Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
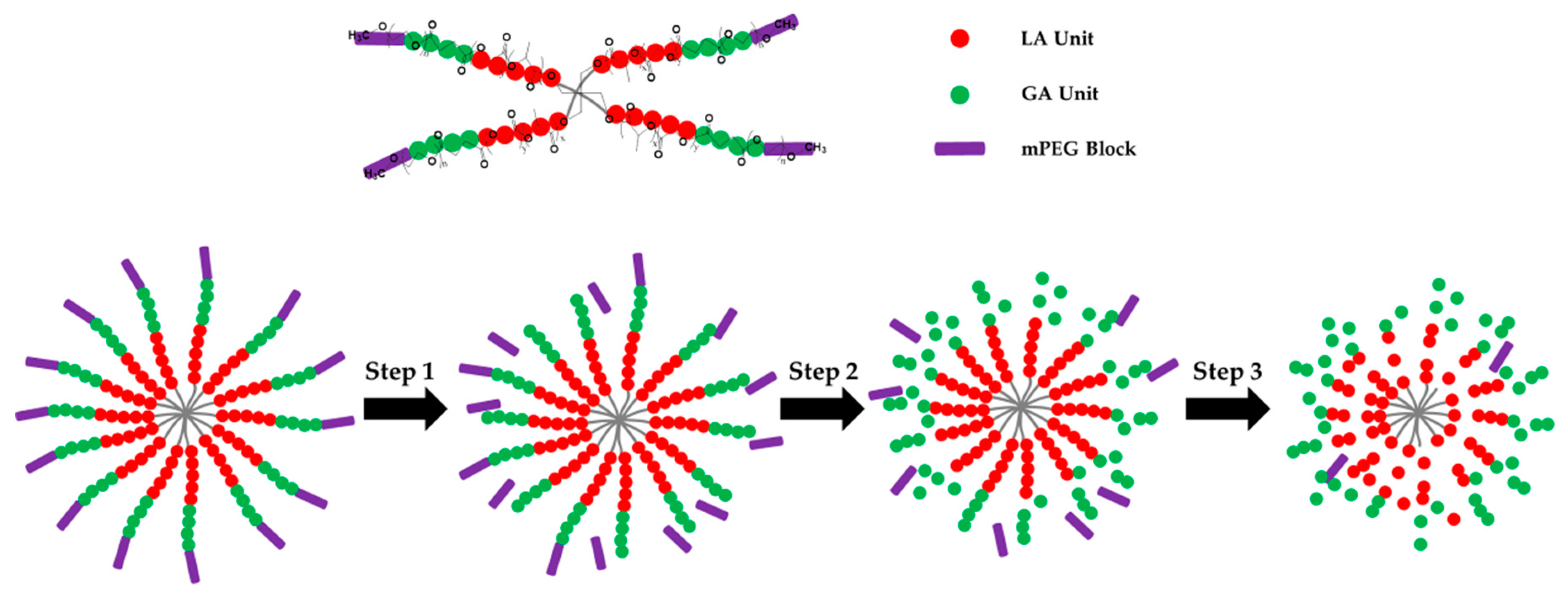
2.2. Preparation of Four-Arm Star-shaped poly(D,L-lactic-co-glycolic acid)-b-methoxy poly(ethylene glycol) (4sPLGA-mPEG) Block Copolymers
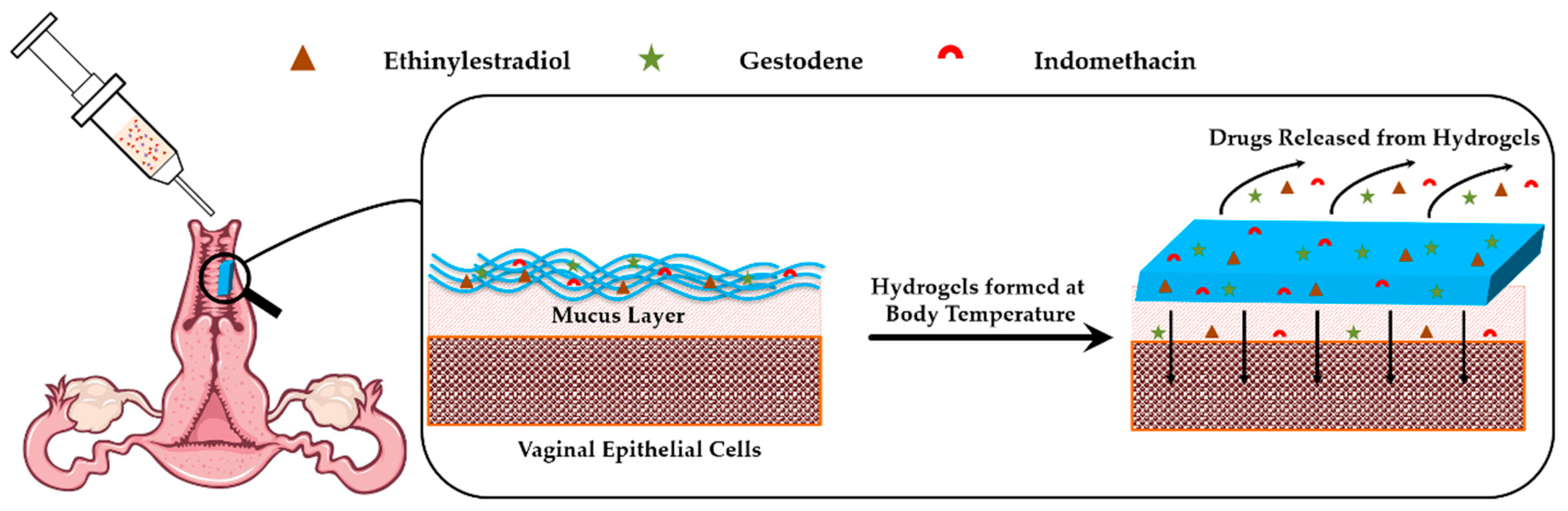
2.3. Preparation of Injectable Hydrogels
2.4. Preparation of Standard Drug Solutions
2.5. High-Performance Liquid Chromatography
2.6. Drug Release Profiles In Vitro
2.7. Animals Experiments
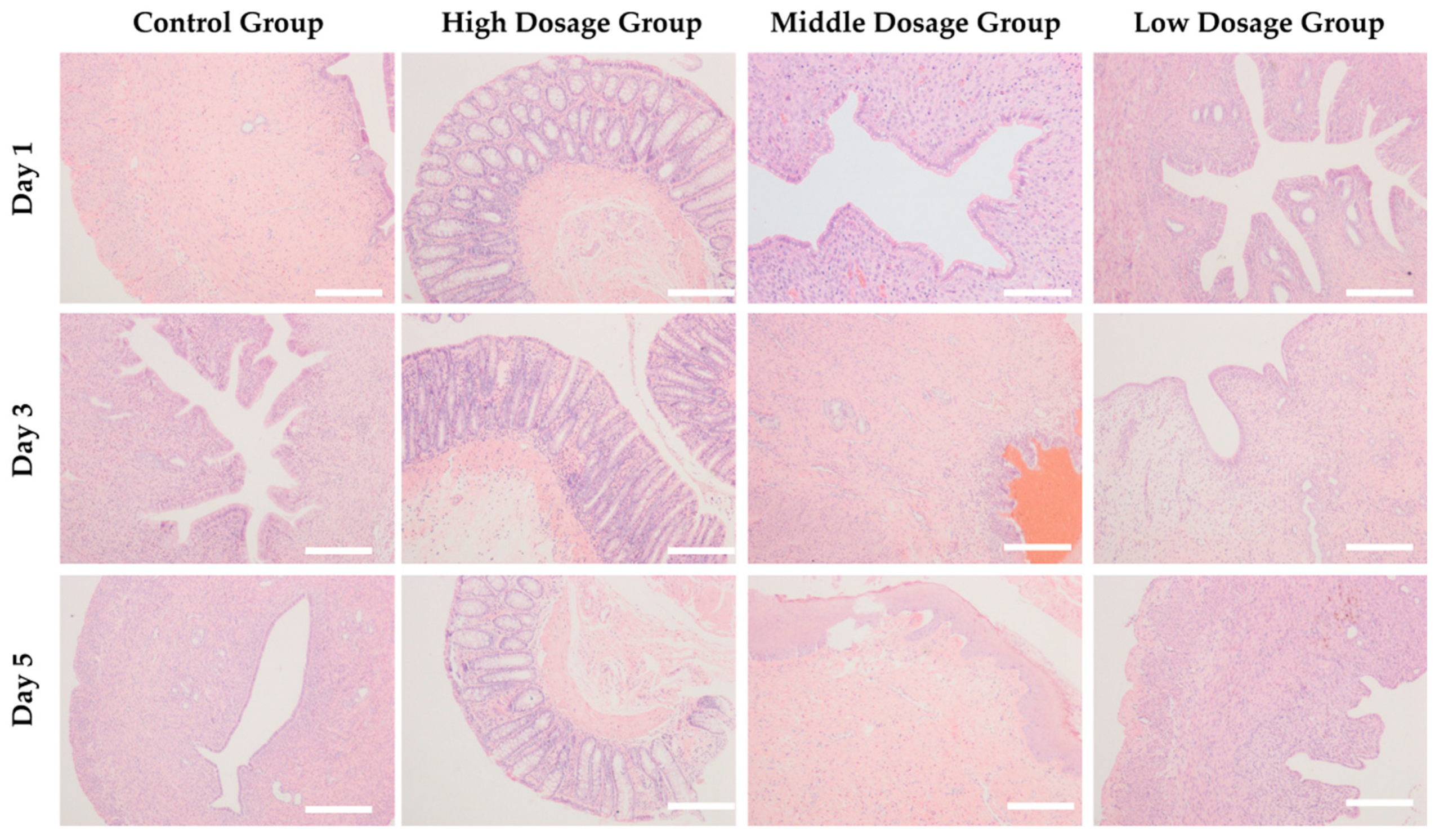
2.8. Histopathological Examinations
2.9. Determination of Estrous Cycle
2.10. Antifertility Experiment
2.11. Statistics
3. Results
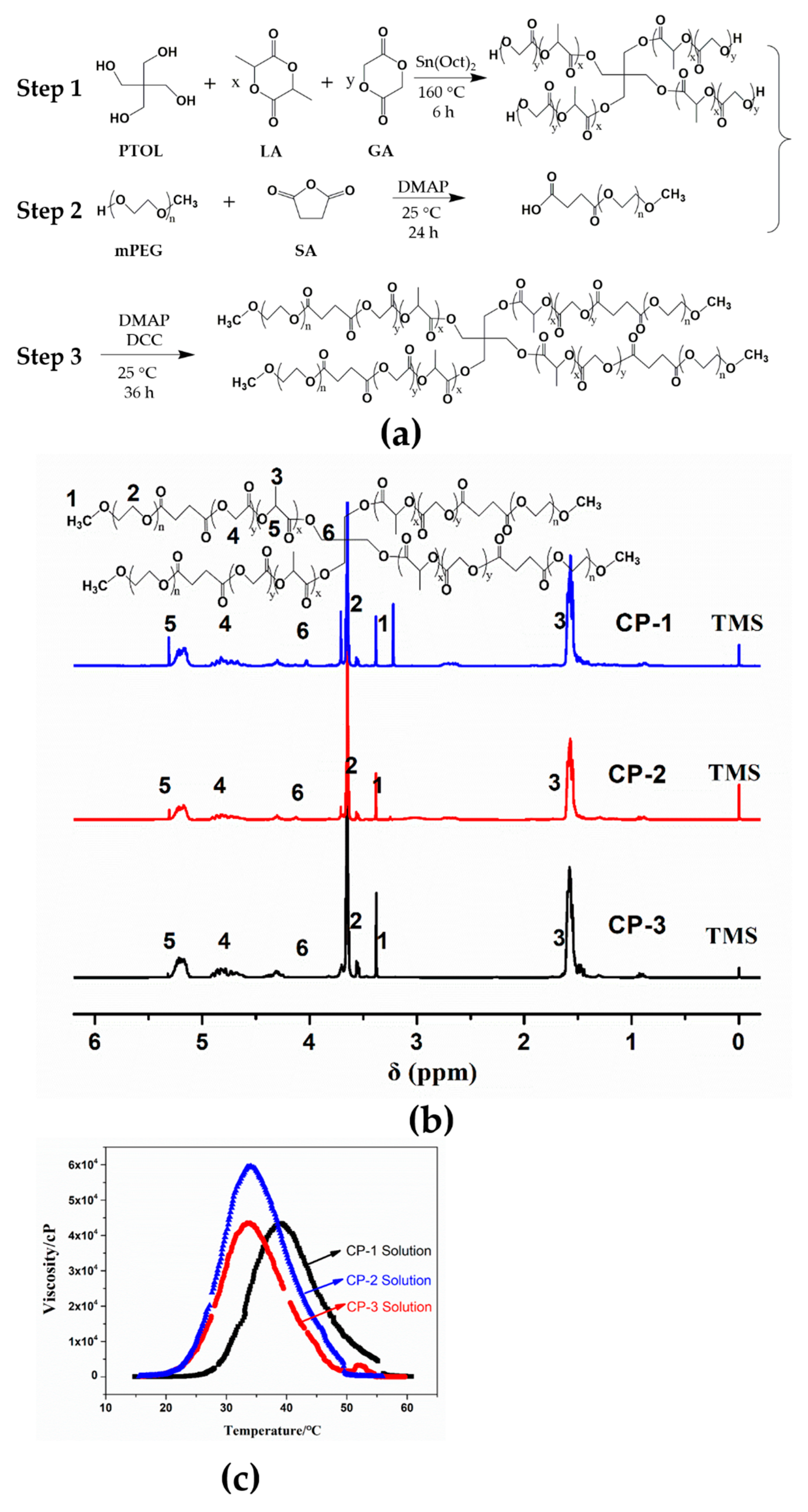
3.1. Characterization of 4sPLGA-mPEG Block Copolymers
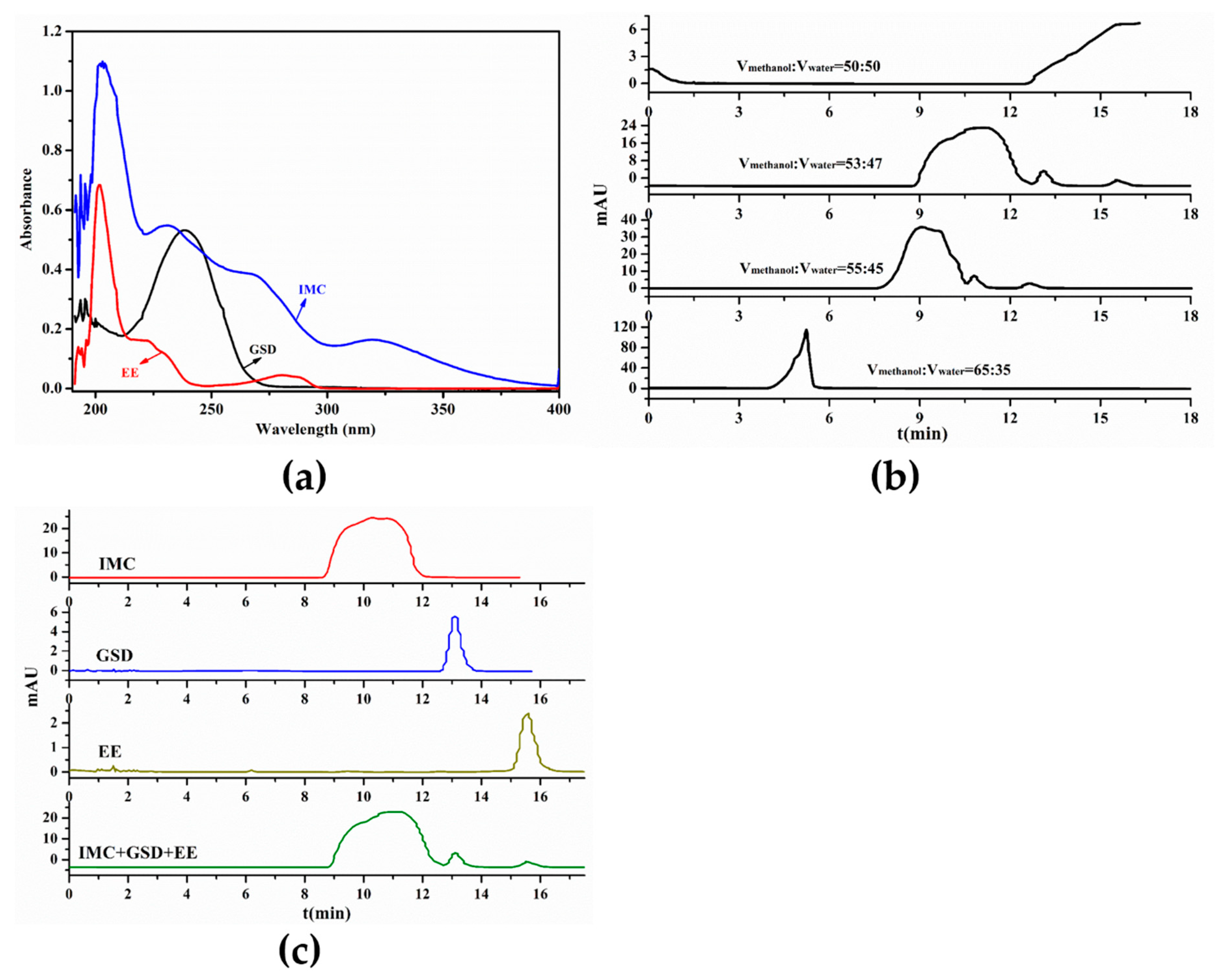
3.2. Determination of HPLC Condition
3.3. In Vitro Drug Release Profiles
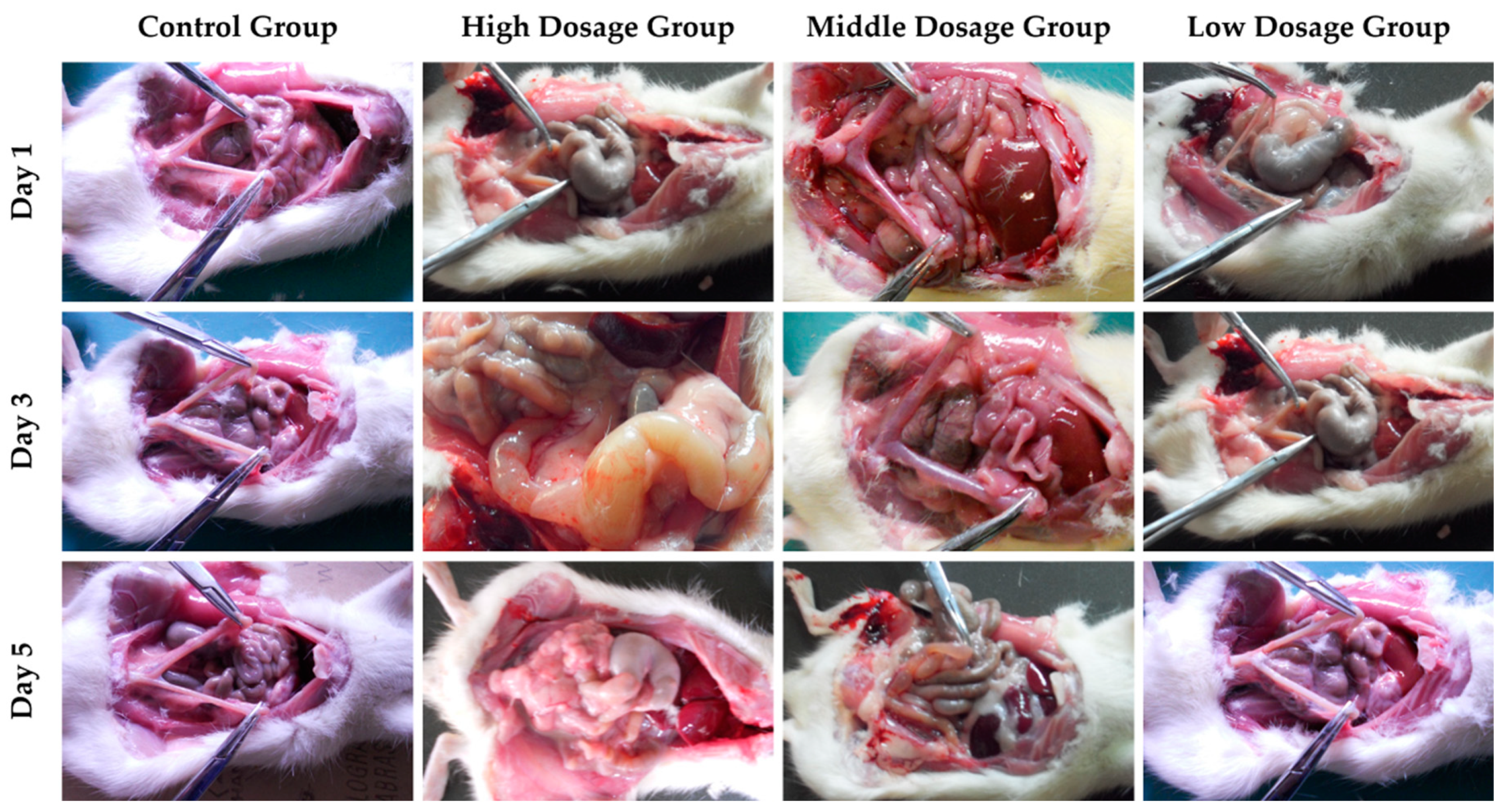
3.4. In Vivo Acute Toxicity of Copolymer Hydrogels with Loading Multi-drug
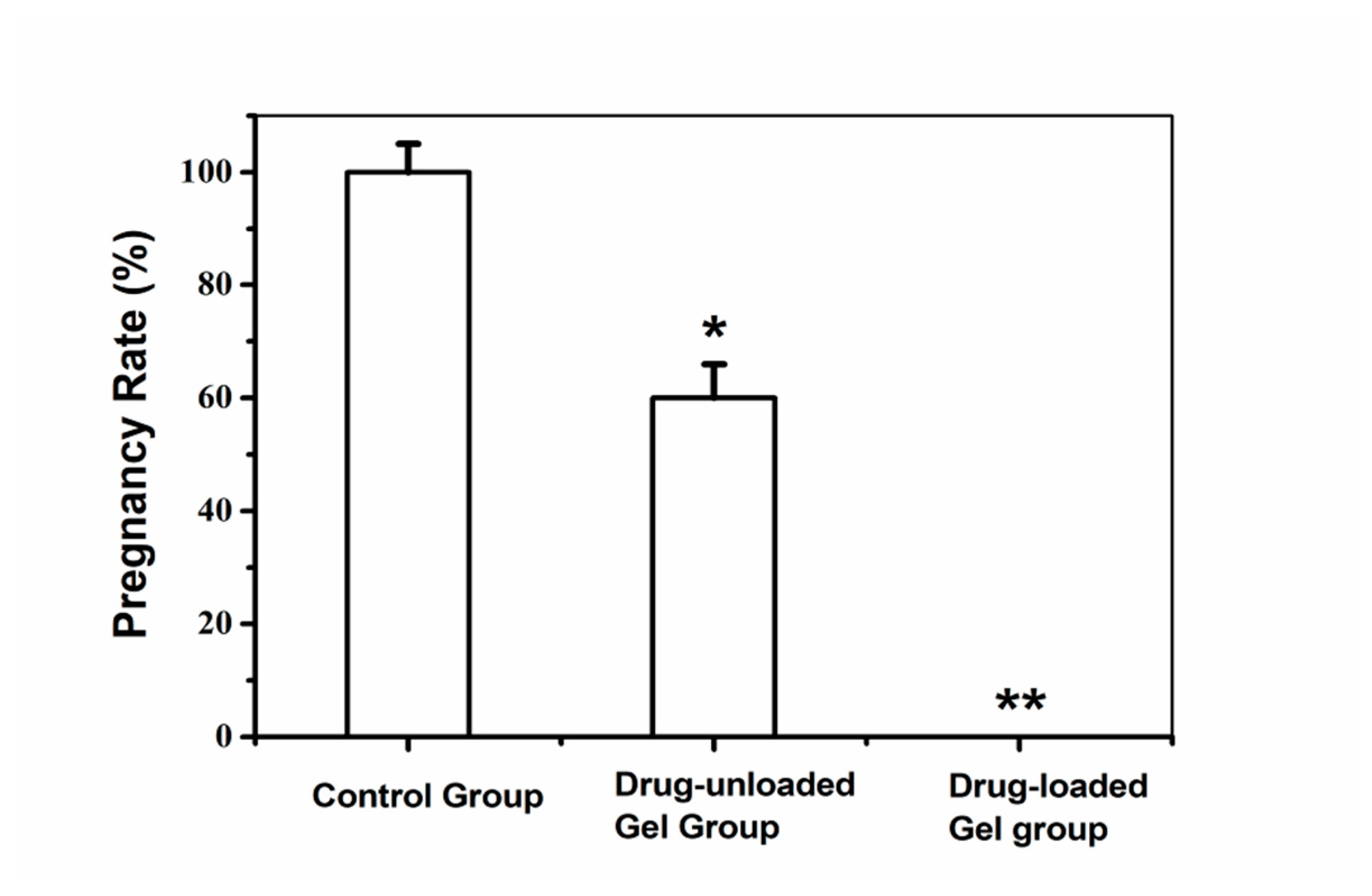
3.5. Antifertility Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cleland, J.; Conde-Agudelo, A.; Peterson, H.; Ross, J.; Tsui, A. Contraception and health. Lancet 2012, 380, 149–156. [Google Scholar] [CrossRef]
- Peipert, J.F.; Madden, T.; Allsworth, J.E.; Secura, G.M. Preventing unintended pregnancies by providing No-Cost contraception. Obstet. Gynecol. 2012, 120, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Peterson, H.B. Sterilization. Obstet. Gynecol. 2008, 111, 189–203. [Google Scholar] [CrossRef]
- Farley, T.M.M.; Rowe, P.J.; Meirik, O.; Rosenberg, M.J.; Chen, J.-H. Intrauterine devices and pelvic inflammatory disease: An international perspective. Lancet 1992, 339, 785–788. [Google Scholar] [CrossRef]
- Beining, R.M.; Dennis, L.K.; Smith, E.M.; Dokras, A. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann. Epidemiol. 2008, 18, 492–499. [Google Scholar] [CrossRef]
- Castellsagué, X.; Díaz, M.; Vaccarella, S.; de Sanjosé, S.; Muñoz, N.; Herrero, R.; Franceschi, S.; Meijer, C.J.L.M.; Bosch, F.X. Intrauterine device use, cervical infection with human papillomavirus, and risk of cervical cancer: A pooled analysis of 26 epidemiological studies. Lancet Oncol. 2011, 12, 1023–1031. [Google Scholar] [CrossRef]
- Cheng, L.; Gülmezoglu, A.M.; Piaggio, G.G.; Ezcurra, E.E.; Look, P.P.V. Interventions for emergency contraception. Cochrane Database Syst. Rev. 2008. [Google Scholar] [CrossRef]
- International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical cancer and hormonal contraceptives: Collaborative reanalysis of individual data for 16 573 women with cervical cancer and 35 509 women without cervical cancer from 24 epidemiological studies. Lancet 2007, 370, 1609–1621. [Google Scholar] [CrossRef]
- Lidegaard, Ø.; Løkkegaard, E.; Svendsen, A.L.; Agger, C. Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ 2009, 339, b2890. [Google Scholar] [CrossRef]
- Curtis, K.M.; Nanda, K.; Kapp, N. Safety of hormonal and intrauterine methods of contraception for women with HIV/AIDS: A systematic review. AIDS 2009, 23, S55. [Google Scholar] [CrossRef]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Furst, T.; Piette, M.; Lechanteur, A.; Evrard, B.; Piel, G. Mucoadhesive cellulosic derivative sponges as drug delivery system for vaginal application. Eur. J. Pharm. Biopharm. 2015, 95, 128–135. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Nanoparticle-based drug delivery to the vagina: A review. J. Control. Release 2014, 190, 500–514. [Google Scholar] [CrossRef]
- Jensen, J.T. Vaginal ring delivery of selective progesterone receptor modulators for contraception. Contraception 2013, 87, 314–318. [Google Scholar] [CrossRef]
- Han, Y.A.; Singh, M.; Saxena, B.B. Development of vaginal rings for sustained release of nonhormonal contraceptives and anti-HIV agents. Contraception 2007, 76, 132–138. [Google Scholar] [CrossRef]
- Machado, R.M.; Palmeira-De-Oliveira, A.; Martinez-De-Oliveira, J.; Palmeira-De-Oliveira, R. Vaginal films for drug delivery. J. Pharm. Sci.-US 2013, 102, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Loxley, A.; Mitchnick, M.; Okoh, O.; McConnell, J.; Goldman, L.; Morgan, C.; Clark, M.; Friend, D.R. Ethylene vinyl acetate intravaginal rings for the simultaneous delivery of the antiretroviral UC781 and contraceptive levonorgestrel. Drug Deliv. Transl. Res. 2011, 1, 247–255. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Nath, A.; Mishell, D.R. Contraception technology: Past, present and future. Contraception 2013, 87, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Amiji, M.; Sarmento, B. Mucoadhesive nanosystems for vaginal microbicide development: Friend or foe? Wires Nanomed. Nanobiotechnol. 2011, 3, 389–399. [Google Scholar] [CrossRef]
- Sigurdsson, H.H.; Kirch, J.; Lehr, C.-M. Mucus as a barrier to lipophilic drugs. Int. J. Pharm. 2013, 453, 56–64. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- das Neves, J.; Bahia, M.F. Gels as vaginal drug delivery systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef]
- Wolinsky, J.B.; Colson, Y.L.; Grinstaff, M.W. Local drug delivery strategies for cancer treatment: Gels, nanoparticles, polymeric films, rods, and wafers. J. Control. Release 2012, 159, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, J.; Nunes, R.; Machado, A.; Sarmento, B. Polymer-based nanocarriers for vaginal drug delivery. Adv. Drug Deliver. Rev. 2015, 92, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Jalalvandi, E.; Shavandi, A. In situ-forming and pH-responsive hydrogel based on chitosan for vaginal delivery of therapeutic agents. J. Mater. Sci. Mater. Med. 2018, 29, 158. [Google Scholar] [CrossRef]
- Singh, V.K.; Sagiri, S.S.; Pal, K.; Khade, S.M.; Pradhan, D.K.; Bhattacharya, M.K. Gelatin-carbohydrate phase-separated hydrogels as bioactive carriers in vaginal delivery: Preparation and physical characterizations. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Yang, J.-A.; Yeom, J.; Hwang, B.W.; Hoffman, A.S.; Hahn, S.K. In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 2014, 39, 1973–1986. [Google Scholar] [CrossRef]
- Almomen, A.; Cho, S.; Yang, C.-H.; Li, Z.; Jarboe, E.A.; Peterson, C.M.; Huh, K.M.; Janát-Amsbury, M.M. Thermosensitive progesterone hydrogel: A safe and effective new formulation for vaginal application. Pharm. Res. 2015, 32, 2266–2279. [Google Scholar] [CrossRef]
- Taurin, S.; Almomen, A.A.; Pollak, T.; Kim, S.J.; Maxwell, J.; Peterson, C.M.; Owen, S.C.; Janát-Amsbury, M.M. Thermosensitive hydrogels a versatile concept adapted to vaginal drug delivery. J. Drug Target. 2018, 26, 533–550. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol–gel reversible hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 154–162. [Google Scholar] [CrossRef]
- Gupta, K.M.; Barnes, S.R.; Tangaro, R.A.; Roberts, M.C.; Owen, D.H.; Katz, D.F.; Kiser, P.F. Temperature and pH sensitive hydrogels: An approach towards smart semen-triggered vaginal microbicidal vehicles. J. Pharm. Sci.-US 2007, 96, 670–681. [Google Scholar] [CrossRef]
- Patel, N.; Thakkar, V.; Moradiya, P.; Gandhi, T.; Gohel, M. Optimization of curcumin loaded vaginal in-situ hydrogel by box-behnken statistical design for contraception. J. Drug Deliv. Sci. Technol. 2015, 29, 55–69. [Google Scholar] [CrossRef]
- Aka-Any-Grah, A.; Bouchemal, K.; Koffi, A.; Agnely, F.; Zhang, M.; Djabourov, M.; Ponchel, G. Formulation of mucoadhesive vaginal hydrogels insensitive to dilution with vaginal fluids. Eur. J. Pharm. Biopharm. 2010, 76, 296–303. [Google Scholar] [CrossRef]
- Bouchemal, K.; Frelichowska, J.; Martin, L.; Lievin-Le Moal, V.; Le Grand, R.; Dereuddre-Bosquet, N.; Djabourov, M.; Aka-Any-Grah, A.; Koffi, A.; Ponchel, G. Note on the formulation of thermosensitive and mucoadhesive vaginal hydrogels containing the miniCD4 M48U1 as anti-HIV-1 microbicide. Int. J. Pharm. 2013, 454, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, F.; Feng, L.; Yang, L.; Chen, L.; Wei, G.; Lu, W. In vivo retention of poloxamer-based in situ hydrogels for vaginal application in mouse and rat models. Acta Pharm. Sin. B 2017, 7, 502–509. [Google Scholar] [CrossRef]
- Ravani, L.; Esposito, E.; Bories, C.; Moal, V.L.-L.; Loiseau, P.M.; Djabourov, M.; Cortesi, R.; Bouchemal, K. Clotrimazole-loaded nanostructured lipid carrier hydrogels: Thermal analysis and in vitro studies. Int. J. Pharm. 2013, 454, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, F.; Feng, L.; Yu, L.; Ding, J. An Injectable Thermogel containing levonorgestrel for long-acting contraception and fertility control of animals. J. Biomed. Nanotechnol. 2017, 13, 1357–1468. [Google Scholar] [CrossRef]
- Janagam, D.R.; Wang, L.; Ananthula, S.; Johnson, J.R.; Lowe, T.L. An accelerated release study to evaluate long-acting contraceptive levonorgestrel-containing in situ forming depot systems. Pharmaceutics 2016, 8, 28. [Google Scholar] [CrossRef]
- Zou, P.; Suo, J.; Nie, L.; Feng, S. Temperature-sensitive biodegradable mixed star-shaped block copolymers hydrogels for an injection application. Polymer 2012, 53, 1245–1257. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Huo, H.; Ji, C.; Sun, M.; Nie, L. Injectable temperature-sensitive hydrogel with VEGF loaded microspheres for vascularization and bone regeneration of femoral head necrosis. Mater. Lett. 2018, 229, 138–141. [Google Scholar] [CrossRef]
- Nie, L.; Chang, P.; Sun, M.; Huo, H.; Zhang, C.; Ji, C.; Wei, X.; Zhou, Q.; Guo, P.; Yuan, H. Composite hydrogels with the simultaneous release of VEGF and MCP-1 for enhancing angiogenesis for bone tissue engineering applications. Appl. Sci. 2018, 8, 2438. [Google Scholar] [CrossRef]
- Merz, M.; Kroll, R.; Lynen, R.; Bangerter, K. Bleeding pattern and cycle control of a low-dose transdermal contraceptive patch compared with a combined oral contraceptive: A randomized study. Contraception 2015, 91, 113–120. [Google Scholar] [CrossRef]
- Nie, L.; Zou, P.; Feng, S.; Suo, J. Temperature-sensitive star-shaped block copolymers hydrogels for an injection application: Phase transition behavior and biocompatibility. J. Mater. Sci. Mater. Med. 2013, 24, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Borges, S.; Silva, J.; Teixeira, P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. Antonie Leeuwenhoek 2012, 101, 677–682. [Google Scholar] [CrossRef]
- Zou, P.; Suo, J.; Nie, L.; Feng, S. Temperature-responsive biodegradable star-shaped block copolymers for vaginal gels. J. Mater. Chem. 2012, 22, 6316–6326. [Google Scholar] [CrossRef]
- Lee, K.-P.; Lee, J.-H.; Kim, T.-S.; Kim, T.-H.; Park, H.-D.; Byun, J.-S.; Kim, M.-C.; Jeong, W.-I.; Calvisi, D.F.; Kim, J.-M.; et al. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 8248–8253. [Google Scholar] [CrossRef]
- Millas, I.; Liquidato, B.M.; de Sousa Buck, H.; Barros, M.D.; Paes, R.A.P.; Dolci, J.E.L. Evaluation of estrogenic receptors in the nasal mucosa of women taking oral contraceptives. Contraception 2011, 83, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes, M.V.; Castro, S.; Marchi, N.M.; Marcovici, M.; Andrade, L.A.L.A.; Fernandes, A.; Bahamondes, L. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception 2014, 90, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Seon, C.S.; Park, Y.S.; Park, S.H.; Ryu, S.R.; Jo, Y.J.; Kim, S.H.; Son, B.K.; Ahn, S.B. A Case of Oral-contraceptive Related Ischemic Colitis in Young Woman. Clin. Endosc. 2011, 44, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Rameshbabu, A.P.; Ghosh, K.; Jha, P.K.; Jha, R.; Murugesan, S.; Chattopadhyay, S.; Dhara, S.; Mondal, K.C.; Basak, P.; et al. Impact of styrene maleic anhydride (SMA) based hydrogel on rat fallopian tube as contraceptive implant with selective antimicrobial property. Mater. Sci. Eng. C 2019, 94, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Turok, D.K.; Gawron, L.M.; Lawson, S. New developments in long-acting reversible contraception: The promise of intrauterine devices and implants to improve family planning services. Fertil. Steril. 2016, 106, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Vanić, Ž.; Škalko-Basnet, N. Nanopharmaceuticals for improved topical vaginal therapy: Can they deliver? Eur. J. Pharm. Biopharm. 2013, 50, 29–41. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Hasan Turabee, M.; Thambi, T.; Trang Duong, H.T.; Hoon Jeong, J.; Sung Lee, D. A pH- and temperature-responsive bioresorbable injectable hydrogel based on polypeptide block copolymers for the sustained delivery of proteins in vivo. Biomater. Sci.-UK 2018, 6, 661–671. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.; Wu, C. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef]
- Zou, P.; Nie, L.; Feng, S.; Suo, J. Synthesis, micellization and gelation of temperature-responsive star-shaped block copolymers. Polym. Adv. Technol. 2013, 24, 460–465. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R. Vaginal delivery of contraceptives. Expert Opin. Drug Deliv. 2005, 2, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Tang, B.C.; Wang, Y.-Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4, 138ra79. [Google Scholar] [CrossRef]
- Maes, M.; Kubera, M.; Leunis, J.-C.; Berk, M.; Geffard, M.; Bosmans, E. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr. Scand. 2013, 127, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Sundara Rajan, S.; Turovskiy, Y.; Singh, Y.; Chikindas, M.L.; Sinko, P.J. Poly(ethylene glycol) (PEG)-lactic acid nanocarrier-based degradable hydrogels for restoring the vaginal microenvironment. J. Control. Release 2014, 194, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Saxena, B.B.; Singh, M.; Gospin, R.M.; Chu, C.C.; Ledger, W.J. Efficacy of nonhormonal vaginal contraceptives from a hydrogel delivery system. Contraception 2004, 70, 213–219. [Google Scholar] [CrossRef]
- Neyts, J.; Kristmundsdóttir, T.; Clercq, E.D.; Thormar, H. Hydrogels containing monocaprin prevent intravaginal and intracutaneous infections with HSV-2 in mice: Impact on the search for vaginal microbicides. J. Med. Virol. 2000, 61, 107–110. [Google Scholar] [CrossRef]





| Group | Drug | Content (mg/mL) |
|---|---|---|
| High Dosage Content | IMC | 22.5 ± 0.7 |
| High Dosage Content | GSD | 9.5 ± 0.2 |
| High Dosage Content | EE | 3.6 ± 0.2 |
| Middle Dosage Content | IMC | 11.6 ± 0.6 |
| Middle Dosage Content | GSD | 4.85 ± 0.35 |
| Middle Dosage Content | EE | 2.0 ± 0.3 |
| Low Dosage Content | IMC | 5.5 ± 0.2 |
| Low Dosage Content | GSD | 2.25 ± 0.35 |
| Low Dosage Content | EE | 0.9 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Zou, P.; Dong, J.; Sun, M.; Ding, P.; Han, Y.; Ji, C.; Zhou, Q.; Yuan, H.; Suo, J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Appl. Sci. 2019, 9, 1638. https://doi.org/10.3390/app9081638
Nie L, Zou P, Dong J, Sun M, Ding P, Han Y, Ji C, Zhou Q, Yuan H, Suo J. Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Applied Sciences. 2019; 9(8):1638. https://doi.org/10.3390/app9081638
Chicago/Turabian StyleNie, Lei, Peng Zou, Jing Dong, Meng Sun, Peng Ding, Yanting Han, Chingching Ji, Qiuju Zhou, Hongyu Yuan, and Jinping Suo. 2019. "Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception" Applied Sciences 9, no. 8: 1638. https://doi.org/10.3390/app9081638
APA StyleNie, L., Zou, P., Dong, J., Sun, M., Ding, P., Han, Y., Ji, C., Zhou, Q., Yuan, H., & Suo, J. (2019). Injectable Vaginal Hydrogels as a Multi-Drug Carrier for Contraception. Applied Sciences, 9(8), 1638. https://doi.org/10.3390/app9081638








