Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process
Abstract
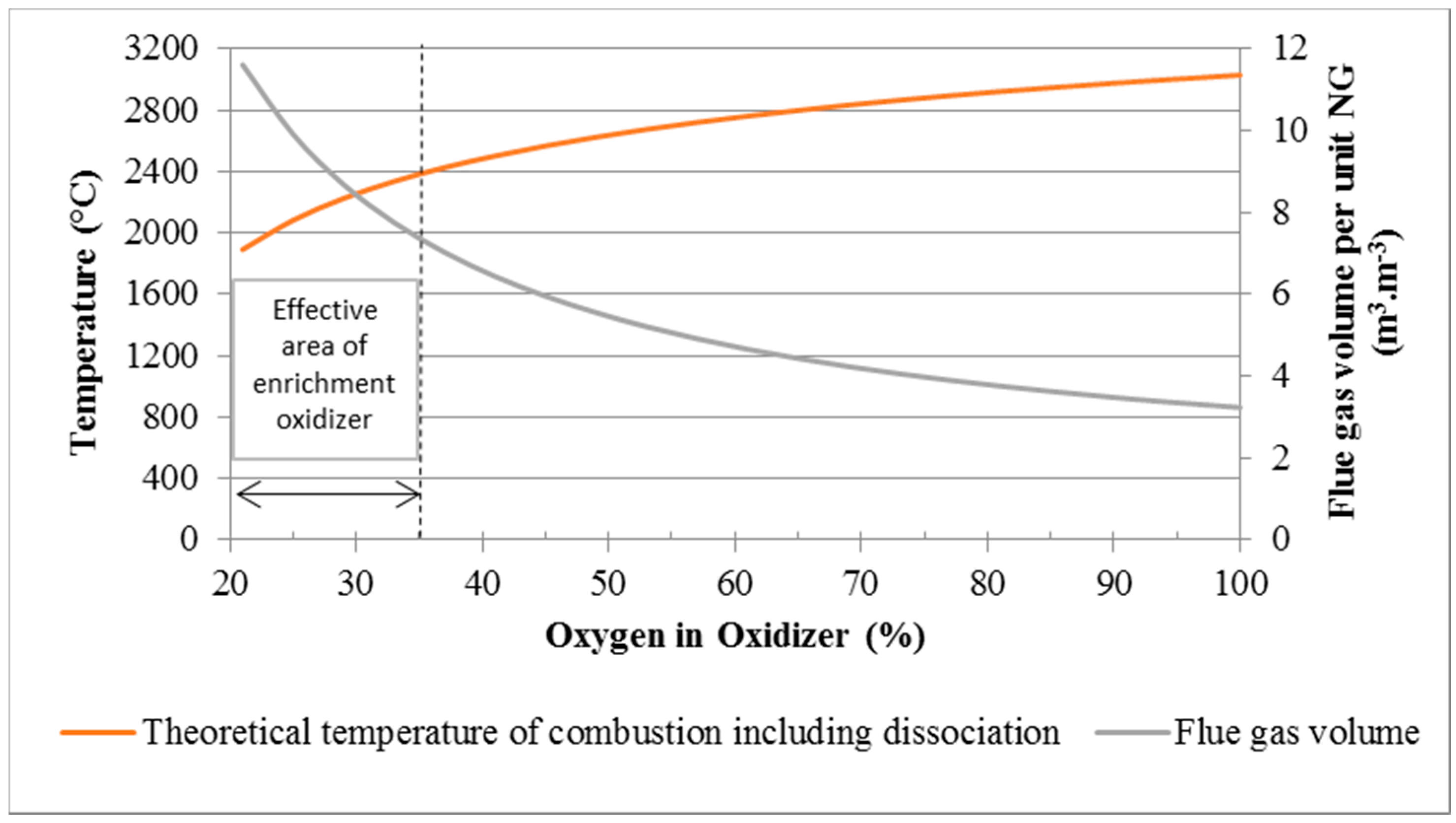
:1. Introduction
2. Experimental Research
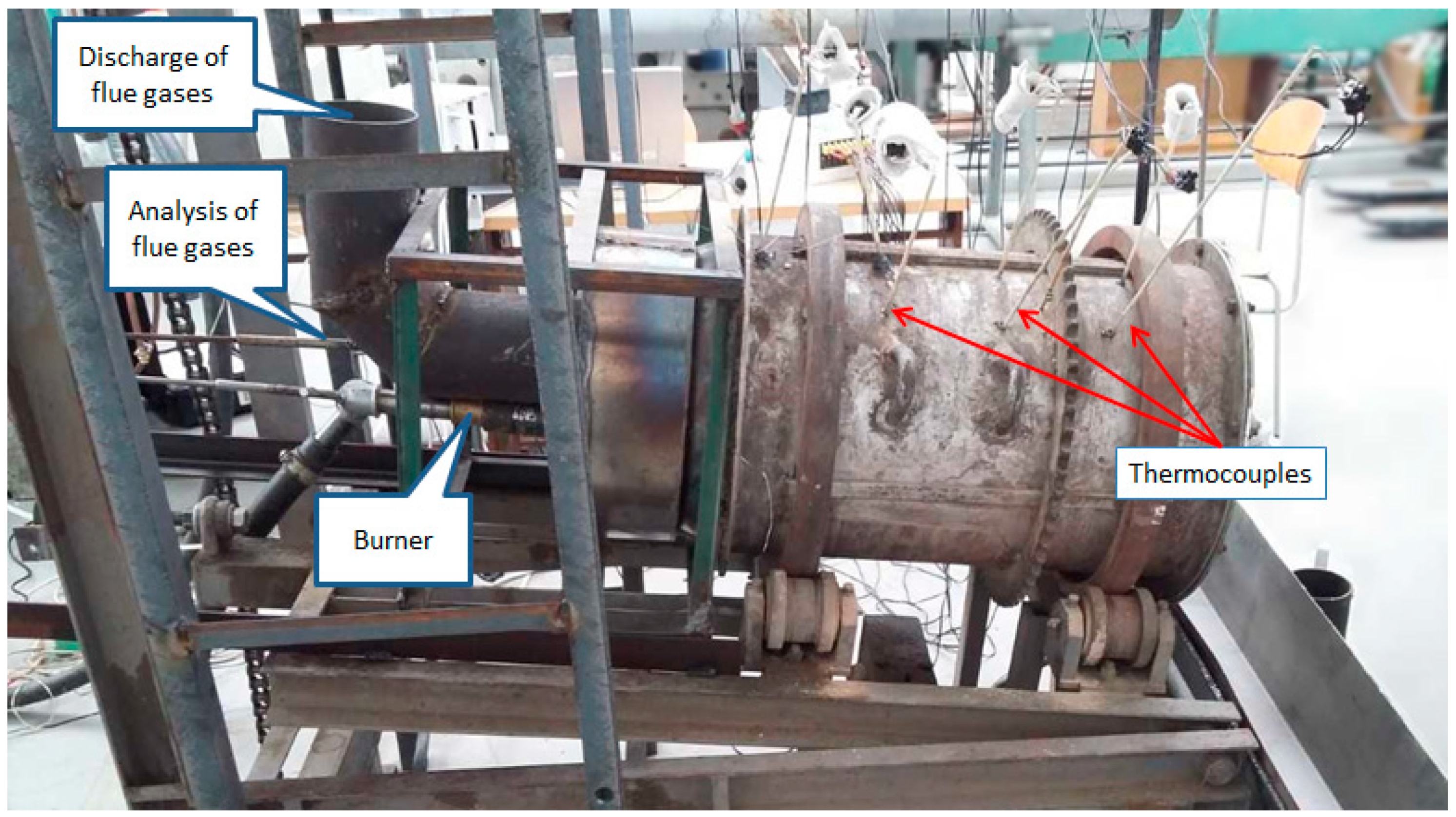
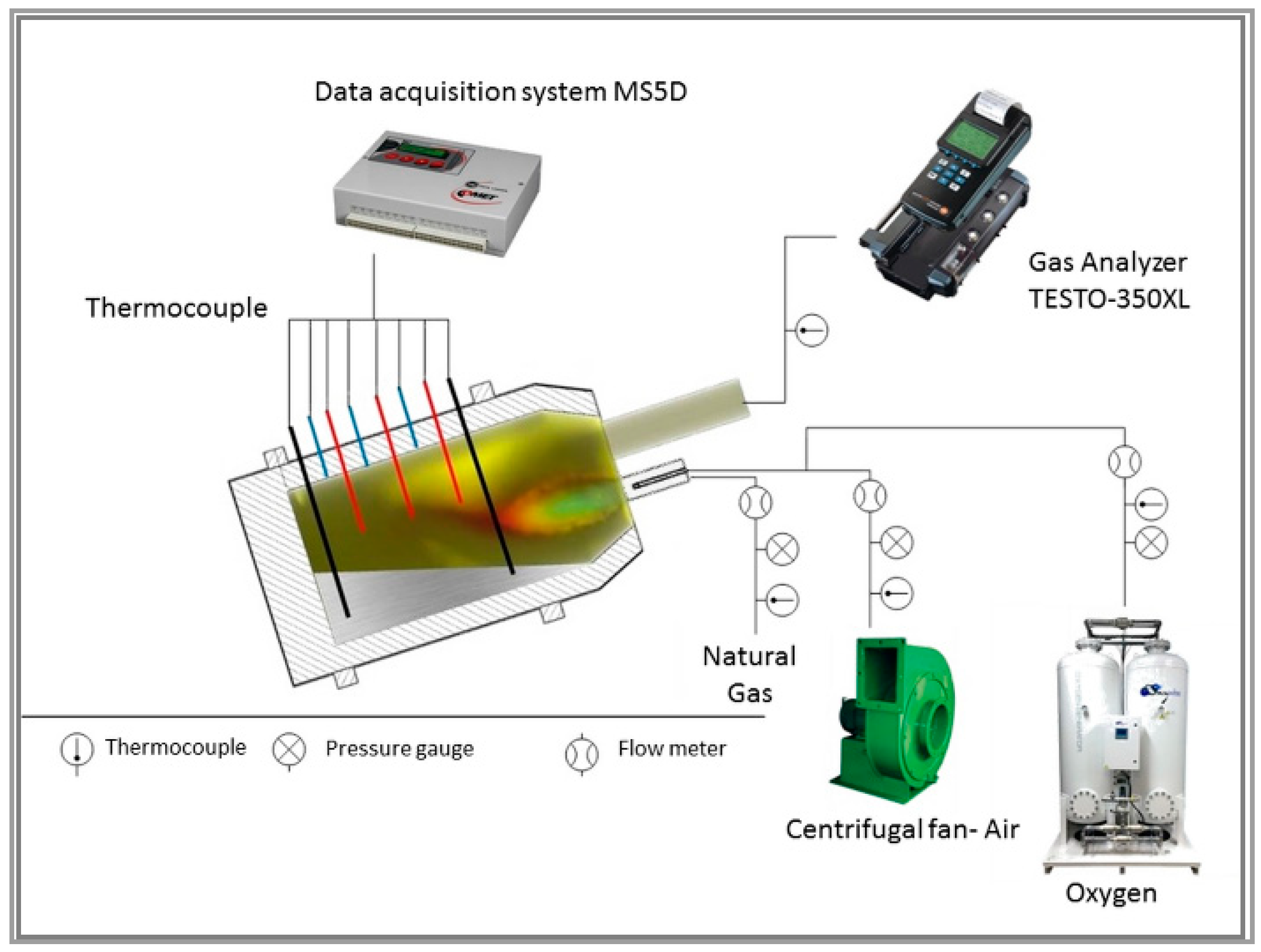
2.1. Experimental Device
2.2. Modification of Burner
2.3. CFD Modeling
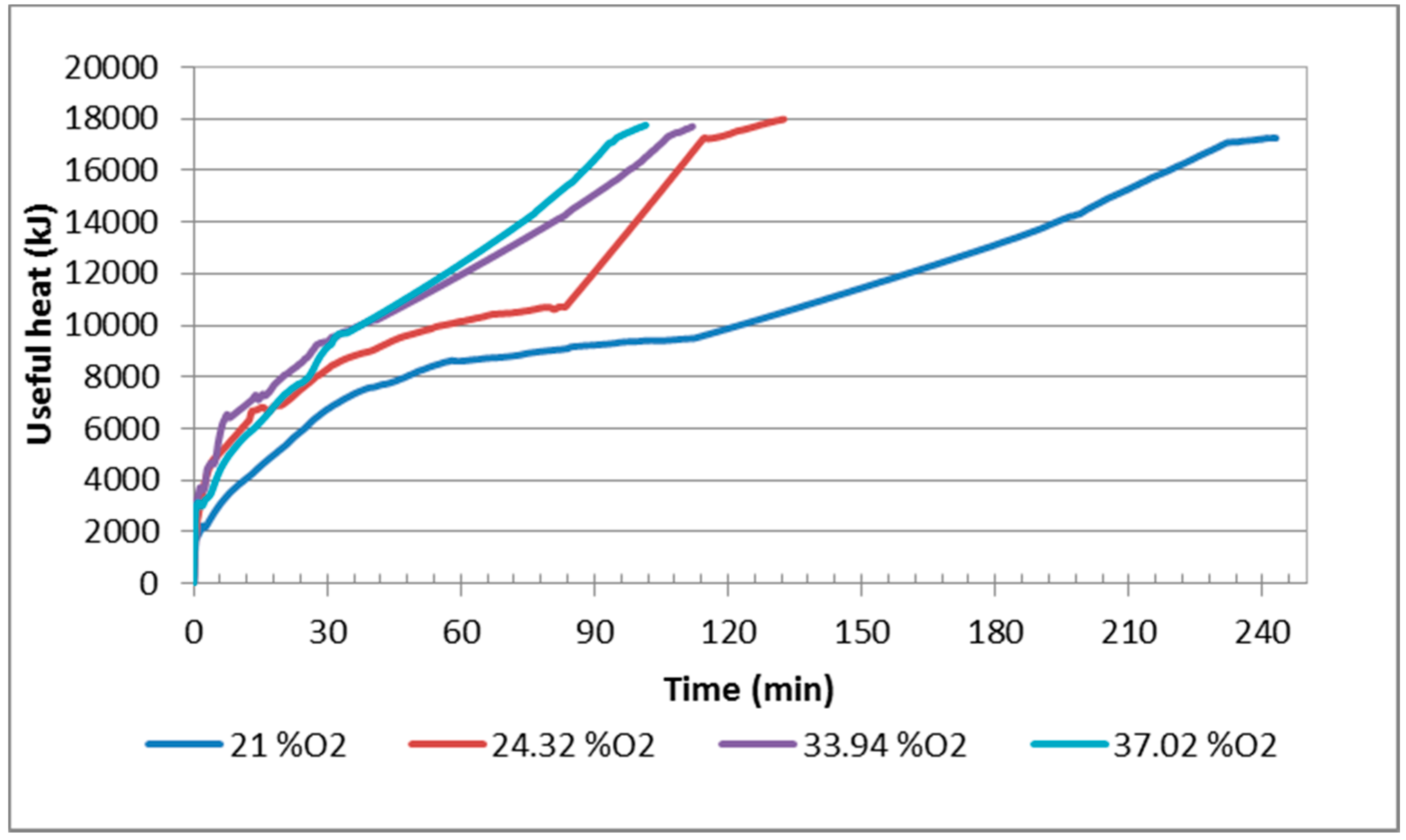
2.4. Heat Balance of Process
3. Results and Discussion
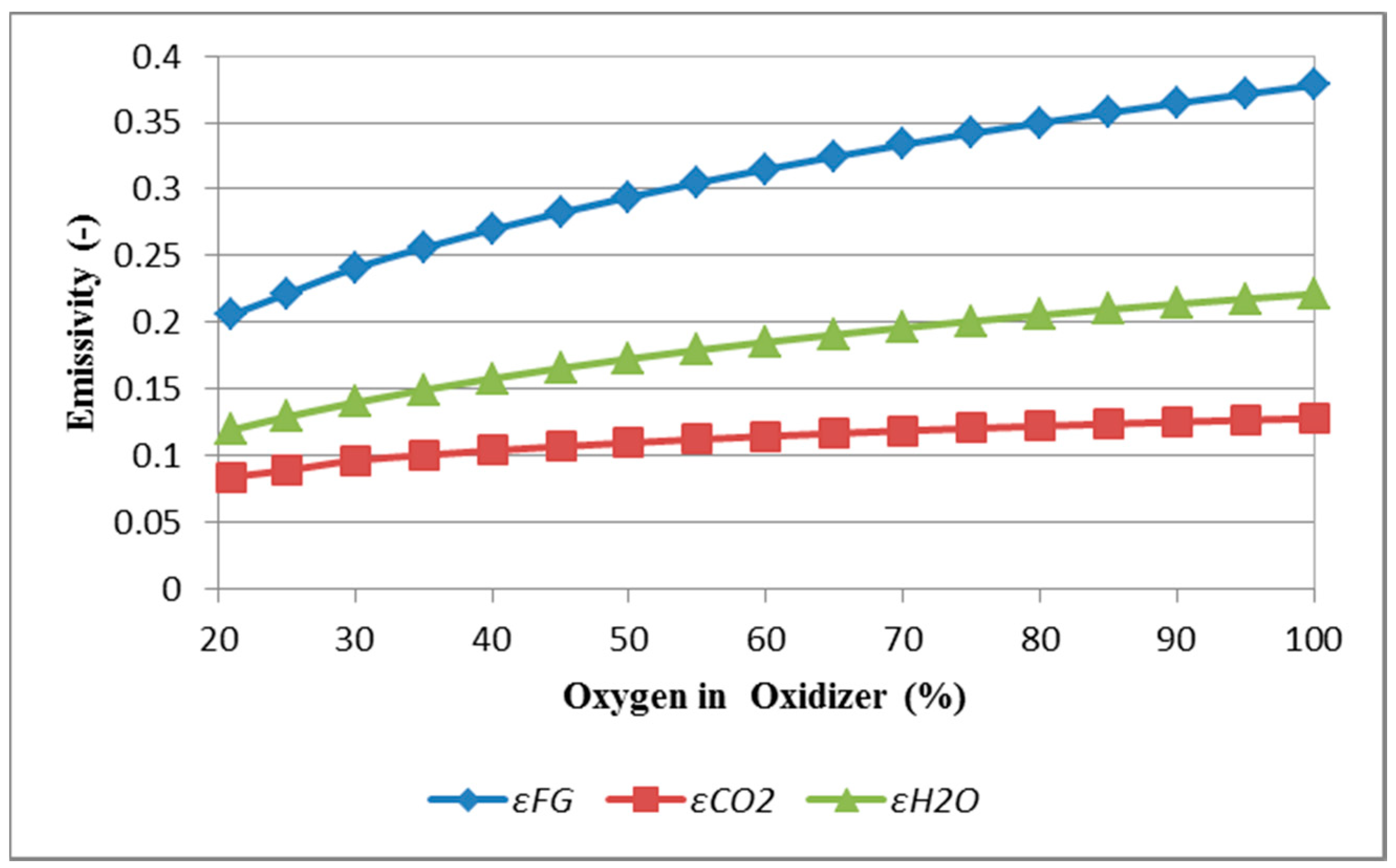
3.1. Results of CFD Modeling
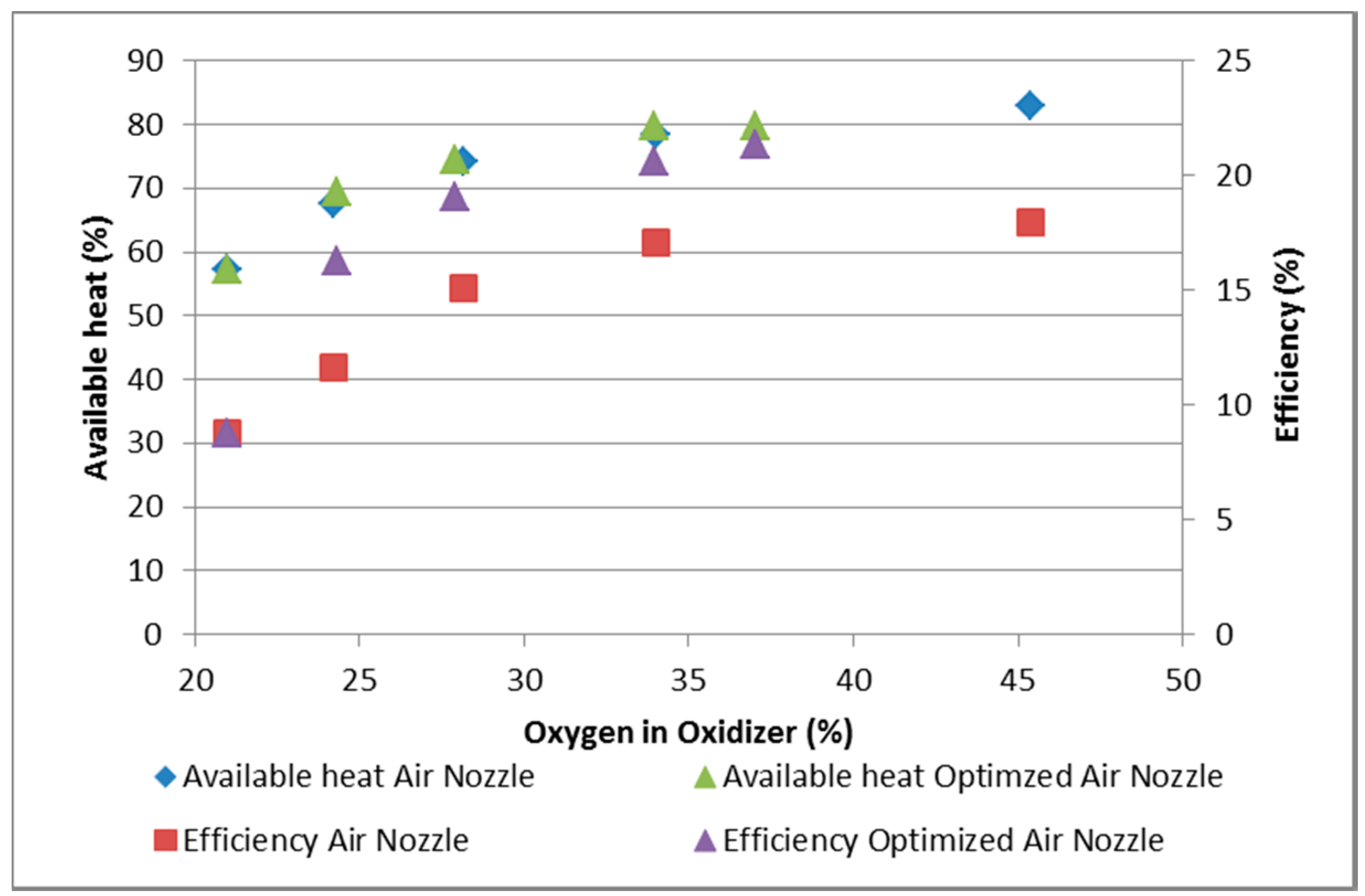
3.2. Results of Experimental Measurements
4. Conclusions
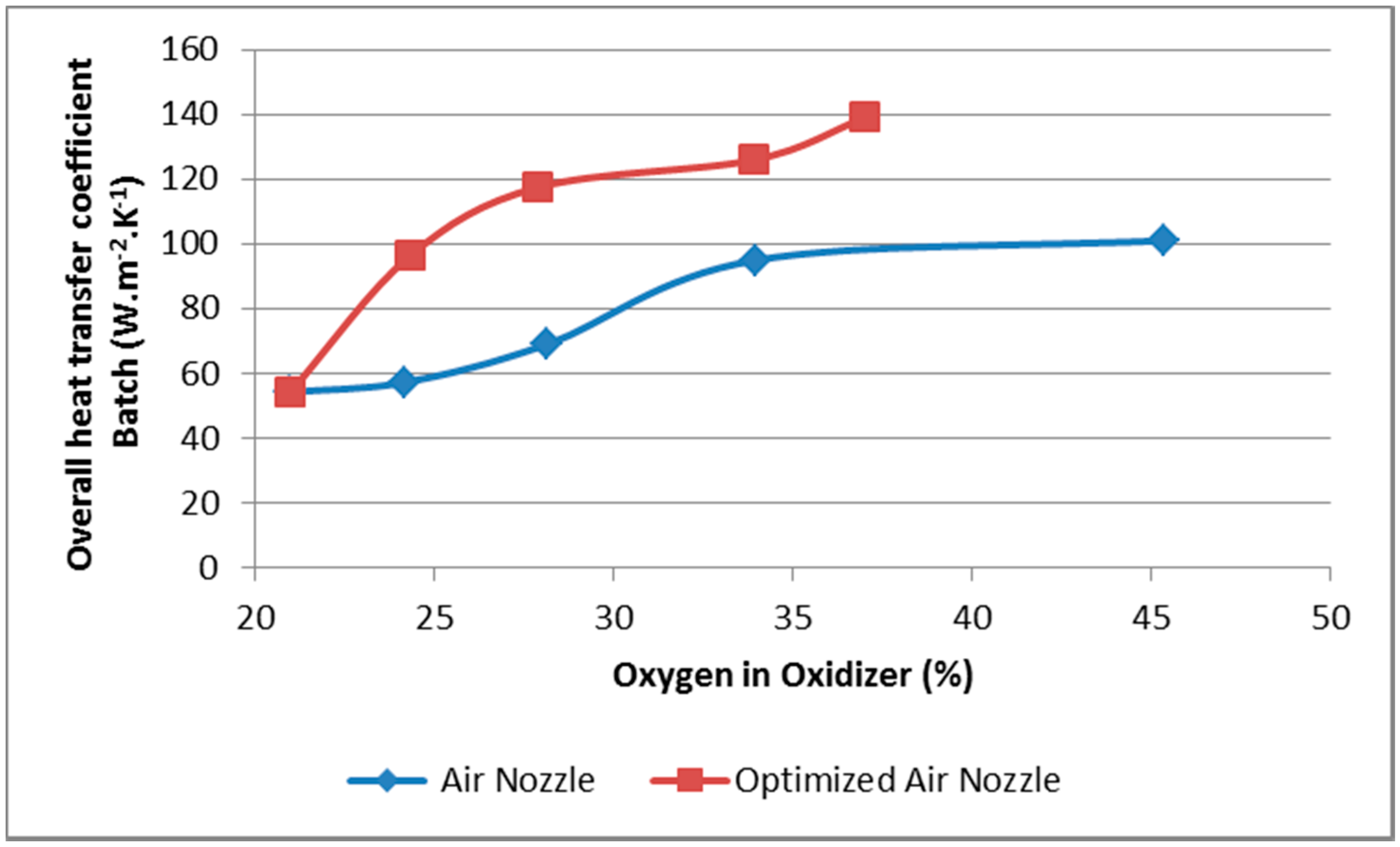
- The increase of the overall heat transfer coefficient to the charge by 32%–70%, depending on the oxygen concentration;
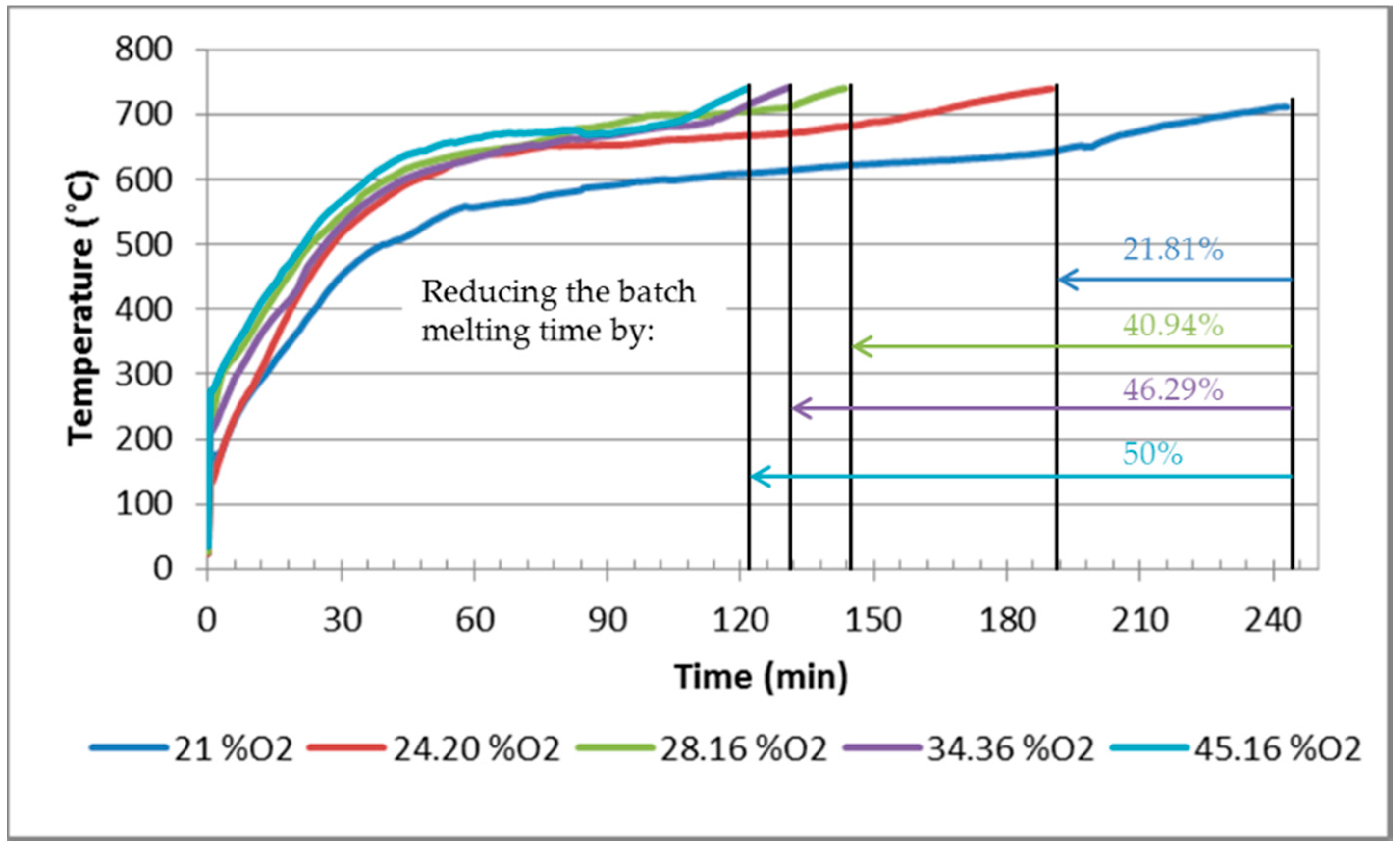
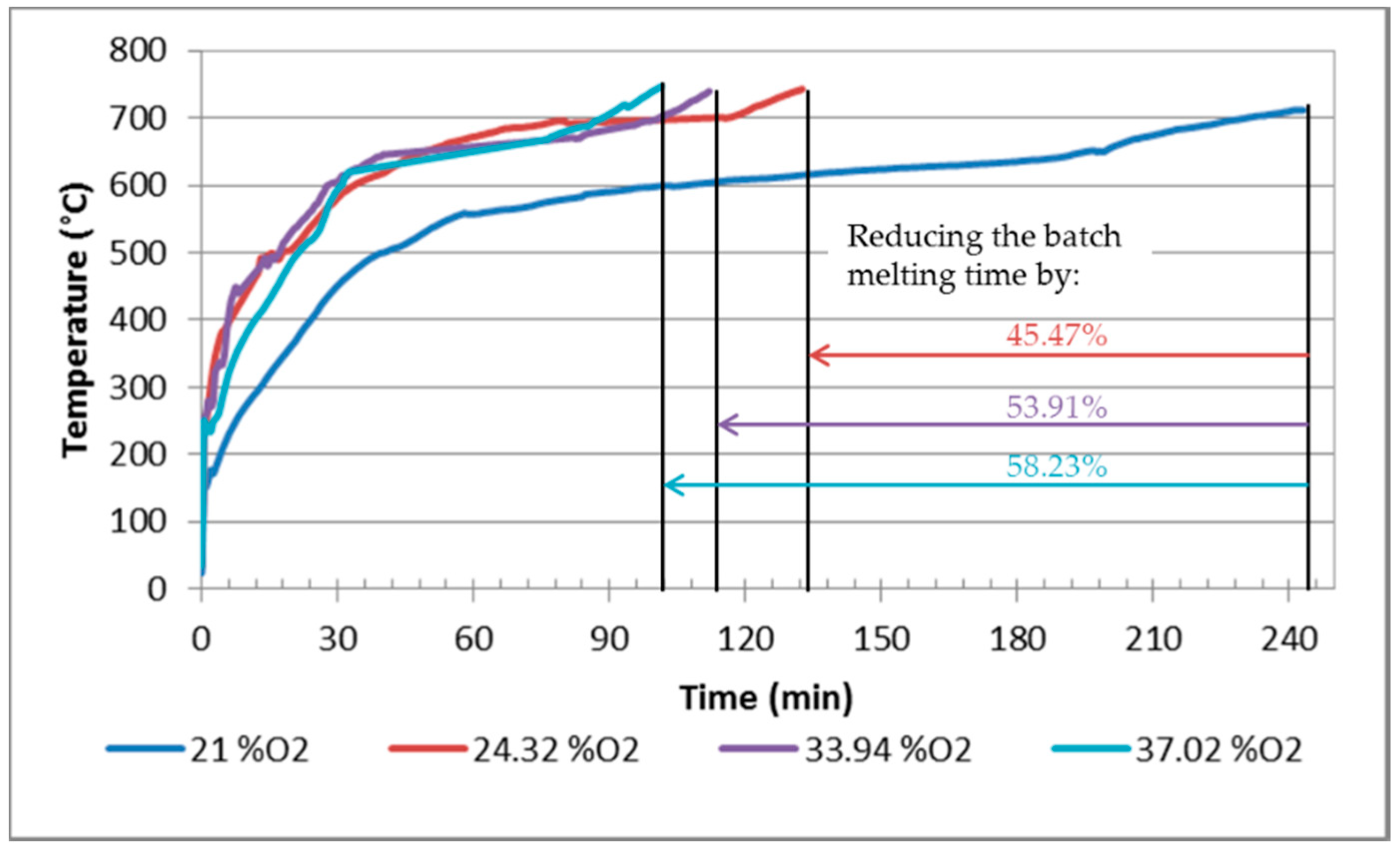
- The shortening of the melting time by 12% and 24% (at an oxygen concentration of 35% and 25%, respectively);
- The increase of the thermal efficiency of devices (by 4% in the experimental device);
- The decrease of the nominal energy consumption for melting the charge by 20%–30%, depending on the oxygen concentration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Organization for Economic Cooperation and Development (OECD). Material Case Study 2: Aluminium, in OECD Global Forum on Environment; OECD: Paris, France, 2010; Available online: https://www.oecd.org/env/waste/46194971.pdf (accessed on 10 October 2018).
- Schlesinger, M.E. Aluminium Recycling, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781138073043. [Google Scholar]
- Gripenberg, H.; Falk, O.; Olausson, R.; Niedermair, F. Controlled melting of secondary aluminum in rotary furnaces. In Proceedings of the Light Metals 2003: Technical Sessions Presented by the TMS Aluminium Committee at the 132nd TMS Annual Meeting, San Diego, CA, USA, 2–6 March 2003; Crepeau, P.N., Minerals, Metals and Materials Society, Eds.; TMS: Warrendale, PA, USA, 2003; pp. 1083–1090. [Google Scholar]
- Furu, J.; Buchholz, A.; Bergstrøm, T.H.; Marthinsen, K. Heating and melting of single Al ingots in an aluminum melting furnace. In Proceedings of the Light Metals 2010: Technical Sessions Presented by the TMS Aluminum Committee at the TMS 2010 Annual Meeting & Exhibition, Seattle, WA, USA, 14–18 February 2010; Johnson, J.A., Ed.; TMS-AIME: Warrendale, PA, USA, 2010; p. 679. [Google Scholar]
- Naranjo, R.J.; Kwon, J.; Majumdar, R.; Choate, W.T. Advanced Melting Technologies: Energy Saving Concepts and Opportunities for the Metal Casting Industry; U.S. Department of Energy: Washington, DC, USA, 2005. Available online: https://www1.eere.energy.gov/manufacturing/resources/metalcasting/pdfs/advancedmeltingtechnologies.pdf (accessed on 12 October 2018).
- Jablonský, G.; Pástor, M.; Dzurňák, R. Enriching the Combustible Mixture with Oxygen in Practice; TU of Košice: Košice, Slovakia, 2015; ISBN 978-80-5532414-2. (In Slovak) [Google Scholar]
- Gel, V.I.; Rudakov, D.N. Development of aluminium scrap melting technology. Non-Ferr. Met. 2013, 2, 35–39. [Google Scholar]
- Gripenberg, H.; Johansson, A.; Eichler, R.; Rangmark, L. Optimised oxyfuel melting process at sapa heat transfer ab. In Proceedings of the Light Metals 2007: Technical Sessions Presented by the TMS Aluminum Committee at the TMS 2007 Annual Meeting & Exhibition, Orlando, FL, USA, 25 February–1 March 2007; Sorlie, M., Minerals, Metals and Materials Society, Eds.; TMS: Warrendale, PA, USA, 2007; pp. 597–601. [Google Scholar]
- Jepson, S.; Kampen, P.V. Oxygen-enhanced combustion provides advantages in al-melting furnaces. Ind. Heat. 2005, 72, 29–35. [Google Scholar]
- Saha, D.; Baukal, C.E. Nonferous Metals. In Oxygen-Enhanced Combustion, 1st ed.; Baukal, C.E., Ed.; CRC Press: Boca Raton, FL, USA, 1998; ISBN 9780849316951. [Google Scholar]
- Gluns, L.; Schemberg, S. Advantages of oxyfuel burner systems for aluminium recycling. Alum. World 2002, 2, 107–110. [Google Scholar]
- Poskart, A.; Radomiak, H.; Niegodajew, P.; Zajemska, M.; Musiał, D. The Analysis of Nitrogen Oxides Formation During Oxygen—Enriched Combustion of Natural Gas. Arch. Metall. Mater. 2016, 61, 1925–1930. [Google Scholar] [CrossRef]
- Wu, K.K.; Chang, Y.C.; Chen, C.H.; Chen, Y.D. High-efficiency combustion of natural gas with 21–30% oxygen-enriched air. Fuel 2010, 89, 2455–2462. [Google Scholar] [CrossRef]
- Baukal, C.E., Jr. Industrial Burners Handbook, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203488805. [Google Scholar]
- Baukal, C.E. Heat Transfer in Industrial Combustion, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780849316999. [Google Scholar]
- Baukal, C.E., Jr. Oxygen-Enhanced Combustion, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439862285. [Google Scholar]
- Redr, M.; Prihoda, M. Fundamental of Thermal Technology, 1st ed.; SNTL: Praha, Czech Republic, 1991; ISBN 80-03-00366-0. (In Czech) [Google Scholar]
- ANSYS® Academic Research Mechanical, Release 18.1, Help System. Coupled Field Analysis Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2018. [Google Scholar]
- Andersen, J.; Rasmussen, C.L.; Giselsson, T.; Glarborg, P. Global combustion mechanisms for use in CFD modeling under oxy-fuel conditions. Energy Fuel 2009, 23, 1379–1389. [Google Scholar] [CrossRef]
- Bibrzycki, J.; Poinsot, T.; Zajdel, A. Investigation of Laminar Flame Speed of CH4/N2/O2 Mixtures Using Reduced Chemical Kinetic Mechanisms. Arch. Combust. 2010, 30, 287–296. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999; ISBN 0-471-25424-X. [Google Scholar]
- SPP-Distribucia. Available online: http://www.spp-distribucia.sk/sk_distribucna-siet/sk_zlozenie-zemneho-plynu-a-emisny-faktor (accessed on 15 October 2018).




| Parameter | Unit | Value | ||||
|---|---|---|---|---|---|---|
| Burner power | kW | 13.5 | ||||
| Excess of combustion air | - | 1.1 | ||||
| Oxygen enrichment | % | 21 | 25 | 30 | 35 | 40 |
| Gas flow | m3 h−1 | 1.32 | 1.32 | 1.32 | 1.32 | 1.32 |
| Air flow | m3 h−1 | 14.33 | 11.42 | 8.89 | 7.07 | 5.71 |
| Oxygen Flow | m3 h−1 | - | 0.61 | 1.14 | 1.52 | 1.8 |
| Diameter of Nozzle (D1) | m | 0.044 | 0.026 | 0.023 | 0.019 | 0.016 |
| CH4 | C2H6 | C3H8 | C4H10 | C5H12 | C6H14 | CO2 | N2 |
|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % |
| 95.171 | 2.7131 | 0.8729 | 0.2772 | 0.0486 | 0.0207 | 0.2266 | 0.6697 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzurňák, R.; Varga, A.; Kizek, J.; Jablonský, G.; Lukáč, L. Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process. Appl. Sci. 2019, 9, 1614. https://doi.org/10.3390/app9081614
Dzurňák R, Varga A, Kizek J, Jablonský G, Lukáč L. Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process. Applied Sciences. 2019; 9(8):1614. https://doi.org/10.3390/app9081614
Chicago/Turabian StyleDzurňák, Róbert, Augustín Varga, Ján Kizek, Gustáv Jablonský, and Ladislav Lukáč. 2019. "Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process" Applied Sciences 9, no. 8: 1614. https://doi.org/10.3390/app9081614
APA StyleDzurňák, R., Varga, A., Kizek, J., Jablonský, G., & Lukáč, L. (2019). Influence of Burner Nozzle Parameters Analysis on the Aluminium Melting Process. Applied Sciences, 9(8), 1614. https://doi.org/10.3390/app9081614







