Investigation of Properties Alternation during Super-Critical CO2 Injection in Shale
Abstract
:1. Introduction
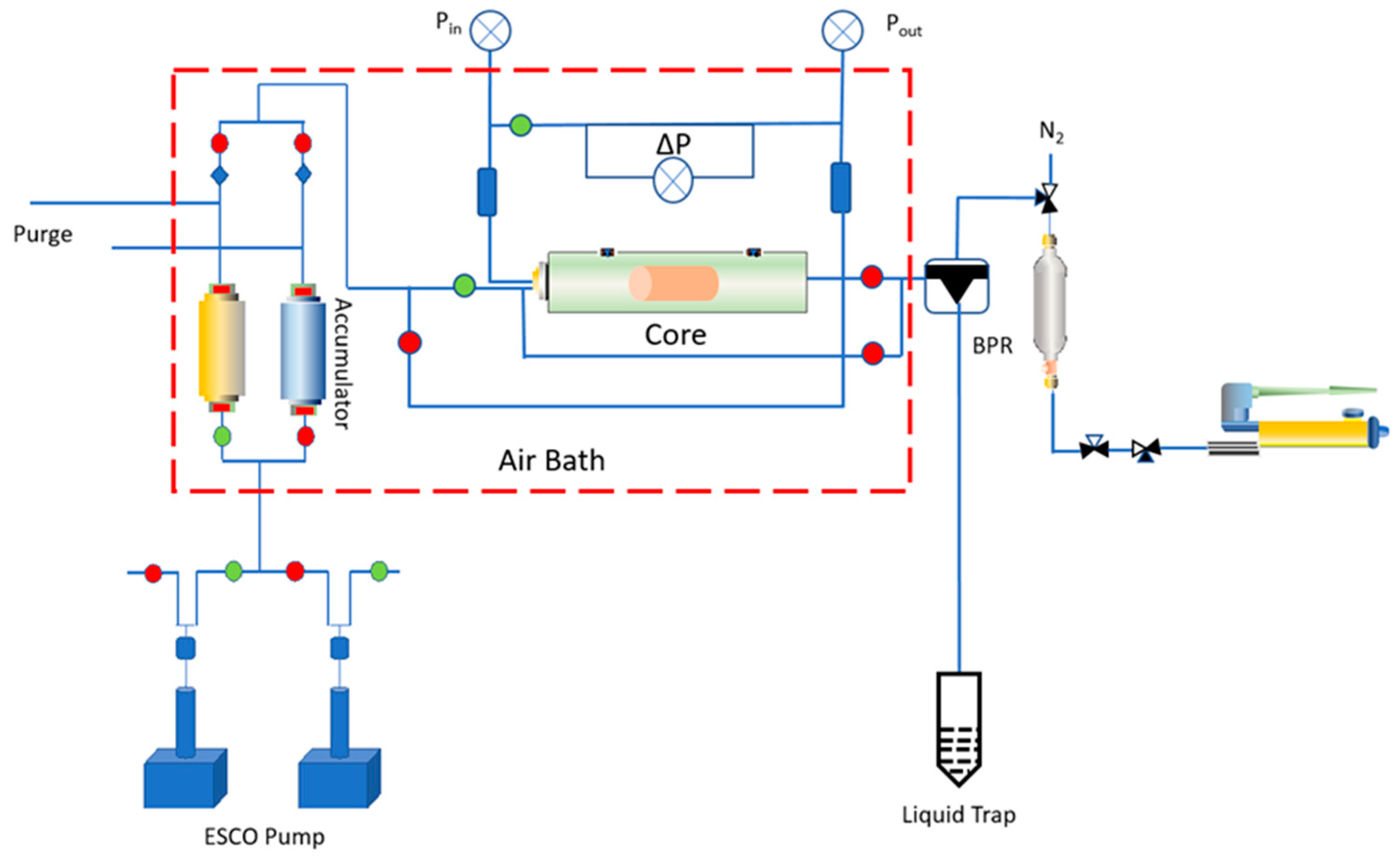
2. Materials and Methods
2.1. Sample Preparation
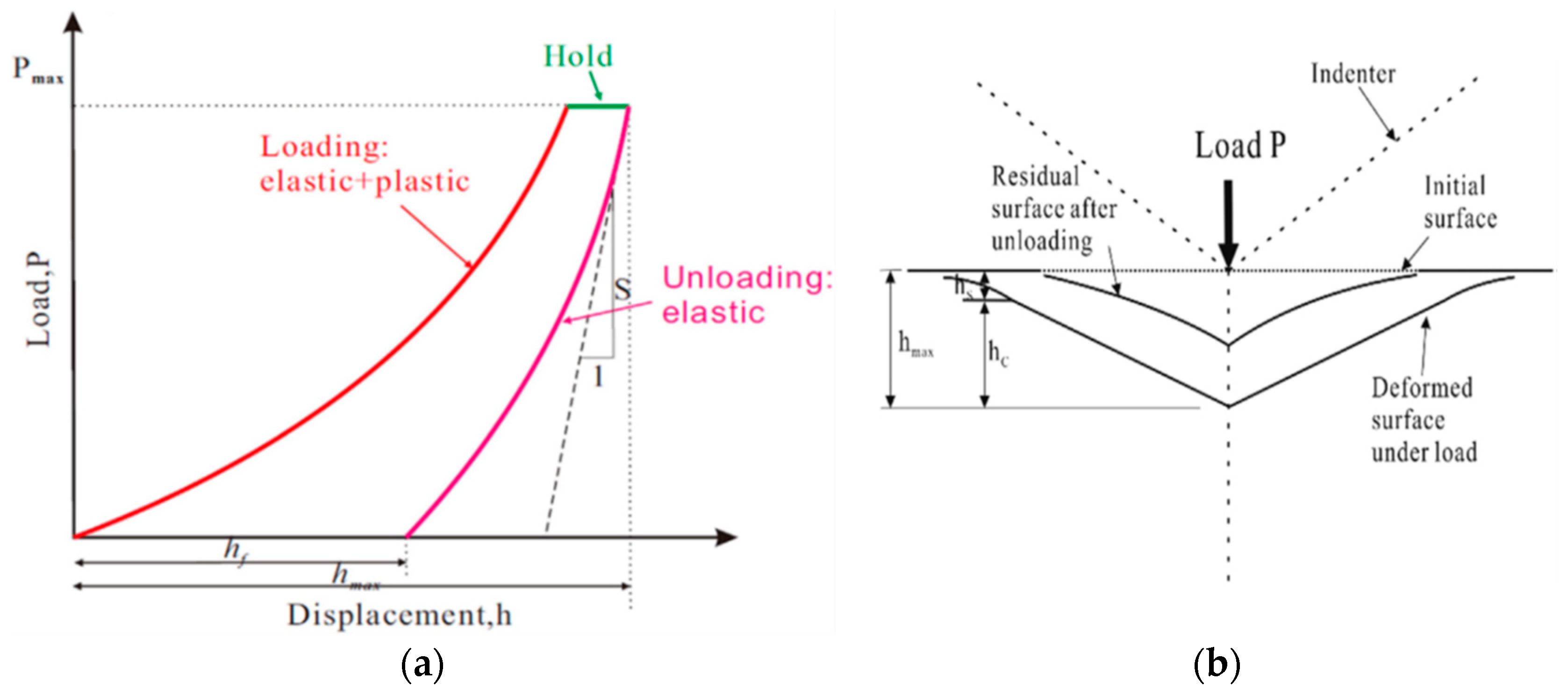
2.2. Properties Analysis Test
3. Results and Discussion
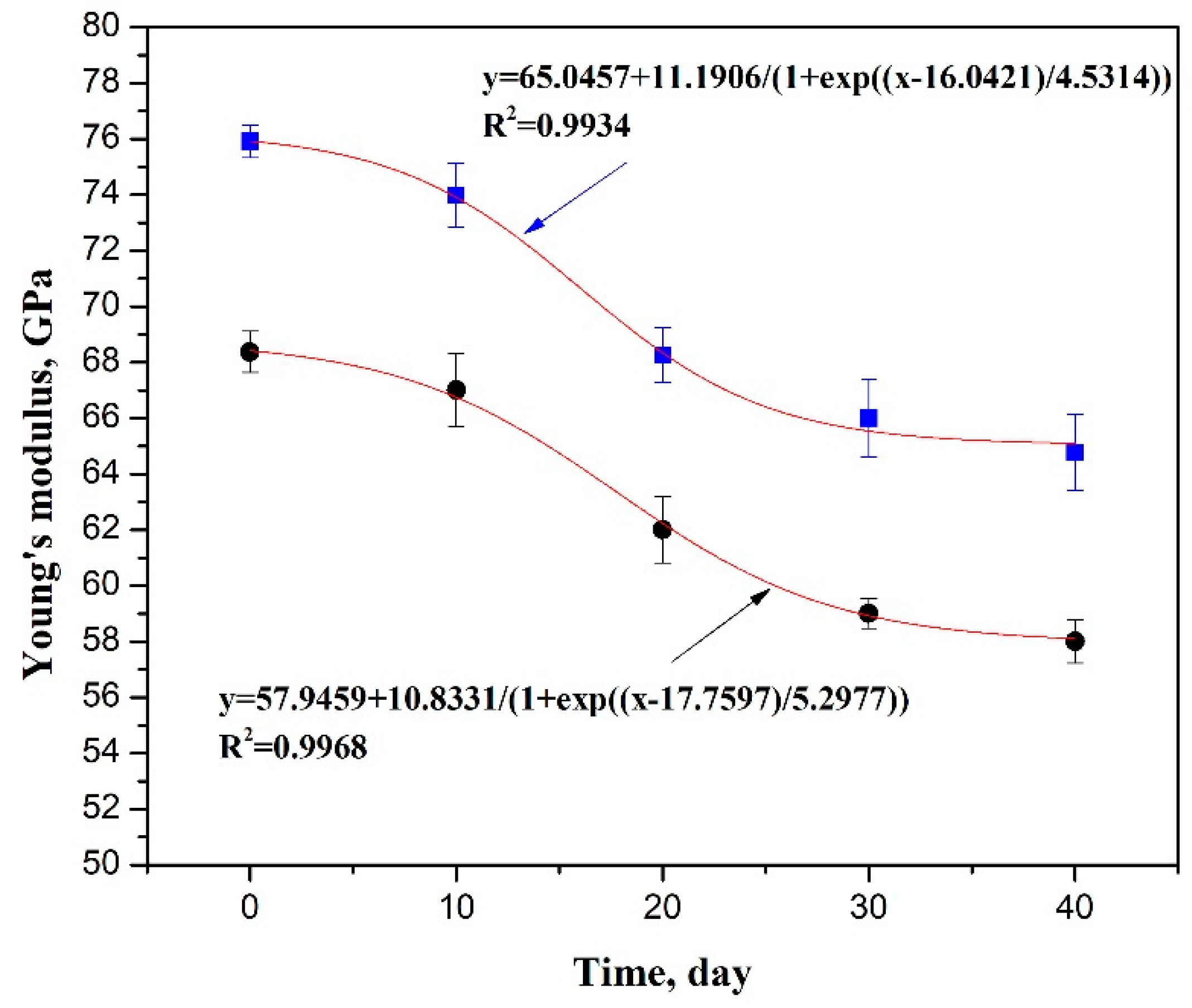
3.1. Mechanical Property Deformation
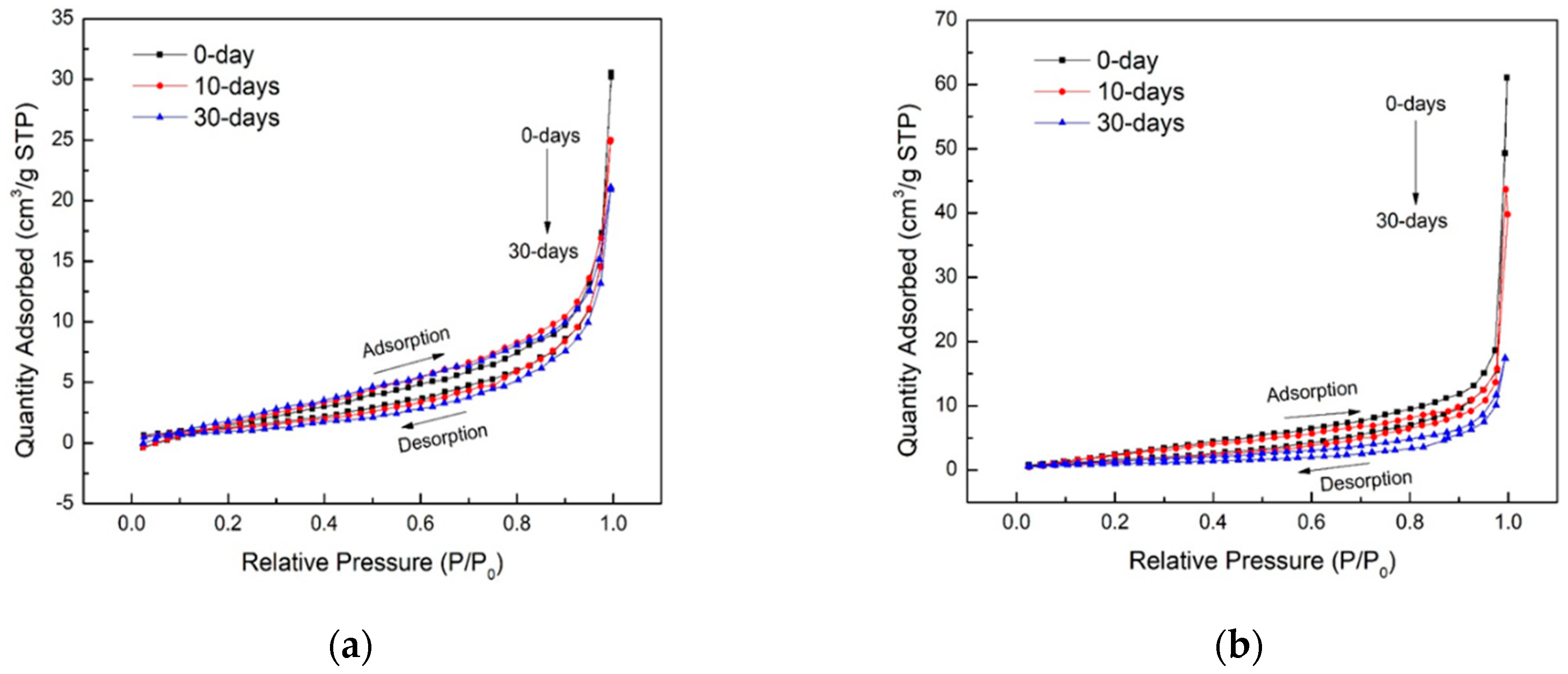
3.2. Pore Structure Alteration
- N: Avogadro’s number (6.0221415 × 1023 molecules/mol);
- Wm: weight of adsorbate constituting a monolayer of surface coverage;
- Acs: molecular cross section of the adsorbate molecule, (16.2 Å2 for N2 at 77 K);
- M: molar mass (molecular weight) of the adsorbate N2 gas (28.0123).
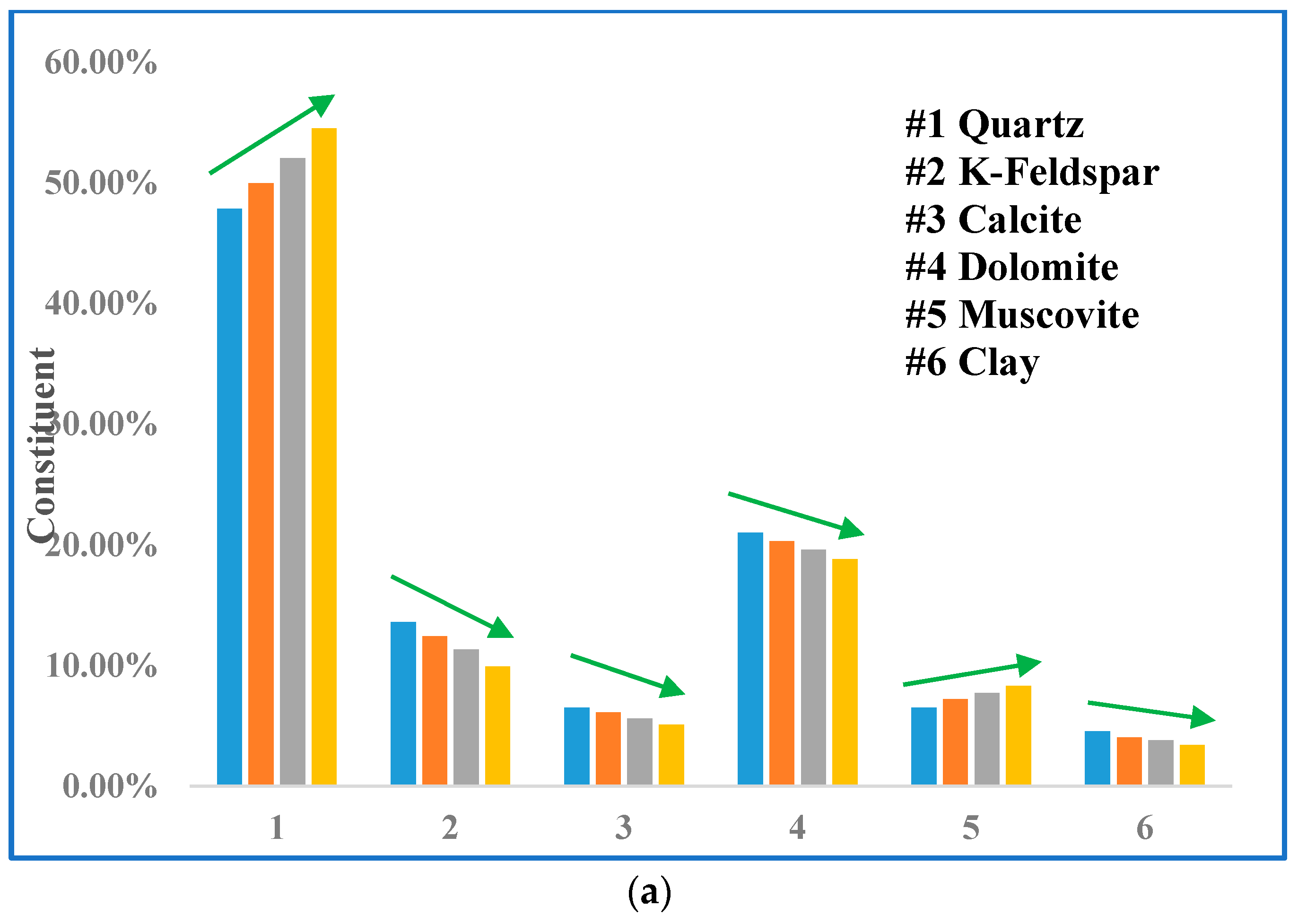
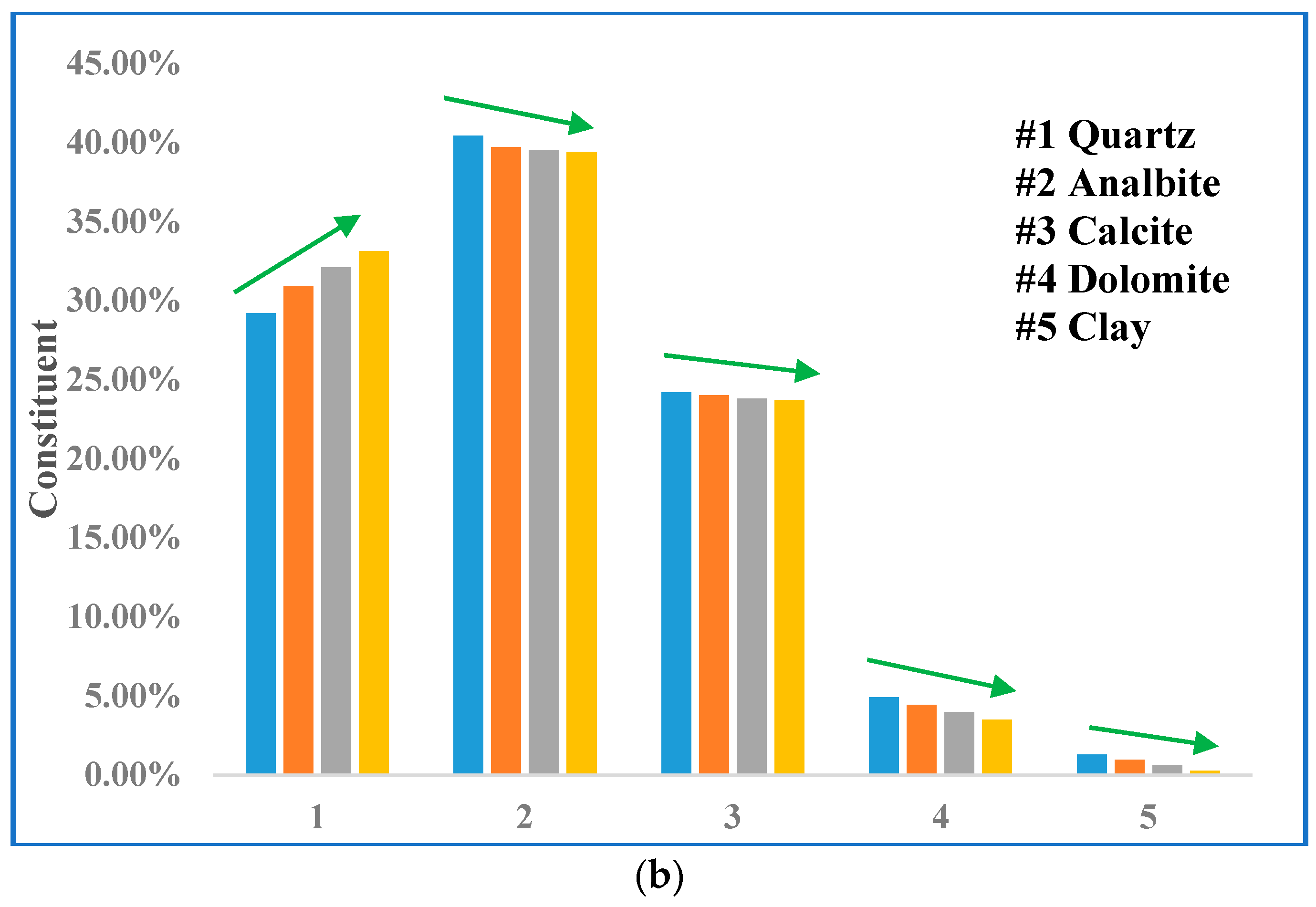
3.3. Mineralogical Alterations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Rabiei, M.; Wang, S.; Cui, G. Fracture Quantification Method with 3D X-ray Image—Entropy-assisted Indicator Kriging Method. In SPE Western Regional Meeting, 22–26 April, Garden Grove, CA, USA, Garden Grove, CA, USA; Society of Petroleum Engineers: Richardson, TX, USA, 26 April 2018. [Google Scholar]
- Ren, B. Local capillary trapping in carbon sequestration: Parametric study and implications for leakage assessment. Int. J. Greenhouse Gas Control 2018, 78, 135–147. [Google Scholar] [CrossRef]
- Ling, K.; Wu, X.; Han, G.; Wang, S. Optimizing the Multistage Fracturing Interval for Horizontal Wells in Bakken and Three Forks Formations. In SPE Asia Pacific Hydraulic Fracturing Conference, 24–26 August 2016, Beijing, China; Society of Petroleum Engineers: Richardson, TX, USA, 26 August 2016. [Google Scholar]
- Jia, B.; Tsau, J.; Barati, R. Role of Molecular Diffusion in Heterogeneous, Naturally Fractured Shale Reservoirs During CO2 Huff-n-puff. J. Pet. Sci. Eng. 2018, 164, 31–42. [Google Scholar] [CrossRef]
- San, J.; Wang, S.; Yu, J.; Liu, N.; Lee, R. Nanoparticle-Stabilized Carbon Dioxide Foam Used. In Enhanced Oil Recovery: Effect of Different Ions and Temperatures. SPE J. 2017, 22, 1416–1423. [Google Scholar] [CrossRef]
- Amirian, E.; Leung, J.Y.; Zanon, S.; Dzurman, P. Integrated cluster analysis and artificial neural network modeling for steam-assisted gravity drainage performance prediction in heterogeneous reservoirs. Expert Syst. Appl. 2015, 42, 723–740. [Google Scholar] [CrossRef]
- Fedutenko, E.; Yang, C.; Card, C.; Nghiem, L.X. Time-Dependent Neural Network Based Proxy Modeling of SAGD Process. In SPE Heavy Oil Conference-Canada, 10–12 June, Calgary, AB, Canada; Society of Petroleum Engineers: Richardson, TX, USA, 12 June 2014. [Google Scholar]
- Li, H.; Lau, H.C.; Huang, S. Coalbed Methane Development in China: Engineering Challenges and Opportunities. In SPE/IATMI Asia Pacific Oil & Gas Conference and Exhibition, 17–19 October, Jakarta, Indonesia; Society of Petroleum Engineers: Richardson, TX, USA, 19 October 2014. [Google Scholar]
- Wang, S.; San, J.; Yu, J.; Lee, R.; Liu, N. A Downhole CO2 Sensor to Monitor CO2 Movement In situ for Geologic Carbon Storage. Int. J. Greenhouse Gas Control 2016, 55, 202–208. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.; Barati, R. A Review of the Current Progress of CO2 Injection EOR and Carbon Storage in Shale Oil Reservoirs. Fuel 2019, 236, 404–427. [Google Scholar] [CrossRef]
- Akrad, O.M.; Miskimins, J.L.; Prasad, M. The Effects of Fracturing Fluids on Shale Rock Mechanical Properties and Proppant Embedment. In SPE Annual Technical Conference and Exhibition, 30 October–2 November, Denver, CO, USA; Society of Petroleum Engineers: Richardson, TX, USA, 2 November 2014. [Google Scholar]
- Liu, Q.-Y.; Tao, L.; Zhu, H.-Y.; Lei, Z.-D.; Jiang, S.; McLennan, J.D. Macroscale Mechanical and Microscale Structural Changes in Chinese Wufeng Shale with Supercritical Carbon Dioxide Fracturing. SPE J. 2018, 23, 691–703. [Google Scholar] [CrossRef]
- Blasingame, T.; Maxwell, S.; Valleau, D. Petrophysical Characterization of the Bakken Shale for Carbon Storage Investigation. In Unconventional Resources Technology Conference, Austin, Texas, 24–26 July 2017; Unconventional Resources Technology Conference: Tulsa, OK, USA, 26 July 2017. [Google Scholar]
- Rezaee, R.; Saeedi, A.; Iglauer, S.; Evans, B. Shale Alteration after Exposure to Supercritical CO2. Int. J. Greenhouse Gas Control 2017, 62, 91–99. [Google Scholar] [CrossRef]
- Busch, A.; Alles, S.; Gensterblum, Y.; Prinz, D.; Dewhurst, D.N.; Raven, M.D.; Stanjek, H.; Krooss, M.B. Carbon dioxide storage potential of shales. Int. J. Greenhouse Gas Control 2008, 2, 297–308. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y.; Wang, Z. Experimental Investigation on the Mechanical Properties of a Low-clay Shale with Different Adsorption Times in Sub-/super-critical CO2. Energy 2018, 147, 1288–1298. [Google Scholar] [CrossRef]
- Lahann, R.; Mastalerz, M.; Rupp, J.A. Influence of CO2 on New Albany Shale Composition and Pore Structure. Int. J Coal Geol. 2013, 108, 2–9. [Google Scholar] [CrossRef]
- Ao, X.; Lu, Y.; Tang, J.; Chen, Y.; Li, H. Investigation on the Physics Structure and Chemical Properties of the Shale Treated by Supercritical CO2. J. CO2 Utilization 2017, 20, 274–281. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, W.; Liang, C.; Wang, Y.; Liu, H.; Chen, X. Basic Characteristics and Evaluation of Shale Oil Reservoirs. Pet. Res. 2016, 1, 149–163. [Google Scholar] [CrossRef]
- Hu, C.; Li, Z. A review on the Mechanical Properties of Cement-based Materials Measured by Nanoindentation. Constr. Build. Mater. 2015, 90, 80–90. [Google Scholar] [CrossRef]
- Liu, K.; Ostadhassan, M.; Bailey, B. Applications of Nano-indentation Methods to Estimate Nanoscale Mechanical Properties of Shale Reservoir rocks. J. Nat. Gas Sci. Eng. 2016, 35, 1310–1319. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved Technique for Determining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Shalabi, F.I.; Cording, E.J.; Al-Hattamleh, O.H. Estimation of Rock Engineering Properties Using Hardness Tests. Eng. Geol. 2007, 90, 138–147. [Google Scholar] [CrossRef]
- Rouquerolb, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the Characterization of Porous Solids (Technical Report). Pure Appl. Chem. 2009, 66, 1739–1758. [Google Scholar] [CrossRef]
- Jia, B.; Tsau, J.-S.; Barati, R. A Workflow to Estimate Shale Gas Permeability Variations during the Production Process. Fuel 2018, 220, 879–889. [Google Scholar] [CrossRef]

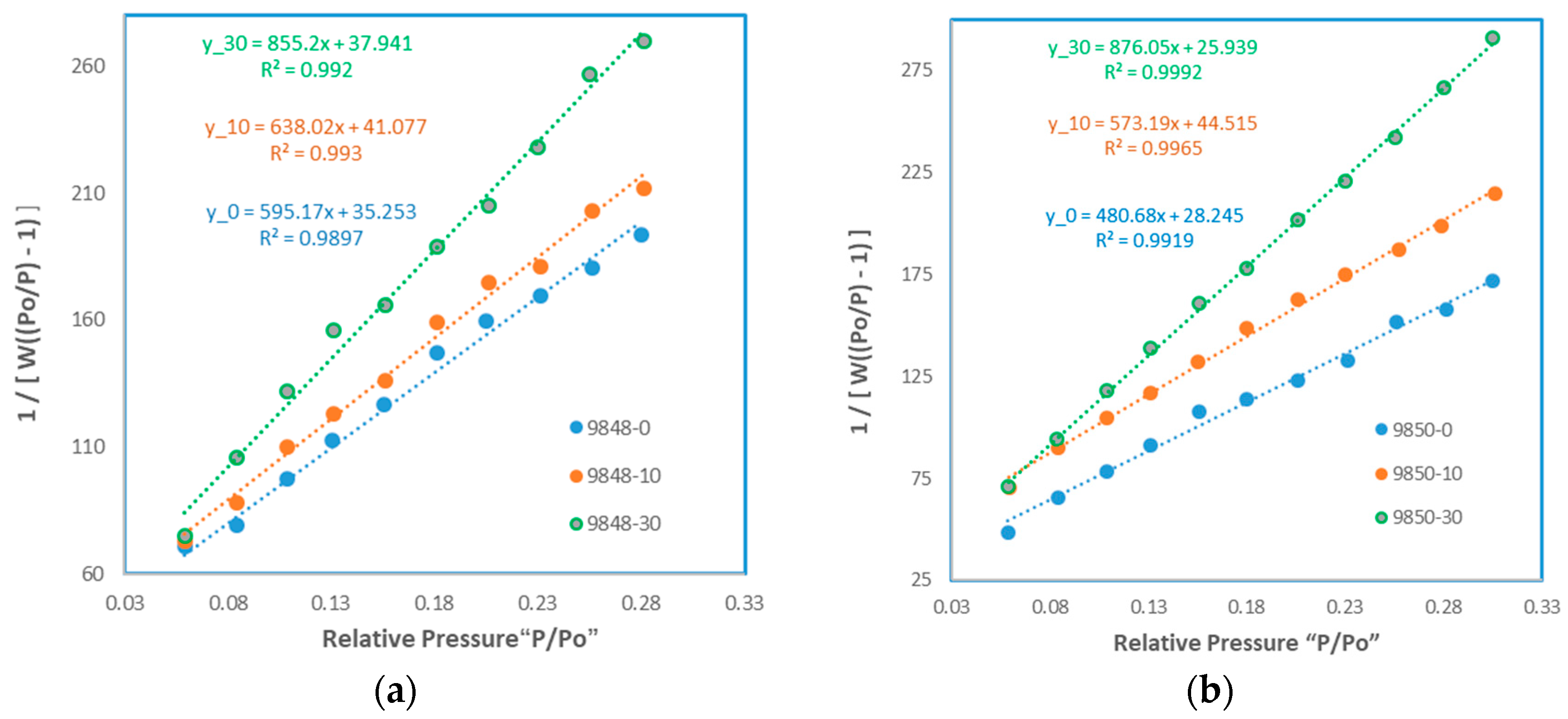
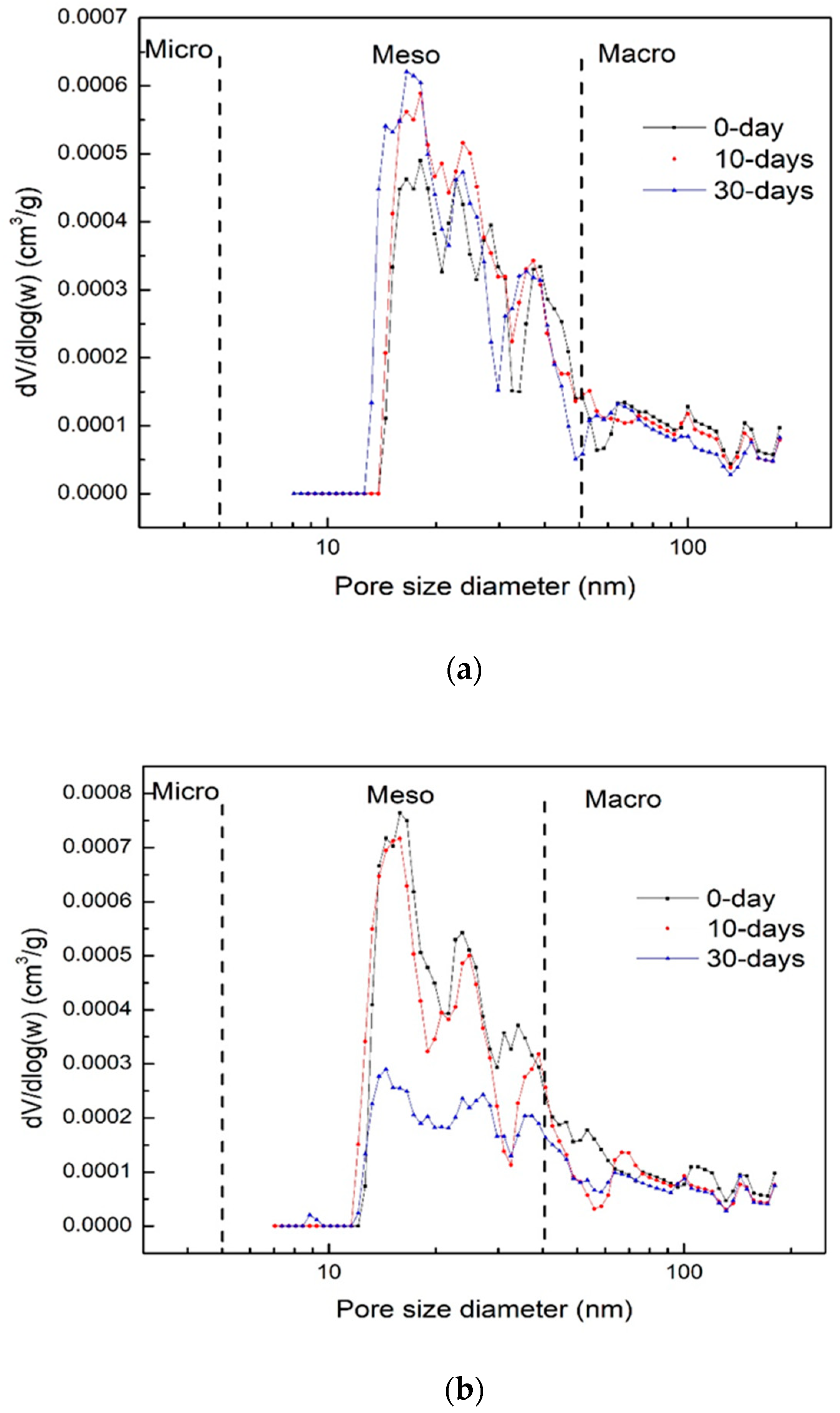
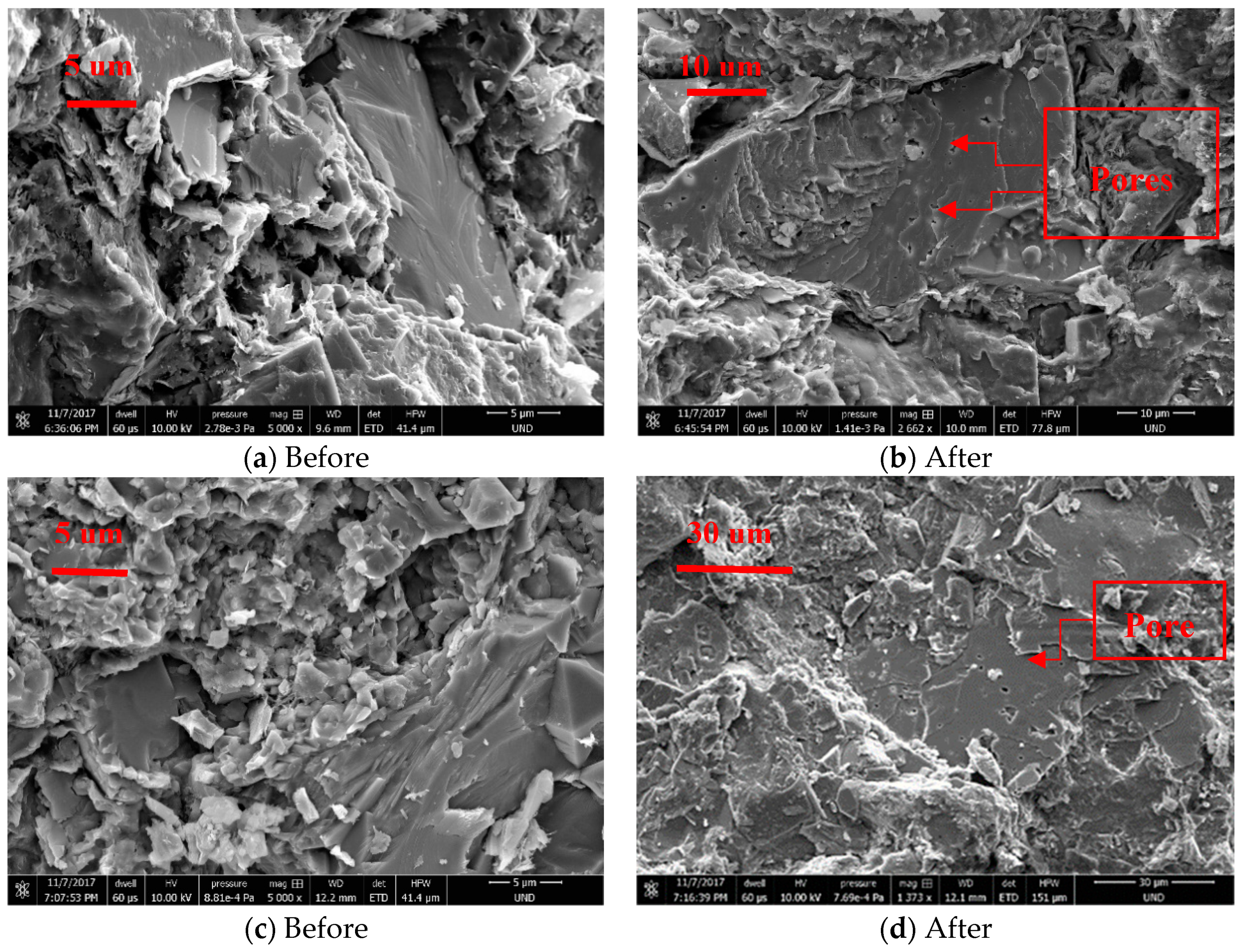
| Weight of sample taken (g) | 0.1 |
| Adsorbate | N2 |
| Bath temperature (K) | 77.00 |
| Outgas temperature (°C) | 120 |
| Outgas time (h) | 10 |
| Relative pressure (P/Po) | 0.01–0.99 |
| Sample ID | ||||
|---|---|---|---|---|
| 9848 ft | 9850 ft | |||
| Time, Day | Surface Area, m2/g | Deduction Per., % | Surface Area, m2/g | Deduction Per., % |
| 0 | 5.595 | - | 6.85 | - |
| 10 | 5.238 | 6.38% | 4.998 | 27.04% |
| 30 | 4.055 | 27.52% | 3.865 | 43.58% |
| Sample ID | Treatment Time (day) | Mineral Content (%) | |||||
|---|---|---|---|---|---|---|---|
| Quartz | Analbite | Calcite | Dolomite | Muscovite | Clay | ||
| 9850 | 0 | 29.21 | 40.4 | 24.2 | 4.9 | - | 1.29 |
| 10 | 30.93 | 39.7 | 24 | 4.41 | - | 0.96 | |
| 20 | 32.11 | 39.5 | 23.8 | 3.96 | - | 0.63 | |
| 30 | 33.13 | 39.4 | 23.7 | 3.5 | - | 0.27 | |
| 9848 | 0 | 47.85 | 13.6 | 6.5 | 21 | 6.5 | 4.55 |
| 10 | 49.96 | 12.4 | 6.1 | 20.3 | 7.2 | 4.04 | |
| 20 | 52.03 | 11.3 | 5.6 | 19.6 | 7.7 | 3.77 | |
| 30 | 54.51 | 9.9 | 5.1 | 18.8 | 8.3 | 3.39 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Liu, K.; Han, J.; Ling, K.; Wang, H.; Jia, B. Investigation of Properties Alternation during Super-Critical CO2 Injection in Shale. Appl. Sci. 2019, 9, 1686. https://doi.org/10.3390/app9081686
Wang S, Liu K, Han J, Ling K, Wang H, Jia B. Investigation of Properties Alternation during Super-Critical CO2 Injection in Shale. Applied Sciences. 2019; 9(8):1686. https://doi.org/10.3390/app9081686
Chicago/Turabian StyleWang, Sai, Kouqi Liu, Juan Han, Kegang Ling, Hongsheng Wang, and Bao Jia. 2019. "Investigation of Properties Alternation during Super-Critical CO2 Injection in Shale" Applied Sciences 9, no. 8: 1686. https://doi.org/10.3390/app9081686




