and Selective Atomic Layer Deposition. J. Am. Chem. Soc. 2004, 126, 28–29. [Google Scholar] [CrossRef]
- Tatsuma, T.; Yoshida, Y.; Shitanda, I.; Notsu, H. Algal biosensor array on a single electrode. Analyst 2009, 134, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Nada, J.; Nishikawa, M.; Notsu, H.; Tatsuma, T.; Sakai, Y. Simultaneous evaluation of toxicities using a mammalian cell array chip prepared by photocatalytic lithography. Anal. Chim. Acta 2009, 653, 222–227. [Google Scholar] [CrossRef]
- Panzarasa, G. The art and science of polymer brushes: Recent developments in patterning and characterization approaches. Chimia 2017, 71, 354–358. [Google Scholar] [CrossRef]
- Panzarasa, G.; Aghion, S.; Soliveri, G.; Consolati, G.; Ferragut, R. Positron annihilation spectroscopy: A new frontier for understanding nanoparticle- loaded polymer brushes. Nanotechnology 2016, 27, 02LT03. [Google Scholar] [CrossRef]
- Panzarasa, G.; Aghion, S.; Marra, G.; Wagner, A.; Liedke, M.O.; Elsayed, M.; Krause-Rehberg, R.; Ferragut, R.; Consolati, G. Probing the Impact of the Initiator Layer on Grafted-from Polymer Brushes: A Positron Annihilation Spectroscopy Study. Macromolecules 2017, 50, 5574–5581. [Google Scholar] [CrossRef]
- Panzarasa, G.; Dübner, M.; Pifferi, V.; Soliveri, G.; Padeste, C. ON/OFF switching of silicon wafer electrochemistry by pH-responsive polymer brushes. J. Mater. Chem. C 2016, 4, 6287–6294. [Google Scholar] [CrossRef] [Green Version]
- Panzarasa, G.; Pifferi, V. On the capacitive behavior of silicon electrodes modified with ultrathin hydrophobic polymer brushes. J. Solid State Electrochem. 2018, 4, 1269–1273. [Google Scholar] [CrossRef]
- Dehghani, E.S.; Aghion, S.; Anwand, W.; Consolati, G.; Ferragut, R.; Panzarasa, G. Investigating the structure of crosslinked polymer brushes (brush-gels) by means of Positron Annihilation Spectroscopy. Eur. Polym. J. 2018, 99, 415–421. [Google Scholar] [CrossRef]
- Braunecker, W.A.; Matyjaszewski, K. Controlled/living radical polymerization: Features, developments, and perspectives. Prog. Polym. Sci. 2007, 32, 93–146. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Dong, H.; Jakubowski, W.; Pietrasik, J.; Kusumo, A. Grafting from Surfaces for “Everyone”: ARGET ATRP in the Presence of Air. Langmuir 2007, 23, 4528–4531. [Google Scholar] [CrossRef]
- Yu, Q.; Ista, L.K.; Gu, R.; Zauscher, S.; López, G.P. Nanopatterned polymer brushes: Conformation, fabrication and applications. Nanoscale 2016, 8, 680–700. [Google Scholar] [CrossRef]
- Panzarasa, G.; Soliveri, G.; Sparnacci, K.; Ardizzone, S.; Information, S. Patterning of polymer brushes made easy using titanium dioxide: Direct and remote photocatalytic lithography. Chem. Commun. 2015, 51, 7313–7316. [Google Scholar] [CrossRef] [PubMed]
- Panzarasa, G.; Soliveri, G.; Ardizzone, S.; Sparnacci, K. Photocatalytic lithography: An innovative approach to obtain patterned pH-responsive polymer brushes. Mater. Today Proc. 2015, 2, 4183–4189. [Google Scholar] [CrossRef]
- Maino, G.; Meroni, D.; Pifferi, V.; Falciola, L.; Soliveri, G.; Cappelletti, G.; Ardizzone, S. Electrochemically assisted deposition of transparent, mechanically robust TiO2 films for advanced applications. J. Nanopart. Res. 2013, 15, 2087. [Google Scholar] [CrossRef]
- Panzarasa, G.; Soliveri, G.; Ardizzone, S. Crafting positive/negative patterns and nanopillars of polymer brushes by photocatalytic lithography. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Panzarasa, G.; Soliveri, G.; Pifferi, V. Tuning the electrochemical properties of silicon wafer by grafted-from micropatterned polymer brushes. J. Mater. Chem. C 2016, 4, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Kettling, F.; Vonhören, B.; Krings, J.A.; Saito, S.; Ravoo, B.J. One-step synthesis of patterned polymer brushes by photocatalytic microcontact printing. Chem. Commun. 2015, 51, 1027–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzarasa, G.; Dübner, M.; Soliveri, G.; Edler, M.; Griesser, T. Branched poly(ethyleneimine): A versatile scaffold for patterning polymer brushes by means of remote photocatalytic lithography. Nanotechnology 2017, 28, 395302. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Dibbell, R.S.; Soja, G.R.; Hoth, R.M.; Watson, D.F. Photocatalytic patterning of monolayers for the site-selective deposition of quantum dots onto TiO2 surfaces. Langmuir 2007, 23, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Panzarasa, G. Just What Is It That Makes Silver Nanoprisms so Different, so Appealing? J. Chem. Educ. 2015, 92, 1918–1923. [Google Scholar] [CrossRef]
- Panzarasa, G.; Soliveri, G.; Marra, G.; Meda, L.; Savoini, A.; Ardizzone, S.; Salvalaggio, M. Sculpturing patterns of plasmonic silver nanoprisms by means of photocatalytic lithography. Nanotechnology 2017, 28, 155302. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; MacDonald, A.H. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Panzarasa, G.; Consolati, G.; Scavini, M.; Longhi, M.; Quasso, F. Convenient Preparation of Graphene Oxide from Expandable Graphite and Its Characterization by Positron Annihilation Lifetime Spectroscopy. C 2019, 5, 6. [Google Scholar] [CrossRef]
- Yao, H.-B.; Wu, L.-H.; Cui, C.-H.; Fang, H.-Y.; Yu, S.-H. Direct fabrication of photoconductive patterns on LBL assembled graphene oxide/PDDA/titania hybrid films by photothermal and photocatalytic reduction. J. Mater. Chem. 2010, 20, 5190. [Google Scholar] [CrossRef]
- Zhang, L.; Diao, S.; Nie, Y.; Yan, K.; Liu, N.; Dai, B.; Xie, Q.; Reina, A.; Kong, J.; Liu, Z. Photocatalytic patterning and modification of graphene. J. Am. Chem. Soc. 2011, 133, 2706–2713. [Google Scholar] [CrossRef]
- Radich, J.G.; Krenselewski, A.L.; Zhu, J.; Kamat, P.V. Is Graphene a Stable Platform for Photocatalysis? Mineralization of Reduced Graphene Oxide with UV-Irradiated TiO2 Nanoparticles. Chem. Mater. 2014, 26, 4662–4668. [Google Scholar] [CrossRef]
- Mun, D.-H.; Lee, H.J.; Bae, S.; Kim, T.-W.; Lee, S.H. Photocatalytic decomposition of graphene over a ZnO surface under UV irradiation. Phys. Chem. Chem. Phys. 2015, 17, 15683–15686. [Google Scholar] [CrossRef]
- Kapitanova, O.O.; Panin, G.N.; Cho, H.D.; Baranov, A.N.; Kang, T.W. Formation of self-assembled nanoscale graphene/graphene oxide photomemristive heterojunctions using photocatalytic oxidation. Nanotechnology 2017, 28, 204005. [Google Scholar] [CrossRef] [PubMed]
- Panzarasa, G.; Sparnacci, K. Glowing teacup demonstration: Trautz-schorigin reaction of natural polyphenols. J. Chem. Educ. 2012, 89, 1297–1300. [Google Scholar] [CrossRef]
- Panzarasa, G. Just Add Luminol to Turn the Spotlight on Radziszewski Amidation. ACS Omega 2018, 3, 13179–13182. [Google Scholar] [CrossRef]
- Breslow, D.S.; Simpson, D.A.; Kramer, B.D.; Schwarz, R.J.; Newburg, N.R. A new photoimaging system based on singlet oxygen. Ind. Eng. Chem. 1987, 10, 2144–2148. [Google Scholar] [CrossRef]
- Lougnot, D.J.; Ritzenthaler, D.; Carre, C.; Fouassier, J.P. A new gated system for two-photon holographic recording in the near infrared. J. Appl. Phys. 1988, 63, 4841–4848. [Google Scholar] [CrossRef]
- Kimel, S.; Tromberg, B.J.; Roberts, W.G.; Berns, M.W. Singlet oxygen generation of porphyrins, chlorins, and phthalocyanines. J. Photochem. Photobiol. 1989, 50, 175–183. [Google Scholar] [CrossRef]
- Suslick, K.S.; Rakow, N.A.; Kosal, M.E.; Chou, J.H. The materials chemistry of porphyrins and metalloporphyrins. J. Porphyr. Phthalocyanines 2000, 4, 407–413. [Google Scholar] [CrossRef] [Green Version]
- Bearinger, J.P.; Stone, G.; Christian, A.T.; Dugan, L.; Hiddessen, A.L.; Wu, K.J.J.; Wu, L.; Hamilton, J.; Stockton, C.; Hubbell, J.A. Porphyrin-based photocatalytic lithography. Langmuir 2008, 24, 5179–5184. [Google Scholar] [CrossRef]
- Bearinger, J.P.; Stone, G.; Hiddessen, A.L.; Dugan, L.C.; Wu, L.; Hailey, P.; Conway, J.W.; Kuenzler, T.; Feller, L.; Cerritelli, S.; et al. Phototocatalytic lithography of poly(propylene sulfide) block copolymers: Toward high-throughput nanolithography for biomolecular arraying applications. Langmuir 2009, 25, 1238–1244. [Google Scholar] [CrossRef]
- Bearinger, J.P.; Stone, G.; Dugan, L.C.; El Dasher, B.; Stockton, C.; Conway, J.W.; Kuenzler, T.; Hubbell, J. a Porphyrin-based photocatalytic nanolithography: A new fabrication tool for protein arrays. Mol. Cell. Proteomics 2009, 8, 1823–1831. [Google Scholar] [CrossRef]
- Fudickar, W.; Linker, T. Photoimaging with singlet oxygen at the solid-air interface. Langmuir 2009, 25, 9797–9803. [Google Scholar] [CrossRef]
- Fudickar, W.; Linker, T. Novel anthracene materials for applications in lithography and reversible photoswitching by light and air. Langmuir 2010, 26, 4421–4428. [Google Scholar] [CrossRef] [PubMed]
- Griesser, T.; Montanuniversität, Leoben, Austria. Personal communication, 2016.

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzarasa, G.; Soliveri, G. Photocatalytic Lithography. Appl. Sci. 2019, 9, 1266. https://doi.org/10.3390/app9071266
Panzarasa G, Soliveri G. Photocatalytic Lithography. Applied Sciences. 2019; 9(7):1266. https://doi.org/10.3390/app9071266
Chicago/Turabian StylePanzarasa, Guido, and Guido Soliveri. 2019. "Photocatalytic Lithography" Applied Sciences 9, no. 7: 1266. https://doi.org/10.3390/app9071266




