Low Temperature Chemoselective Hydrogenation of Aldehydes over a Magnetic Pd Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Catalyst Synthesis and Characterization
2.3. Generalprocedure of the Reduction of Carbonyl Compounds
2.4. Analyticmethods
- Aldehyde conversion and Alcoholselectivitywere defined as follows:
- Aldehyde conversion = moles of convertedaldehyde/moles of starting aldehyde×100%
- Alcohol yield = moles of generated alcohol/moles of starting aldehyde×100%
- Alcohol selectivity = alcohol yield/aldehyde conversion×100%
3. Results and Discussion
3.1. Effect of the Reaction Solvents on the Hydrogenation of Benzaldehyde
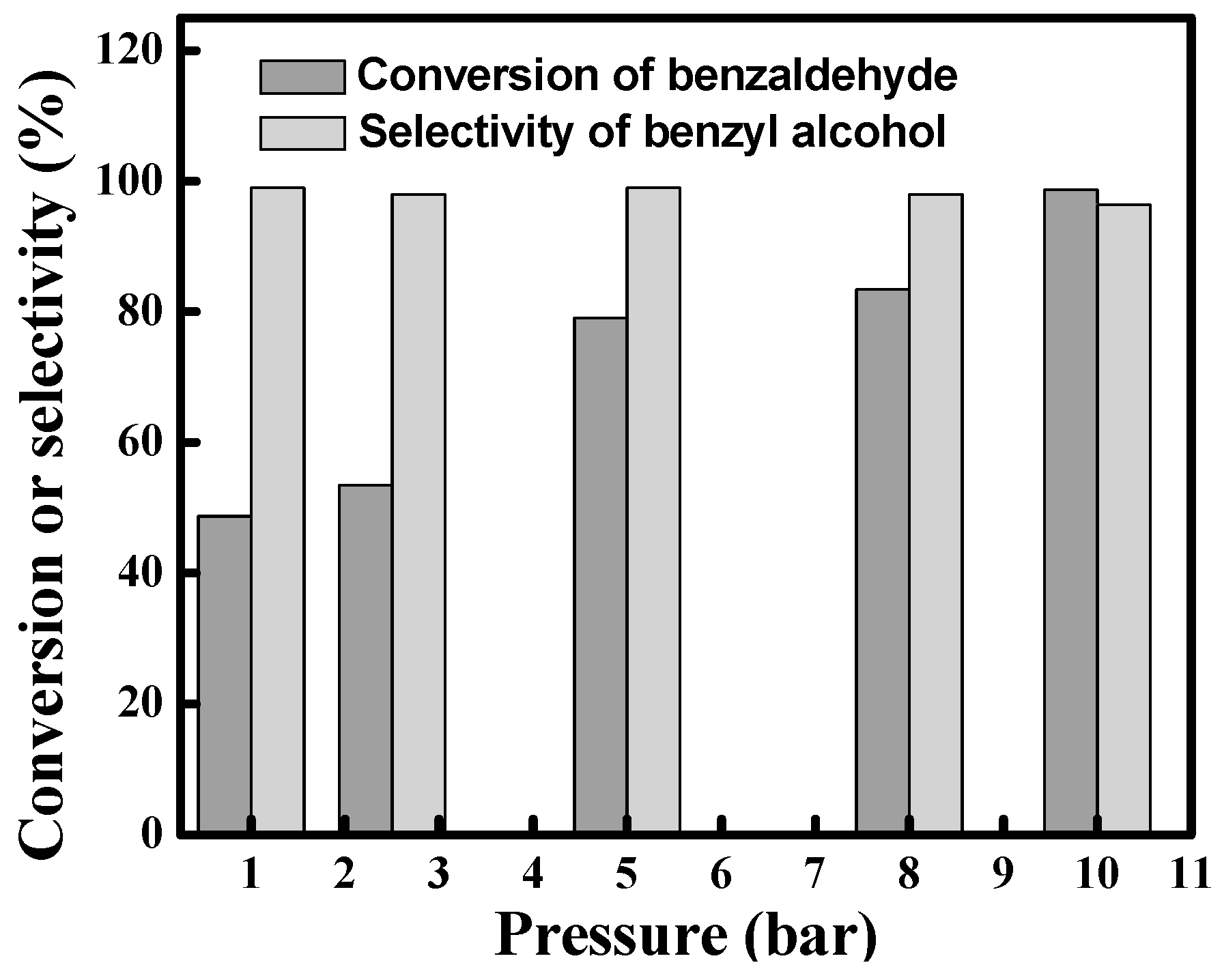
3.2. Effect of the Hydrogen Pressure on the Hydrogenation of Benzaldehyde
3.3. Substrate Scope of the Reduction of Aldehydes to Alcohols
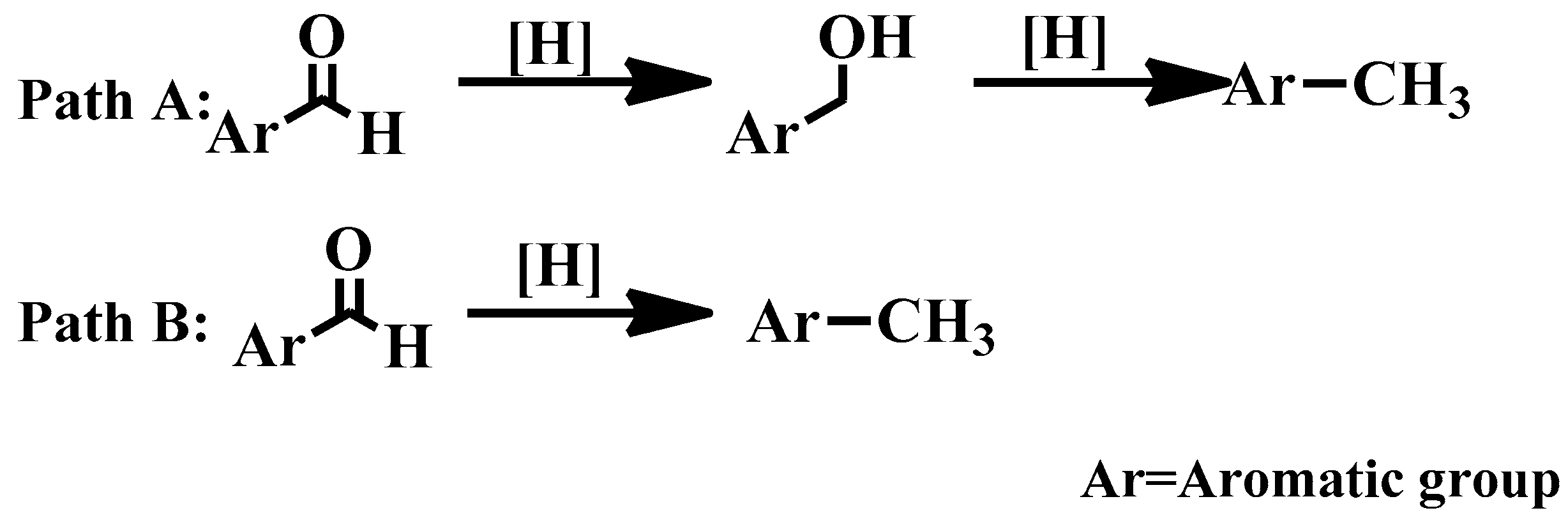
3.4. Effect of the Temperature on the Deoxygenation of Benzaldehyde
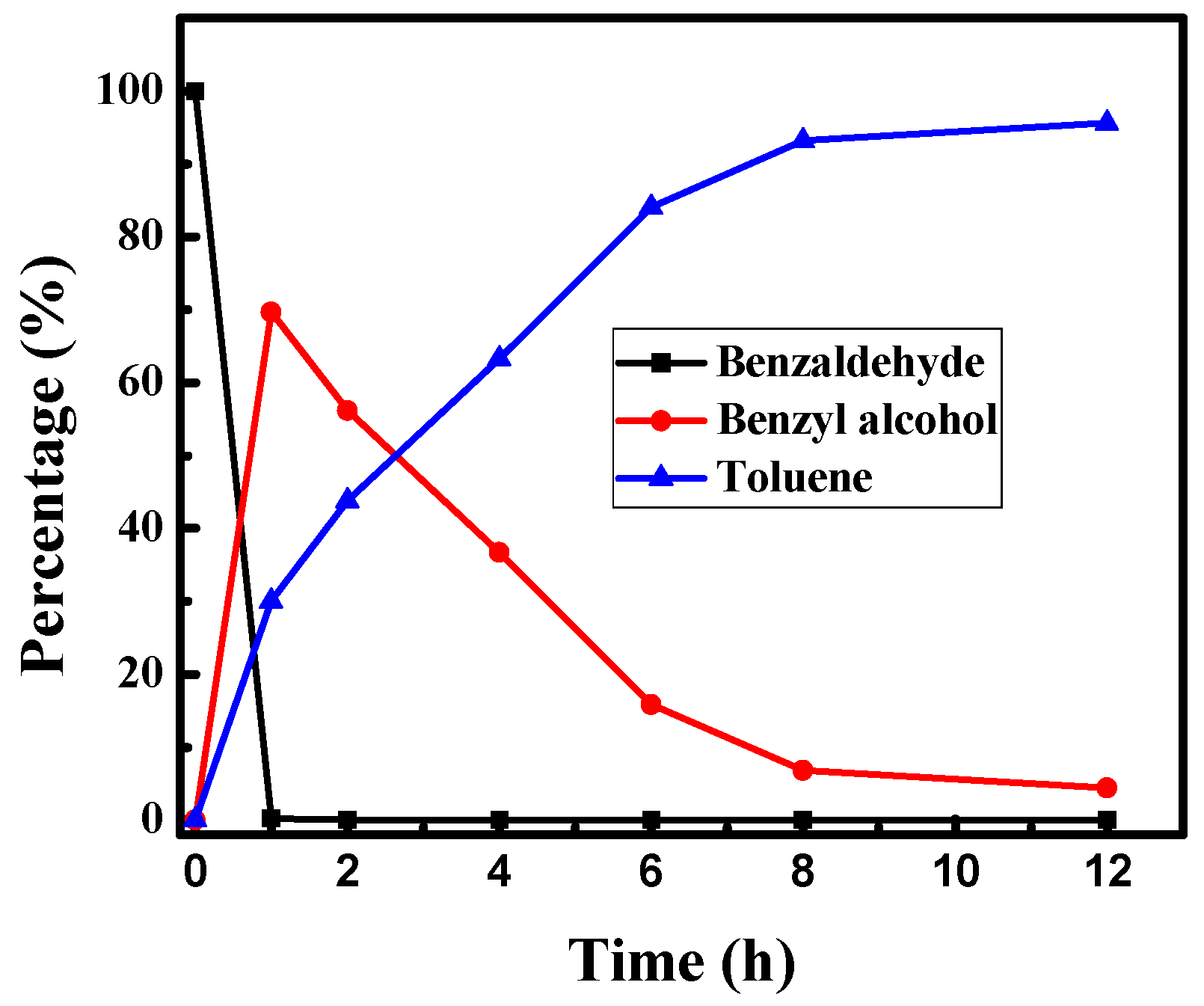
3.5. Time Course of the Product Distributions
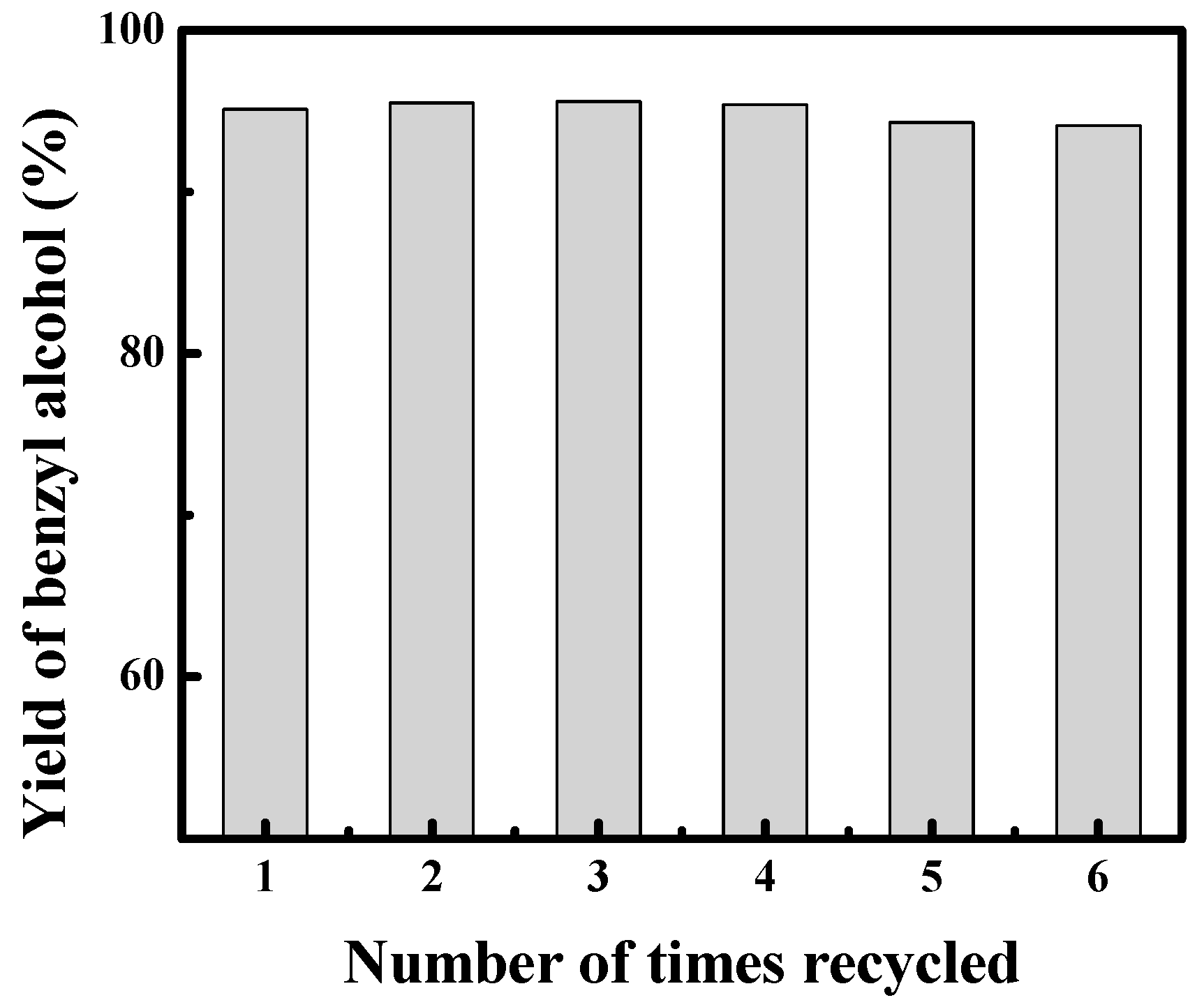
3.6. Recycling of the γ-Fe2O3@HAP-Pd Catalyst
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Seemala, B.; Cai, C.M.; Kumar, R.; Wyman, C.E.; Christopher, P. Effects of Cu-Ni bimetallic catalyst composition and support on activity, selectivity and stability for furfural Cconversion to 2-methyfuran. ACS Sustain. Chem. Eng. 2018, 6, 2152–2161. [Google Scholar] [CrossRef]
- Kogan, V.; Aizenshtat, Z.; Neumann, R. Polyoxometalates as reduction catalysts: Deoxygenation and hydrogenation of carbonyl compounds. Angew. Chem. Int. Ed. 1999, 38, 3331–3334. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic reduction of biomass-derived furanic compounds with hydrogen. ACSCatal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Kumar, B.S.; Puthiaraj, P.; Amali, A.J.; Pitchumani, K. Ultrafine Bimetallic PdCo Alloy Nanoparticles on Hollow Carbon Capsules: An efficient heterogeneous catalyst for transfer hydrogenation of carbonyl compounds. ACS Sustain. Chem. Eng. 2018, 6, 491–500. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharya, P.; Dai, H.G.; Guan, H.R. Nickel and iron pincer complexes as catalysts for the reduction of carbonyl compounds. Acc. Chem. Res. 2015, 48, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Holthausen, M.H.; Mallov, I.; Perez, M.; Qu, Z.W.; Grimme, S.D.; Stephan, W. Catalytic nketone hydrodeoxygenation mediated by highly electrophilic phosphoniumcations. Angew. Chem. Int. Ed. 2015, 54, 8250–8254. [Google Scholar] [CrossRef]
- Volkov, A.; Gustafson, K.P.J.; Tai, C.W.; Verho, O.; Bäckvall, J.E.; Adolfsson, H. Mild deoxygenation of aromatic ketones and aldehydes over Pd/C using polymethylhydrosiloxane as the reducing agent. Angew. Chem. Int. Ed. 2015, 54, 5122–5126. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.A.; Bernardo, J.R.; Fernandes, A.C. Direct reductive deoxygenation of aryl ketones catalyzed by oxo-rhenium complexes. ChemCatChem 2015, 7, 1177–1183. [Google Scholar] [CrossRef]
- Bernardo, J.R.; Fernandes, A.C. Deoxygenation of carbonyl compounds using an alcohol as an efficient reducing agent catalyzed by oxo-rhenium complexes. Green Chem. 2016, 18, 2675–2681. [Google Scholar] [CrossRef]
- Ye, W.; Zhao, M.; Yu, Z. Ruthenium (ii) pyrazolyl–pyridyl–oxazolinyl complex catalysts for the asymmetric transfer hydrogenation of ketones. Chem. Eur. J. 2012, 18, 10843–10846. [Google Scholar] [CrossRef] [PubMed]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Weidner, V.L.; Barger, C.J.; Delferro, M.; Lohr, T.L.; Marks, T.J. Rapid, mild and selective ketone and aldehyde hydroboration/reduction mediated by a simple lanthanide latalyst. ACSCatal. 2017, 7, 1244–1247. [Google Scholar]
- Morris, R.H. Exploiting metal–ligand bifunctional reactions in the design of iron asymmetric hydrogenation catalysts. Acc. Chem. Res. 2015, 48, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Werkmeister, S.; Junge, K.; Wendt, B.; Alberico, E.; Jiao, H.; Baumann, W.; Junge, H.; Gallou, F.; Beller, M. Hydrogenation of esters to alcohols with a Well-defined iron complex. Angew. Chem. Int. Ed. 2014, 53, 8722–8726. [Google Scholar] [CrossRef]
- Yang, P.K.; Liu, Y.W.; Chai, L.; Lai, Z.Z.; Fang, X.M.; Liu, B.Y.; Zhang, W.K.; Lu, M.H.; Xu, Y.Q.; Xu, H. Nmp-Based Ionic Liquids: Recyclable Catalysts for Both Hetero-Michael Addition and Knoevenagel Condensation in Water. Synth. Commun. 2018, 48, 1060–1067. [Google Scholar] [CrossRef]
- Li, Z.X.; Luo, D.; Li, M.M.; Xing, X.F.; Ma, Z.Z.; Xu, H. Recyclable Fe3O4 Nanoparticles Catalysts for Aza-Michael Addition of Acryl Amides by Magnetic Field. Catalysts 2017, 7, 219. [Google Scholar] [CrossRef]
- Kotha, S.S.; Sharma, N.; Sekar, G. An efficient, stable and reusable Palladium nanocatalyst: Chemoselective reduction of aldehydes with molecular hydrogen in water. Adv. Synth. Catal. 2016, 358, 1694–1698. [Google Scholar] [CrossRef]
- Tan, J.; Cui, J.; Cui, X.; Deng, T.; Li, X.; Zhu, Y.; Li, Y. Graphene-modified Runanocatalyst for low-temperature hydrogenation of carbonyl groups. ACS Catal. 2015, 5, 7379–7384. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Selective hydrogenation of crotonaldehyde to crotyl alcohol over metal oxide modified Ir catalysts and mechanistic insight. ACS Catal. 2016, 6, 3600–3609. [Google Scholar] [CrossRef]
- Osako, T.; Torii, K.; Hirata, S.; Uozumi, Y. Chemoselectivecontinuous-Flow hydrogenation of aldehydes catalyzed by platinum nanoparticles dispersed in an amphiphilicresin. ACS Catal. 2017, 7, 7371–7377. [Google Scholar] [CrossRef]
- Kalutharage, N.K.; Yi, C.S. Scope and mechanistic analysis for chemoselectivehydrogenolysis of carbonyl compounds catalyzed by a cationic ruthenium hydride complex with a tunable phenol ligand. J. Am. Chem. Soc. 2015, 137, 11105–11114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Zhen, J.D.; Liu, B.; Lv, K.L.; Deng, K.J. Selective aerobic oxidation of the biomass-derived precursor 5-hydroxymethylfurfural to 2, 5-furandicarboxylic acid under mild conditions over a magnetic palladium nanocatalyst. Green Chem. 2015, 1, 1308–1317. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Liu, B.; Zhou, P.; Zhang, Z.H.; Chi, Q. Aerobic Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran with Cesium-Doped Manganese Dioxide. Catal. Sci. Technol. 2018, 8, 4430–4439. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Lv, K.L.; Sun, J.; Zhang, Z.H.; Chi, Q. Selective and metal-free oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran over nitrogen-doped carbonmaterials. Green. Chem. 2018, 20, 4946–4956. [Google Scholar]
- Xu, X.; Li, Y.; Gong, Y.T.; Zhang, P.F.; Li, H.R.; Wang, Y. Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade. J. Am. Chem. Soc. 2012, 134, 16987–16990. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Bo, X.K.; Tian, Z.F.; Wang, Y.Z.; Guo, X.K.; Xie, M.J.; Gao, F.; Lin, M.; Guo, X.F.; Ding, W.P. Fabrication of highly dispersed/active ultrafine Pd nanoparticle supported catalysts: a facile solvent-free in situ dispersion/reduction method. Green Chem. 2017, 19, 2646–2652. [Google Scholar] [CrossRef]
- Sever, R.; Root, T.W. DFT study of solvent coordination rffects on titanium-basedepoxidationcatalysts. Part One: Formation of the titanium hydroperoxointermediate. J. Phys. Chem. B 2003, 107, 4080–4089. [Google Scholar] [CrossRef]
- Ouyang, R.H.; Jiang, D.E. Understanding selective hydrogenation of α,β–unsaturated ketones to unsaturated alcohols on the Au25(SR)18cluster. ACSCatal. 2015, 5, 6624–6629. [Google Scholar]
- Chen, J.Z.; Liu, R.L.; Guo, Y.Y.; Chen, L.M.; Gao, H. Selective hydrogenation of biomass-based 5-hydroxymethylfurfural over catalyst of Palladium immobilized on amine-functionalized Metal–Organic Frameworks. ACS Catal. 2015, 5, 722–733. [Google Scholar] [CrossRef]
- Heiba, E.I.; Dessau, R.M.; Koehl, W.J. Oxidation by metal salts. III. Reaction of manganic acetate with aromatic hydrocarbons and the reactivity of the carboxymethylradical. J. Am. Chem. Soc. 1969, 91, 138–145. [Google Scholar] [CrossRef]
- Fukui, M.; Tanaka, A.; Hashimoto, K.; Kominami, H. Visible light-induced heterogeneous Meerwein–Ponndorf–Verley-type reduction of an aldehyde group over an organically modified titanium dioxide photocatalyst. Chem. Comm. 2017, 53, 4215–4218. [Google Scholar] [CrossRef] [PubMed]




| Entry | Catalyst | Solvent | Con. of Benzyl Aldehyde(%) | Sel. of Benzyl Alcohol(%) |
|---|---|---|---|---|
| 1 | γ-Fe2O3@HAP-Pd | Hexane | 17.2 | 90.1 |
| 2 | γ-Fe2O3@HAP-Pd | THF | 34.2 | 90.9 |
| 3 | γ-Fe2O3@HAP-Pd | i-PrOH | 56.9 | 99.0 |
| 4 | γ-Fe2O3@HAP-Pd | EtOH | 95.6 | 96.5 |
| 5 | γ-Fe2O3@HAP-Pd | H2O | 98.7 | 96.4 |
| 6b | γ-Fe2O3@HAP-Pd | H2O | 100 | >99 |
| 7c | Pd-BNP | H2O | 90d | |
- aReaction condition: benzyl aldehyde (1 mmol), 10 mgγ-Fe2O3@HAP-Pd catalyst (2 wt.% by ICP-OES analysis); S/C= 530, solvent (10 mL), H2(10 bar), 25 °C, 1h.
- bS/C= 530, H2(1 bar), 25 °C, 4h.
- cfrom ref 15, S/C=100, H2 (1 bar), 25 °C, 6h.
- dIsolated yield.
 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | Product | Time (h) | Conv. (%) | Yield (%) |
| 1 |  |  | 1.5 | 100 | 99.1 |
| 2 |  |  | 1.5 | 100 | 99.3 |
| 3 | 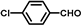 | 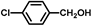 | 2 | 100 | 99.8 |
| 4 | 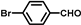 |  | 2.5 | 67.9 | 58.9(55.3b) |
| 5 | 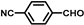 |  | 1.5 | 100 | 99.7(96.1 b) |
| 6 |  | 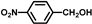 | 4 | 79.3 | 79.3(75.3 b) |
| 7 |  | 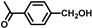 | 6 | 96.5 | 82.6(80.5 b) |
| 8 | 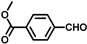 |  | 8 | 93.1 | 88.7 |
| 9 | 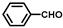 | 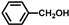 | 1 | 100 | 95.1 |
| 10 |  | 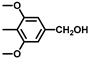 | 21 | 100 | 100 |
| 11 | 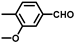 |  | 11 | 100 | 99.3 |
| 12 | 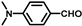 |  | 6 | 100 | 99.8 |
| 13 | 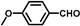 | 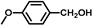 | 8 | 100 | 100 |
| 14 |  |  | 5 | 100 | 99.0 |
| 15 | 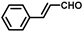 |  | 2 | 100 | 71.6(68.4 b) |
| 16 |  |  | 2 | 97.5 | 97.5(94.8 b) |
| 17 | 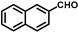 | 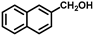 | 7.5 | 100 | 99.6 |
| 18 |  |  | 21 | 46.2 | 46.2(45.1b) |
- aReaction conditions: substrate (1 mmol), γ-Fe2O3@HAP-Pd (10 mg)catalyst, 25 °C, H2 (10 bar), H2O (10 mL).
- b Isolated yield.
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Wang, X.; Gao, D.; Wang, L.; Cheng, J.; Wang, A.; Zhang, Z. Low Temperature Chemoselective Hydrogenation of Aldehydes over a Magnetic Pd Catalyst. Appl. Sci. 2019, 9, 1792. https://doi.org/10.3390/app9091792
Liu A, Wang X, Gao D, Wang L, Cheng J, Wang A, Zhang Z. Low Temperature Chemoselective Hydrogenation of Aldehydes over a Magnetic Pd Catalyst. Applied Sciences. 2019; 9(9):1792. https://doi.org/10.3390/app9091792
Chicago/Turabian StyleLiu, Anqiu, Xiaochen Wang, Daming Gao, Le Wang, Junjie Cheng, An Wang, and Zehui Zhang. 2019. "Low Temperature Chemoselective Hydrogenation of Aldehydes over a Magnetic Pd Catalyst" Applied Sciences 9, no. 9: 1792. https://doi.org/10.3390/app9091792






