Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2Core–Shell Powder for Lithium Batteries
Abstract
1. Introduction
- The synthesis takes place at room temperature in a single step.
- The process is scalable and uses cheap, non-toxic and easily available materials.
- The particles obtained are compatible with the traditional techniques to produce lithium batteries and enables the use of electrolytes, binder and conductive materials used in industry today.
- The conditions of micro-mixing can be obtained within the liquid film over the disc surface by using a low amount of energy.
- The reduced residence time and the high local supersaturation values limits the growth after the nucleation allowing the production of nanometric size particles.
- The use of concentrated solutions allows high yields to be obtained. In addition to this, the synthesis via SDR is a continuous production process that can be easily scaled up for industrial applications.
2. Experimental
2.1. Instruments
2.2. Method
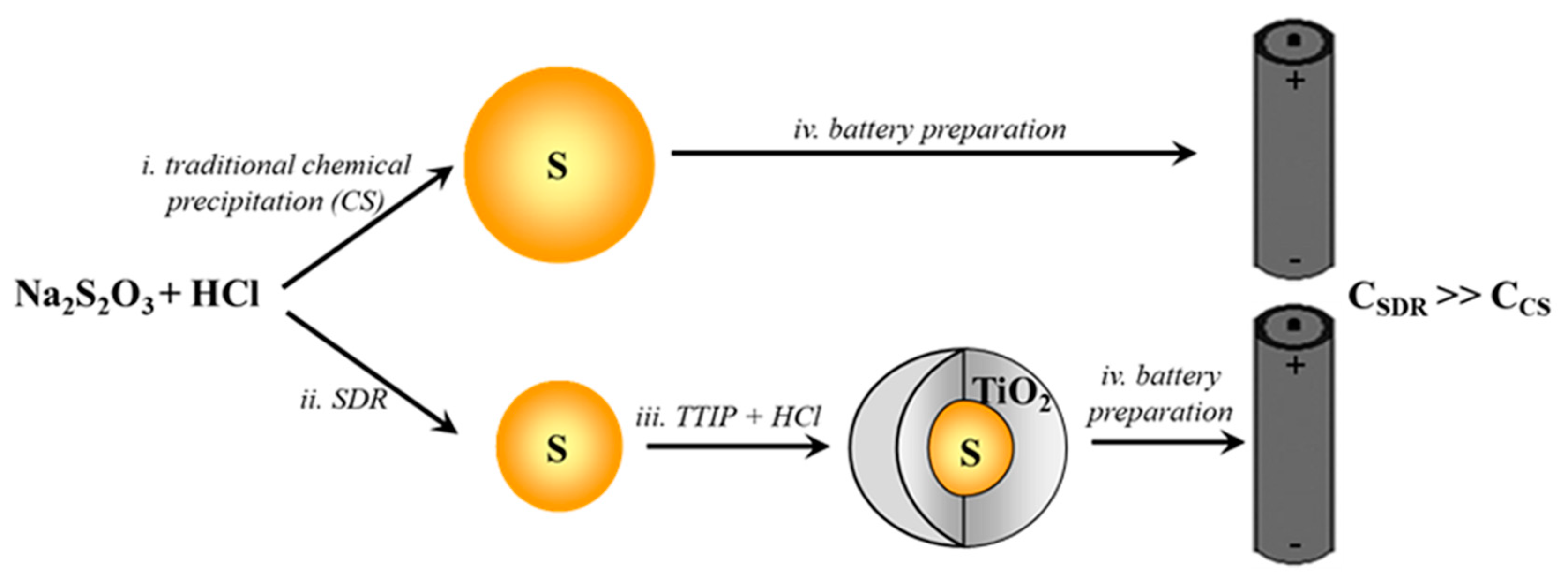
2.2.1. Sulfur Synthesis by Means of Traditional Chemical Precipitation from Aqueous Solution (CS)
2.2.2. Synthesis by Means of SDR
2.3. Core–Shell Synthesis (S–TiO2)
3. Results and Discussion
3.1. DLS Analysis
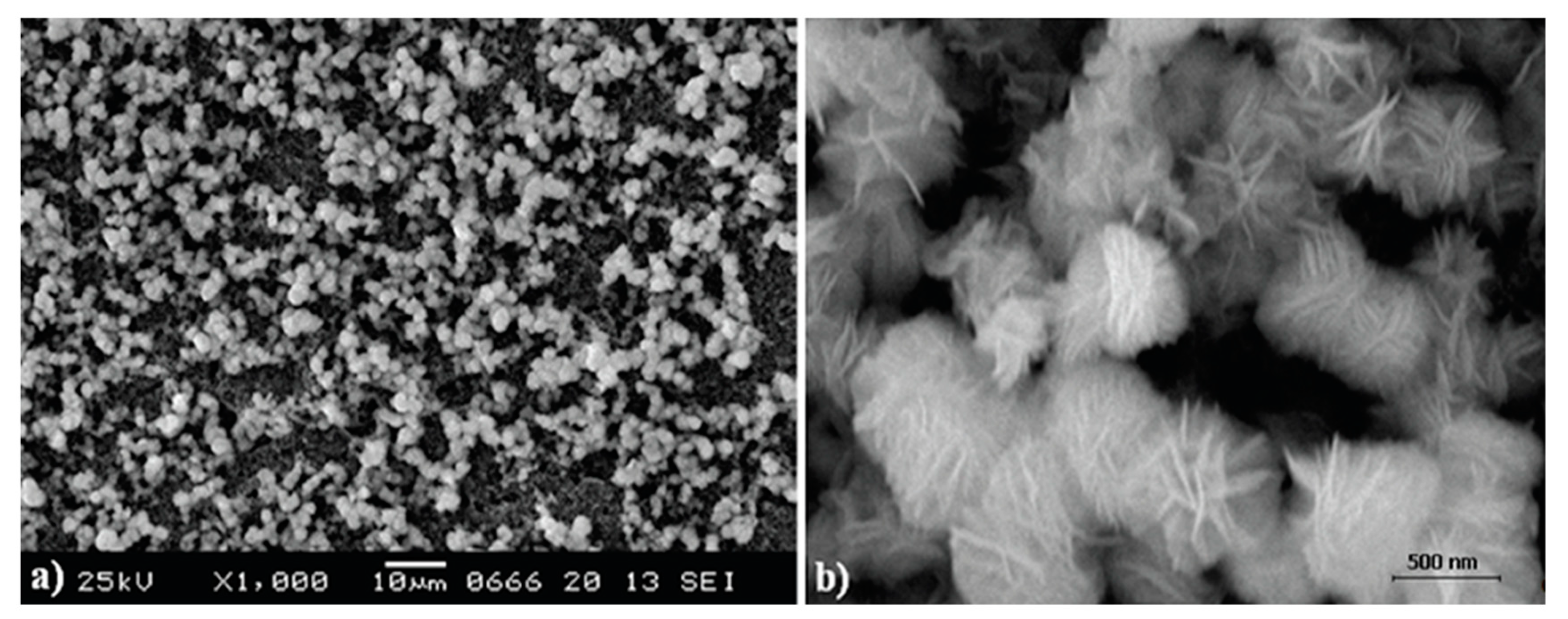
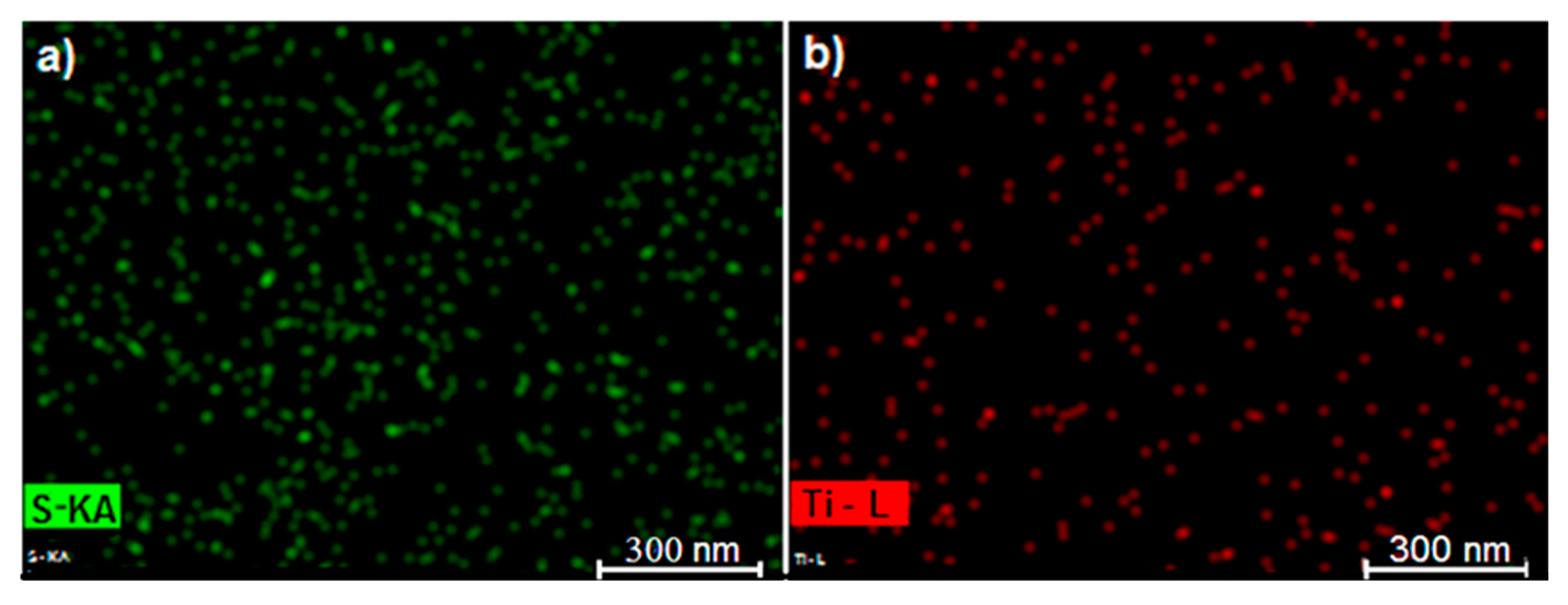
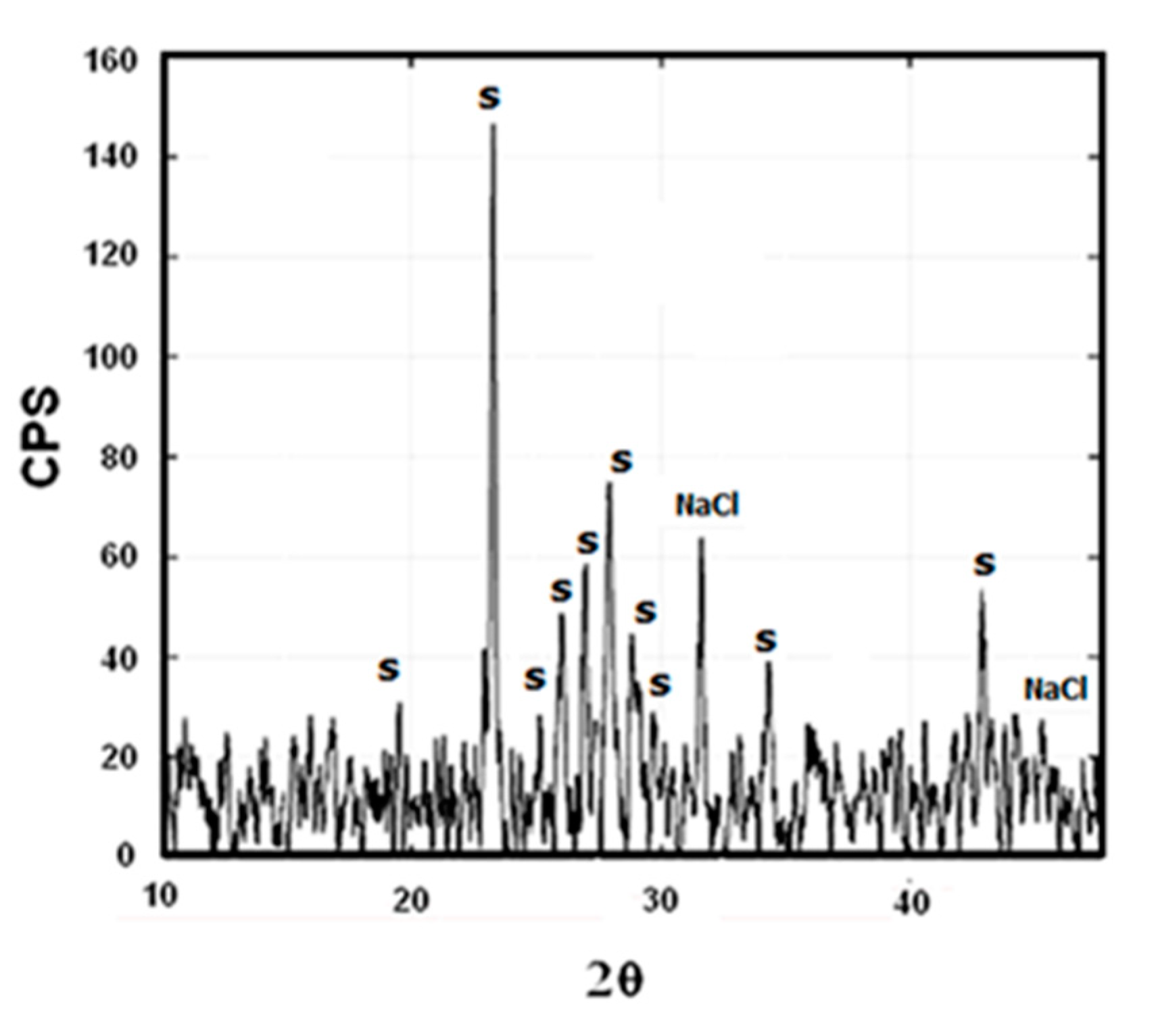
3.2. SEM and XRD Characterization
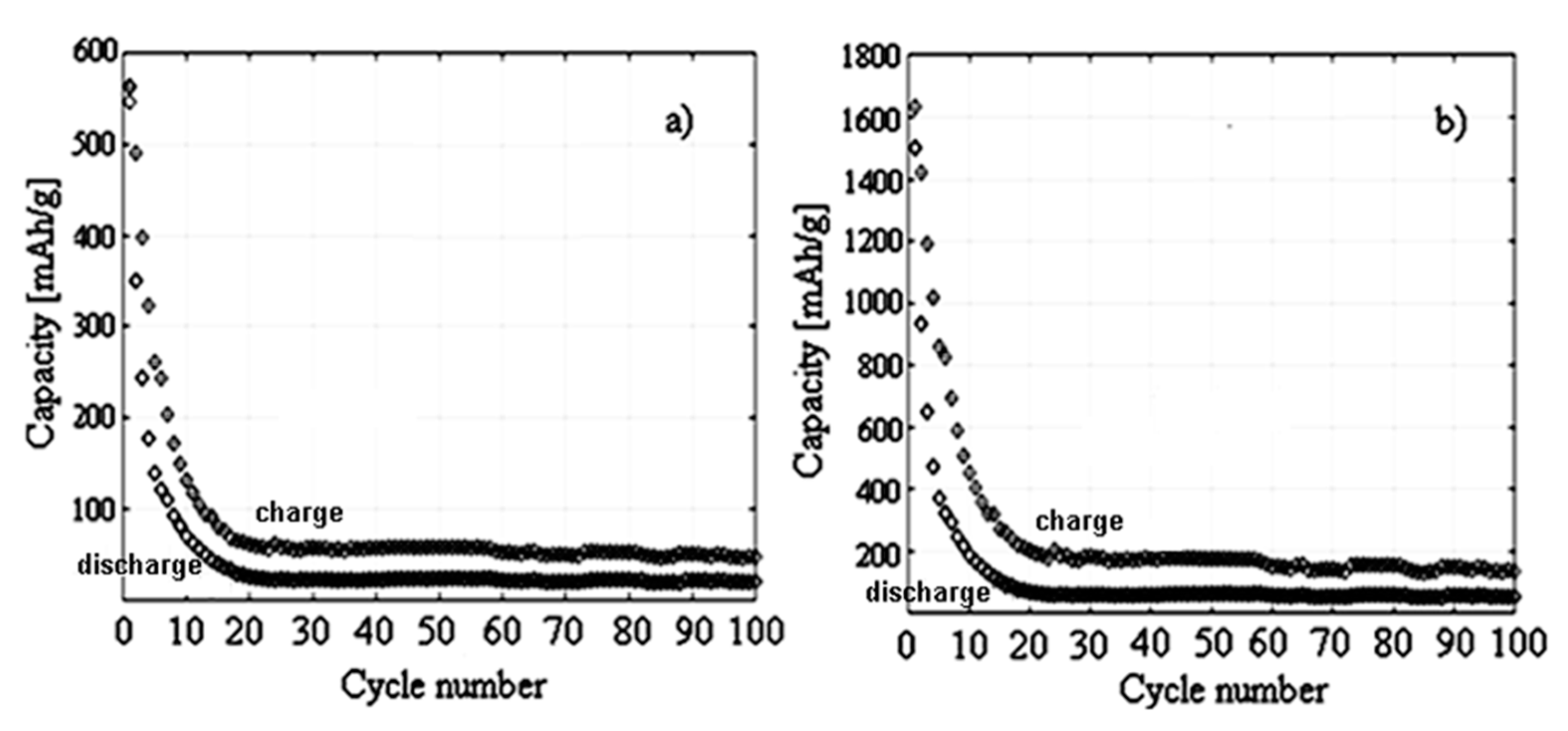
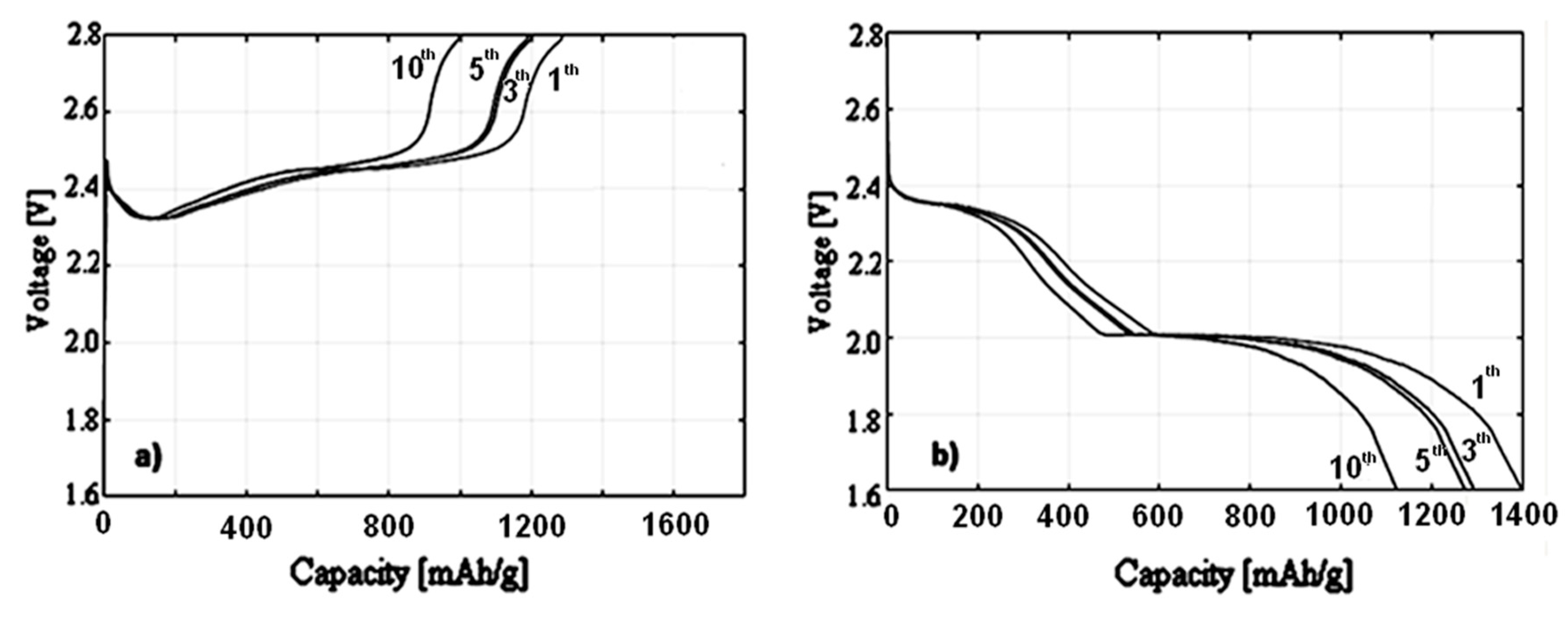
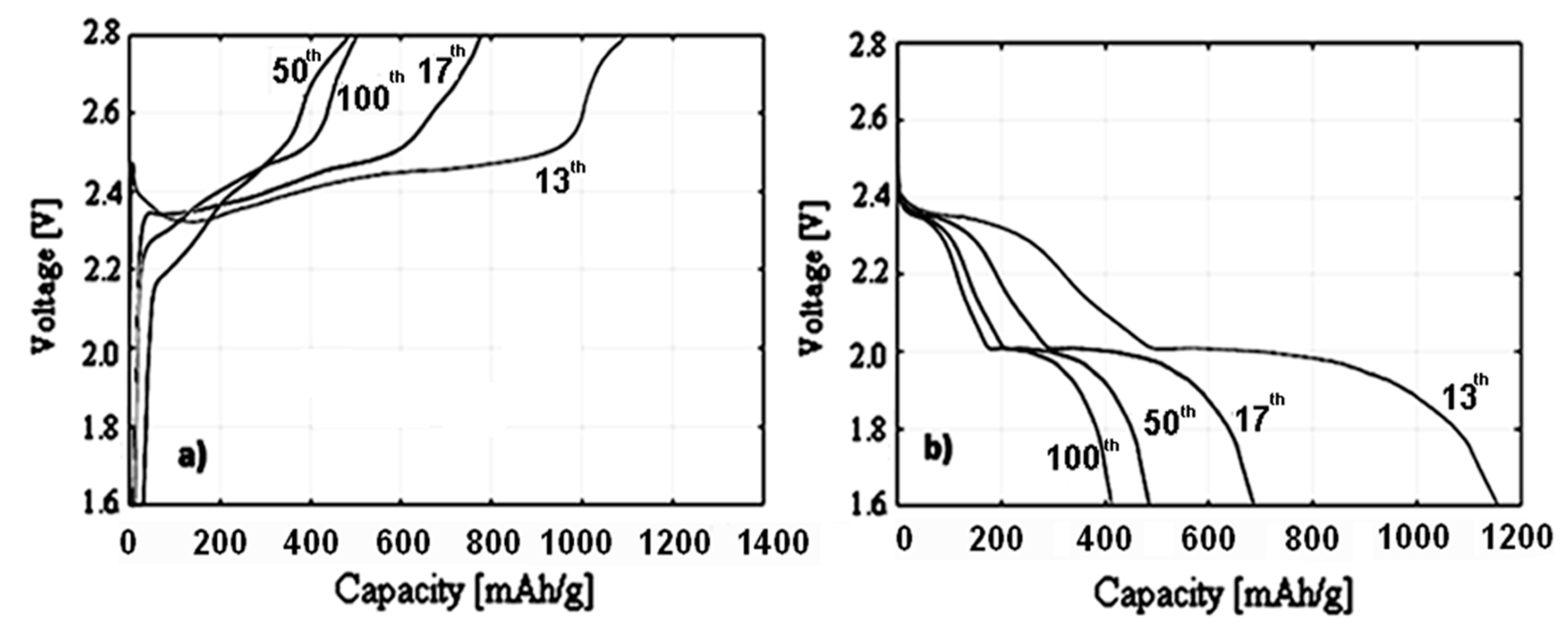
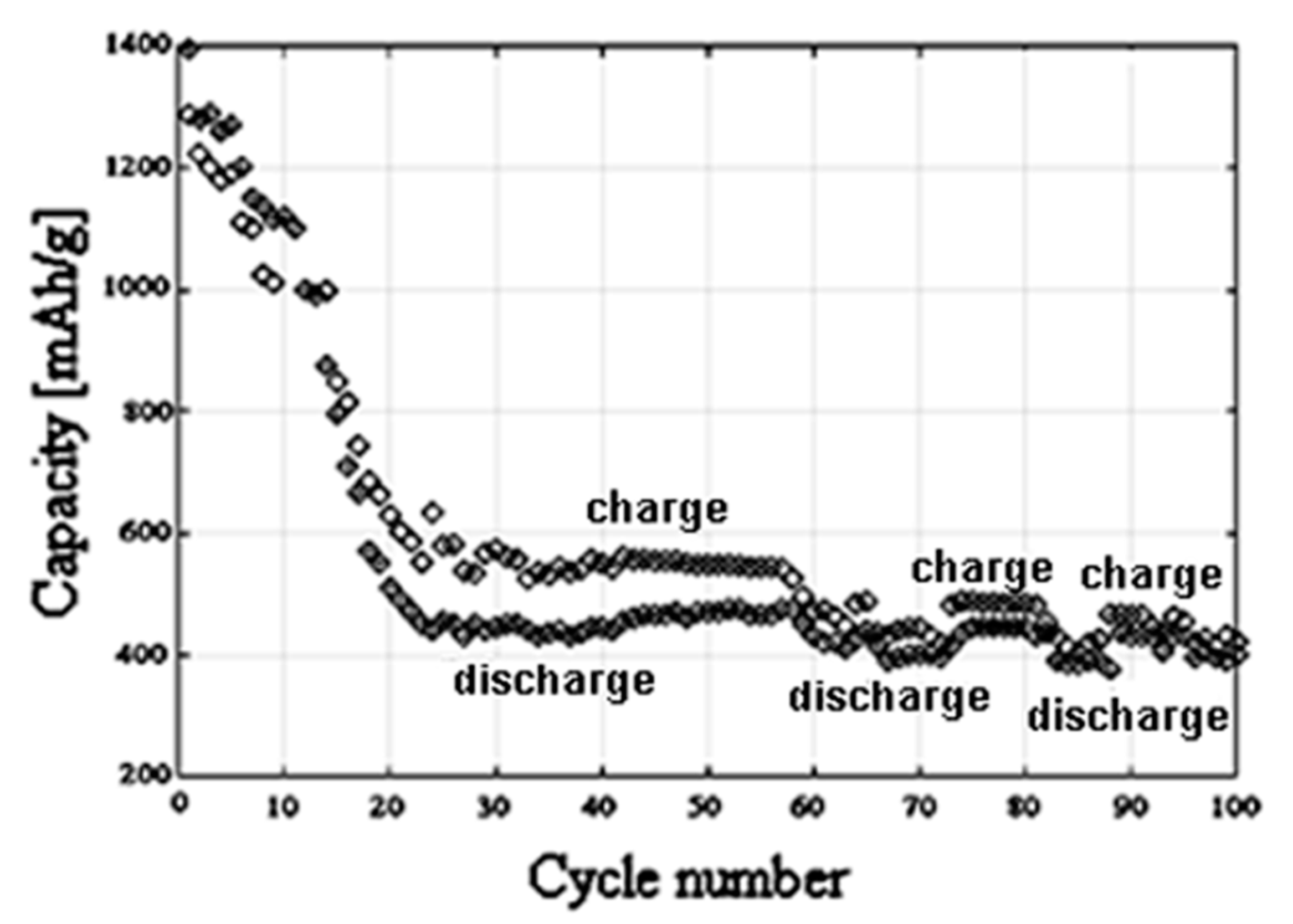
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deshpande, A.S.; Khomane, R.B.; Vaidya, B.K.; Joshi, R.M.; Harle, A.S.; Kulkarni, B.D. Sulfur Nanoparticles Synthesis and Characterization from H2S Gas, Using Novel Biodegradable Iron Chelates in W/O Microemulsion. NanoscaleRes. Lett. 2008, 3, 221–229. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, J.; Yang, S.; Yu, K.; Wang, Z.; Zhang, H. Preparation and characterization of monoclinic sulfur nanoparticles by water-in-oil microemulsions technique. Powder Technol. 2006, 162, 83–86. [Google Scholar] [CrossRef]
- Guo, Y.; Deng, Y.; Zhao, J.; Wang, Z.; Zhang, H. Synthesis and Characterization of Sulfur Nanoparticles by Liquid Phase Precipitation Method. ActaChim. Sin. 2005, 63, 337–340. [Google Scholar]
- Xie, X.Y.; Zheng, W.J.; Bai, Y.; Liu, J. Cystine modified nano-sulfur and its spectral properties. Mater. Lett. 2009, 63, 1374–1376. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Synthesis of sulfur nanoparticlesin aqueous surfactant solutions. J. Colloid Interface Sci. 2010, 343, 439–446. [Google Scholar] [CrossRef]
- Li, W.; Zheng, G.; Yang, Y.; Seh, Z.W.; Liu, N.; Cui, Y. High-performance hollow sulfur nanostructured battery cathode through a scalable, room temperature, one-step, bottom-up approach. Proc. Natl. Acad. Sci. USA 2013, 110, 7148–7153. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, M.; Ali, A.A.; Hussein, A.; Hammouti, B.; Hadda, T.B.; Warad, I. Sulfur Nanoparticles: Synthesis, Characterizations and their Applications. J. Mater. Environ. Sci. 2013, 4, 1029–1033. [Google Scholar]
- Cheng, X.; Cheng, K.; Liu, J.; Sun, X. Synthesis and characterizations of nanoparticle sulfur Using eggshell membrane as template. Mater. Sci. Forum 2011, 675–677, 279–282. [Google Scholar] [CrossRef]
- Chaudhuri, R.G.; Paria, S. Growth kinetics of sulfur nanoparticles in aqueous surfactant solutions. J. Colloid Interface Sci. 2011, 354, 563–569. [Google Scholar] [CrossRef]
- De Caprariis, B.; Di Rita, M.; Stoller, M.; Verdone, N.; Chianese, A. Reaction-precipitation by a spinning disc reactor: Influence of hydrodynamics on nanoparticles production. Chem. Eng. Sci. 2012, 76, 73–80. [Google Scholar] [CrossRef]
- De Caprariis, B.; Stoller, M.; Chianese, A.; Verdone, N. CFD model of a spinning disk reactor for nanoparticle production. Chem. Eng. Trans. 2015, 43, 757–762. [Google Scholar]
- Heng, G.; Yang, Y.; Cha, J.J.; Hong, S.S.; Cui, Y. Hollow carbon nanofiber-encapsulated sulfur cathodes for high specific capacity rechargeable lithiumbatteries. Nano Lett. 2011, 11, 4462–4467. [Google Scholar]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nano-tubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Liwen, J.; Rao, M.; Aloni, S.; Wang, L.; Cairns, E.J.; Zhang, Y. Porous carbon nanofiber-sulfur composite electrodes forlithium/sulfur cells. Energy Environ. Sci. 2011, 4, 5053–5059. [Google Scholar]
- Elazari, R.; Salitra, G.; Garsuch, A.; Panchenko, A.; Aurbach, D. Sulfur-impregnated activated carbon fiber cloth as a binder-free cathodefor rechargeable Li-S batteries. Adv. Mater. 2011, 23, 5641–5644. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Morcrette, M.; Nouar, F.; Davoisne, C.; Devic, T.; Gonbeau, D.; Dominko, R.; Serre, C.; Férey, G.; Tarascon, J.M. Cathode composites for Li-S batteries via the use of oxygenated porous architectures. J. Am. Chem. Soc. 2011, 133, 16154–16160. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, Y.; Xiao, J.; Schwenzer, B.; Engelhard, M.H.; Saraf, L.V.; Nie, Z.; Exarhos, G.J.; Liu, J. A soft approach to encapsulate sulfur: Polyaniline nanotubes for lithium-sulfur batteries with long cycle life. Adv. Mater. 2012, 24, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Liwen, J.; Rao, M.; Zheng, H.; Zhang, L.; Dua, Y.L.W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in highperformance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeablelithium-sulfur battery cathode material with high capacity and cyclingstability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Poroushollow carbon-sulfur composites for high-power lithium-sulfur batteries. Angew. Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.C.; Cui, Y. Sulphur-TiO2 yolk-shell nanoarchitecture with internal void space for long-cycle lithium-sulphur batteries. Nat. Commun. 2013, 4, 1331. [Google Scholar] [CrossRef]
- Sun, T.; King, H.E. Aggregation behavior in the semidilutepoly(N-vinyl-2-pyrrolidone)/water system. Macromolecules 1996, 29, 3175–3181. [Google Scholar] [CrossRef]
- Qiu, P.; Mao, C. Biomimetic branched hollow fibers templated byself-assembled fibrous polyvinylpyrrolidone structures in aqueous solution. ACS Nano 2010, 4, 1573–1579. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xia, Y.; Wei, M.; Lu, Y. One-step fabrication of conductive poly(3,4 ethylenedioxythiophene) hollow spheres in the presence ofpoly(vinylpyrrolidone). Synth. Met. 2009, 159, 372–376. [Google Scholar] [CrossRef]
- Aurbach, D.; Pollak, E.; Elazari, R.; Salitra, G.; Kelley, C.S.; Affinito, J. On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries. J. Electrochem. Soc. 2009, 156, A694–A702. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Bauer, E.M.; Ciprioti Vecchio, S.; Scaramuzzo, F.A.; Lupi, C. Synthesis, characterization, and electrochemical behavior of LiMnxFe(1-x)PO4 composites obtained from phenylphosphonate-based organic-inorganic hybrids. Materials 2018, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Parola, V.; Liveri, V.T.; Todaro, L.; Lombardo, D.; Bauer, E.M.; Dell’Era, A.; Longo, A.; Caschera, D.; De Caro, T.; Toro, R.G.; et al. Iron and lithium-iron alkyl phosphates as nanostructured material for rechargeable batteries. Mater. Lett. 2018, 220, 58–61. [Google Scholar] [CrossRef]
- Dell’Era, A.; Pasquali, M.; Tarquini, G.; Scaramuzzo, F.A.; De Gasperis, P.; Prosini, P.P.; Mezzi, A.; Tuffi, R.; Cafiero, L. Carbon powder material obtained from an innovative high pressure water jet recycling process of tires used as anode in alkali ion (Li, Na) batteries. Solid State Ionics 2018, 324, 20–27. [Google Scholar] [CrossRef]
- Dell’Era, A.; Mura, F.; Pasquali, M.; Pozio, A.; Zaza, F. Synthesis and characterization of TiO2 nanotubes as anodic material in lithium-ion batteries. Nuovo CimentoSoc. Ital. Fis. C 2013, 36, 65–72. [Google Scholar]
- Pasquali, M.; Dell’Era, A.; Prosini, P.P. Fitting of the Voltage-Li+ insertion curve of LiFePO4. J. Solid State Electrochem. 2009, 13, 1859–1865. [Google Scholar] [CrossRef]
- Orecchini, F.; Santiangeli, A.; Dell’Era, A. EVs and HEVs Using Lithium-Ion Batteries. In Lithium-Ion Batteries: Advances and Applications; Pistoia, G., Ed.; Elsevier, B.V.: Amsterdam, The Netherlands, 2014; pp. 205–248. [Google Scholar]
- Scaramuzzo, F.A.; Pasquali, M.; Mura, F.; Pozio, A.; Dell’Era, A.; Curulli, A. TiO2 nanotubes photo-anode: An innovative cell design. Chem. Eng. Trans. 2014, 41, 223–228. [Google Scholar]
- Scaramuzzo, F.A.; Pozio, A.; Masci, A.; Mura, F.; Dell’Era, A.; Curulli, A.; Pasquali, M. Efficientphotocurrent generation using a combined Ni-TiO2 nanotubesanode. J. Appl. Electrochem. 2015, 45, 727–733. [Google Scholar] [CrossRef]
- Scaramuzzo, F.A.; Dell’Era, A.; Tarquini, G.; Caminiti, R.; Ballirano, P.; Pasquali, M. Phasetransition of TiO2nanotubes: A X-ray study as a function of temperature. J. Phys. Chem. C 2017, 121, 24871–24876. [Google Scholar] [CrossRef]
- Santiangeli, A.; Fiori, C.; Zuccari, F.; Dell’Era, A.; Orecchini, F.; D’Orazio, A. Experimental analysis of the auxiliaries consumption in the Energy balance of a pre-series plug-in hybrid-electric vehicle. Energy Procedia 2014, 45, 779–788. [Google Scholar]
- Pasquali, M.; Dell’Era, A.; Bauer, E.M.; Bellitto, C.; Righini, G.; Prosini, P.P. A versatile method of preparation of Carbon-rich LiFePO4: A promising cathode material for Li-ion batteries. J. Power Sources 2005, 146, 544–549. [Google Scholar] [CrossRef]
- Turganbay, S.; Aidarova, S.B.; Bekturganova, N.E.; Alimbekova, G.K.; Musabekov, K.B.; Kumargalieva, S.S. Surface-modification of sulfur nanoparticle with surfactants and application in agriculture. Adv. Mater. Res. 2013, 785–786, 475–479. [Google Scholar] [CrossRef]
- Martynková, G.S.; Chrobáček, K.; Pazourková, L.; Aydina, S.; Pivoňka, D.; Novosad, B. Sulfur cathode material conductivity improving by carbons admixture: Technology and characterization. Mater. Today Proc. 2018, 5, S109–S114. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable Lithium-Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporouspolysulde sorbents for the Li-S battery. J. Phys. Chem. C 2012, 116, 19653–19658. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Era, A.; Scaramuzzo, F.A.; Stoller, M.; Lupi, C.; Rossi, M.; Passeri, D.; Pasquali, M. Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2Core–Shell Powder for Lithium Batteries. Appl. Sci. 2019, 9, 1913. https://doi.org/10.3390/app9091913
Dell’Era A, Scaramuzzo FA, Stoller M, Lupi C, Rossi M, Passeri D, Pasquali M. Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2Core–Shell Powder for Lithium Batteries. Applied Sciences. 2019; 9(9):1913. https://doi.org/10.3390/app9091913
Chicago/Turabian StyleDell’Era, Alessandro, Francesca A. Scaramuzzo, Marco Stoller, Carla Lupi, Marco Rossi, Daniele Passeri, and Mauro Pasquali. 2019. "Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2Core–Shell Powder for Lithium Batteries" Applied Sciences 9, no. 9: 1913. https://doi.org/10.3390/app9091913
APA StyleDell’Era, A., Scaramuzzo, F. A., Stoller, M., Lupi, C., Rossi, M., Passeri, D., & Pasquali, M. (2019). Spinning Disk Reactor Technique for the Synthesis of Nanometric Sulfur TiO2Core–Shell Powder for Lithium Batteries. Applied Sciences, 9(9), 1913. https://doi.org/10.3390/app9091913









