Thermal Fatigue Properties of Ultrasonically Bonded Copper Joints
Abstract
:1. Introduction
2. Materials and Methods
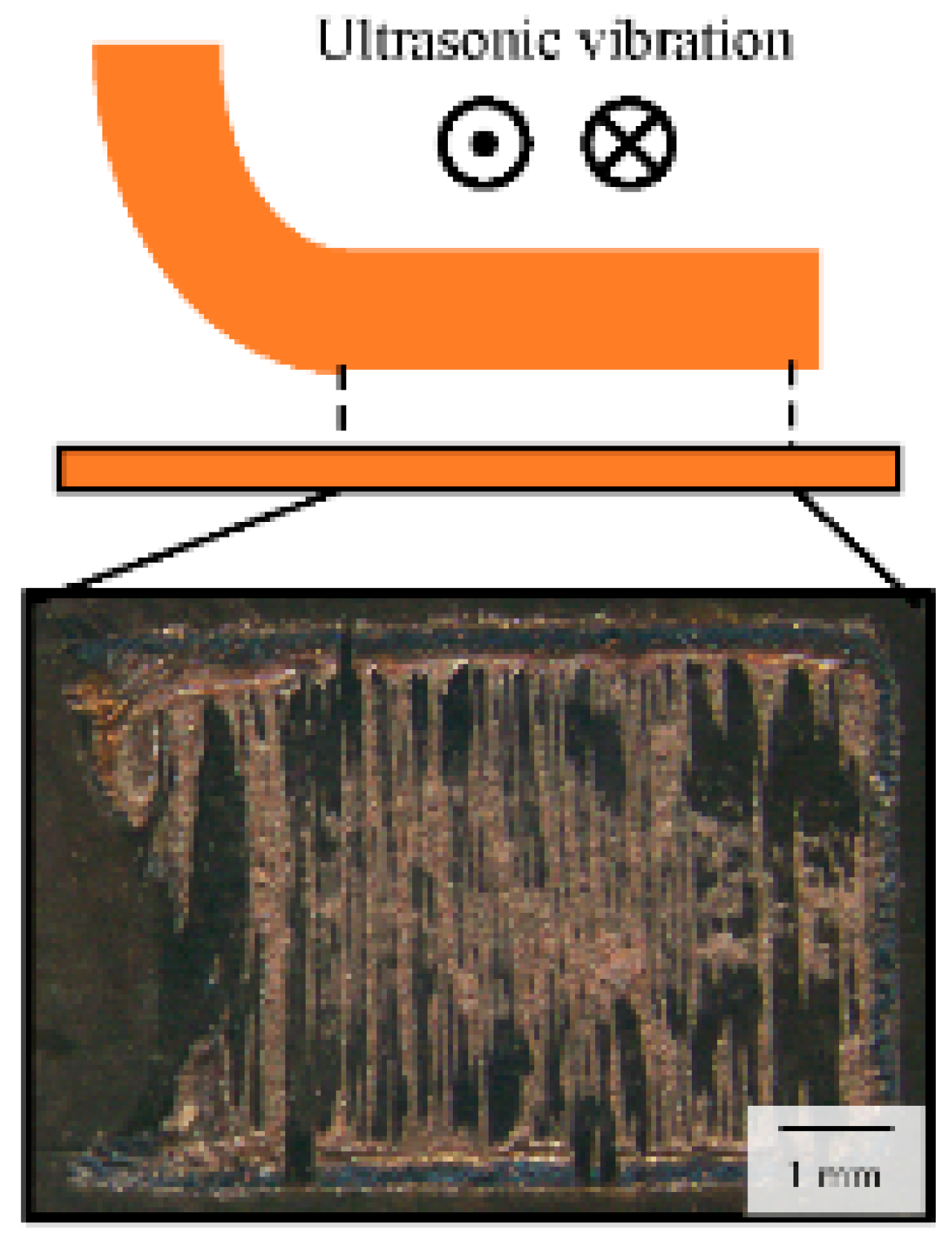
2.1. Materials and Procedure
2.2. Thermal Cycling Test
2.3. Observation of Microstructure
3. Results and Discussion
3.1. Effect of Thermal Fatigue Fracture on Mechanical Properties
3.2. Crack Propagation Behavior during Thermal Fatigue Fracture
3.3. Suppression of Thermal Fatigue Fracture in C1940 Joints
3.4. Discussion on the Suppression of Thermal Fatigue Fracture in C1940 Joints
4. Conclusions
- Microstructural observations of the C1020 joints confirmed that the bonding interface was formed by fine grains. As the grain boundary can be an obstacle to crack propagation, the cracks caused by thermal fatigue did not progress to the fine grain region and propagated by avoiding that region.
- C1940 joints with a harder substrate and finer grain size than C1020 joints resisted thermal fatigue fracture during thermal cycling tests. As the vicinity of the bonding interface was constituted by finer grains in the C1940 joint, crack propagation in the bonding interface of C1940 joints was suppressed because of the presence of more grain boundaries.
- Grain refinement was also confirmed at the terminal of the C1940 joint containing the same material as that used in the terminal of the C1020 joint. It was considered that the time for which the terminal was subjected to sliding friction increased owing to hardening of the substrate, and the grains of the terminal that were subjected to longer processing times were finer.
Author Contributions
Funding
Conflicts of Interest
References
- Kim, J.; Jung, K.-H.; Kim, J.-H.; Lee, C.-J.; Jung, S.-B. Electromigration behaviors of Sn58%Bi solder containing Ag-coated MWCNTs with OSP surface finished PCB. J. Alloys Compd. 2019, 775, 581–588. [Google Scholar] [CrossRef]
- Chan, Y.C.; Yang, D. Failure mechanisms of solder interconnects under current stressing in advanced electronic packages. Prog. Mater. Sci. 2010, 55, 428–475. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, Y.-C.; Lee, S.-M.; Jung, S.-B. Effect of surface finishes on electromigration reliability in eutectic Sn–58Bi solder joints. Microelectron. Eng. 2014, 120, 77–84. [Google Scholar] [CrossRef]
- Bashir, M.N.; Haseeb, A.S.; Rahman, A.Z.; Fazal, M.A.; Kao, C.R. Reduction of electromigration damage in SAC305 solder joints by adding Ni nanoparticles through flux doping. J. Mater. Sci. 2015, 50, 6748–6756. [Google Scholar] [CrossRef]
- Lu, Y.-D.; He, X.-Q.; En, Y.-F.; Wang, X.; Zhuang, Z.-Q. Polarity effect of electromigration on intermetallic compound formation in SnPb solder joints. Acta Mater. 2009, 57, 2560–2566. [Google Scholar] [CrossRef]
- Tang, H.; Tang, Y.; Wan, Z.; Li, J.; Yuan, W.; Lu, L.; Li, Y.; Tang, K. Review of applications and developments of ultra-thin micro heat pipes for electronic cooling. Appl. Energy 2018, 223, 383–400. [Google Scholar] [CrossRef]
- Tanaka, Y.; Fujiwara, S.; Ogura, T.; Sano, T.; Hirose, A. Interfacial microstructure evolution and thermal reliability of copper/nickel joints formed by ultrasonic bonding. Q. J. Jpn. Weld. Soc. 2013, 31, 192–196. [Google Scholar] [CrossRef]
- Maeda, M.; Sato, T.; Inoue, N.; Yagi, D.; Takahashi, Y. Anomalous microstructure formed at the interface between copper ribbon and tin-deposited copper plate by ultrasonic bonding. Microelectron. Reliab. 2011, 51, 130–136. [Google Scholar] [CrossRef]
- Yamanaka, K.; Kobayashi, K.; Hayashi, K.; Fukui, M. Advanced Surface Laminar Circuit Packaging with low Coefficient of Thermal Expansion and high wiring density. In Proceedings of the 2009 59th Electronic Components and Technology Conference, San Diego, CA, USA, 26–29 May 2009; pp. 325–332. [Google Scholar]
- Arjmand, E.; Agyakwa, P.A.; Corfield, M.R.; Li, J.; Mouawad, B.; Johnson, C.M. A thermal cycling reliability study of ultrasonically bonded copper wires. Microelectron. Reliab. 2016, 59, 126–133. [Google Scholar] [CrossRef]
- Myung, W.-R.; Kim, K.-Y.; Kim, Y.; Jung, S.-B. The reliability of ultrasonic bonded Cu to Cu electrode for 3D TSV stacking. J. Mater. Sci. Mater. Electron. 2017, 28, 16467–16475. [Google Scholar] [CrossRef]
- Tanimoto, S.; Tanisawa, H.; Watanabe, K.; Matsui, K.; Sato, S. Power Module Package Structure Capable of Surviving Greater ΔTj Thermal Cycles. Mater. Sci. Forum 2013, 740–742, 1040–1043. [Google Scholar] [CrossRef]
- Lu, W.; Chakravarthula, S.S.; Chen, J.; Qiao, Y. Propagation of a cleavage crack front across a field of persistent grain boundaries. Int. J. Solids Struct. 2012, 49, 584–589. [Google Scholar] [CrossRef]
- Kanninen, M.F.; Popelar, C.H. Advanced Fracture Mechanics; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Zhang, C.; Li, L. A Coupled Thermal-Mechanical Analysis of Ultrasonic Bonding Mechanism. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2007, 40, 196–207. [Google Scholar] [CrossRef]
- Daniels, H.P.C. Ultrasonic welding. Ultrasonics 1965, 3, 190–196. [Google Scholar] [CrossRef]
- Deng, S.Q.; Godfrey, A.; Liu, W.; Zhang, C.L. Microstructural evolution of pure copper subjected to friction sliding deformation at room temperature. Mater. Sci. Eng. A 2015, 639, 448–455. [Google Scholar] [CrossRef]








| Chemical Composition (wt %) | Tensile Strength (N/mm2) | Vickers Hardness (HV) | Linear Expansion Coefficient (10−6/K) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Fe | Zn | P | ||||
| C1020 | 99.96 min | - | - | - | 195–255 | 60 Max. | 17.7 |
| C1940 | 97.6 | 2.3 | 0.12 | 0.03 | 345–415 | 100–125 | 17.6 |
| Apparent CTE of the Substrate (10−6/K) | Average Grain Size of the Substrate (µm) | |
|---|---|---|
| C1020 substrate | 4.4 | 46.2 |
| C1940 substrate | 4.4 | 15.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fushimi, T.; Tanaka, Y.; Soda, S.; Matsuda, T.; Sano, T.; Hirose, A. Thermal Fatigue Properties of Ultrasonically Bonded Copper Joints. Appl. Sci. 2019, 9, 1556. https://doi.org/10.3390/app9081556
Fushimi T, Tanaka Y, Soda S, Matsuda T, Sano T, Hirose A. Thermal Fatigue Properties of Ultrasonically Bonded Copper Joints. Applied Sciences. 2019; 9(8):1556. https://doi.org/10.3390/app9081556
Chicago/Turabian StyleFushimi, Takahito, Yo Tanaka, Shinnosuke Soda, Tomoki Matsuda, Tomokazu Sano, and Akio Hirose. 2019. "Thermal Fatigue Properties of Ultrasonically Bonded Copper Joints" Applied Sciences 9, no. 8: 1556. https://doi.org/10.3390/app9081556
APA StyleFushimi, T., Tanaka, Y., Soda, S., Matsuda, T., Sano, T., & Hirose, A. (2019). Thermal Fatigue Properties of Ultrasonically Bonded Copper Joints. Applied Sciences, 9(8), 1556. https://doi.org/10.3390/app9081556






