Nanosized Ni/SBA-15 Catalysts for CO2 Reforming of CH4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalytic Testing
2.3. Catalyst Characterization
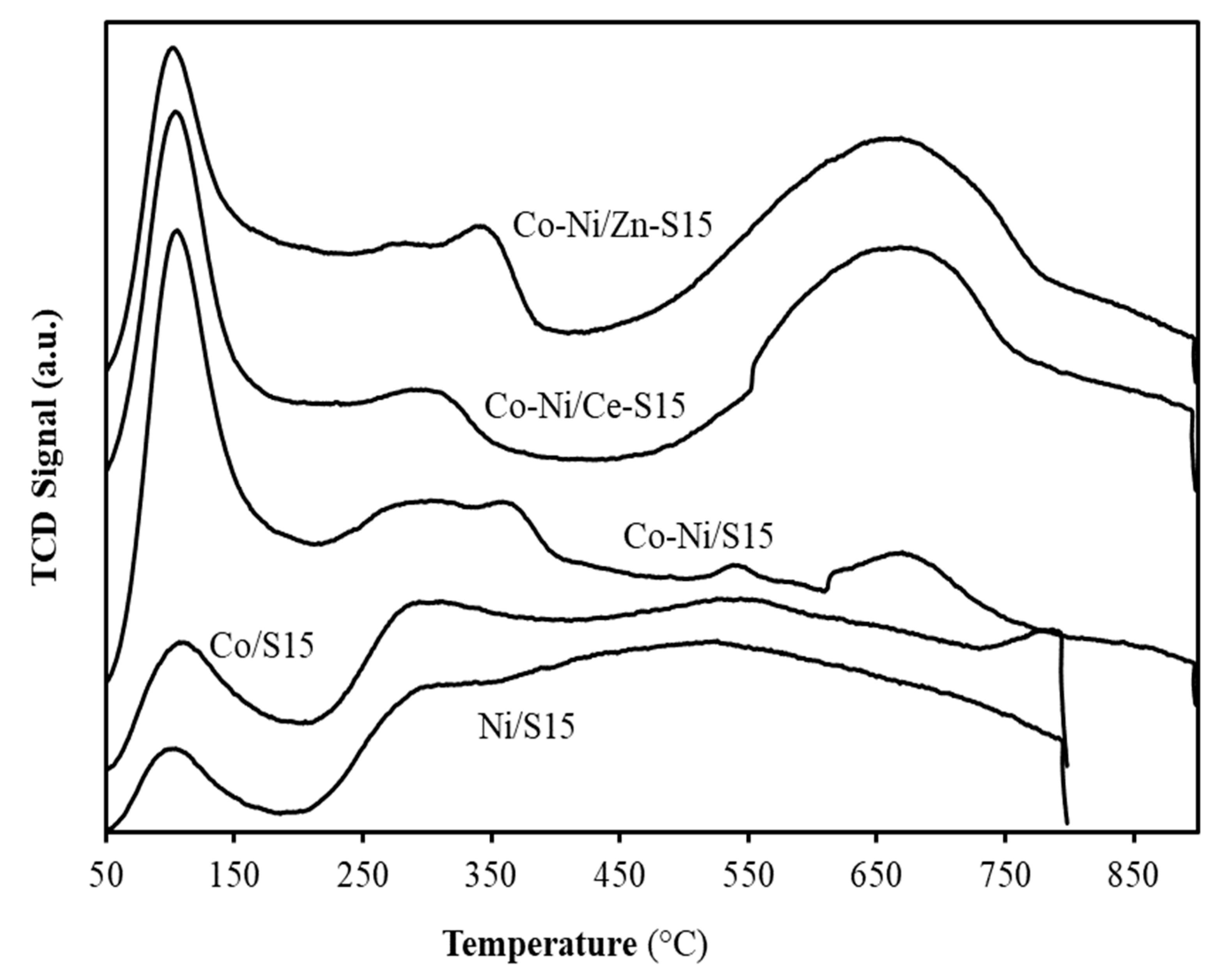
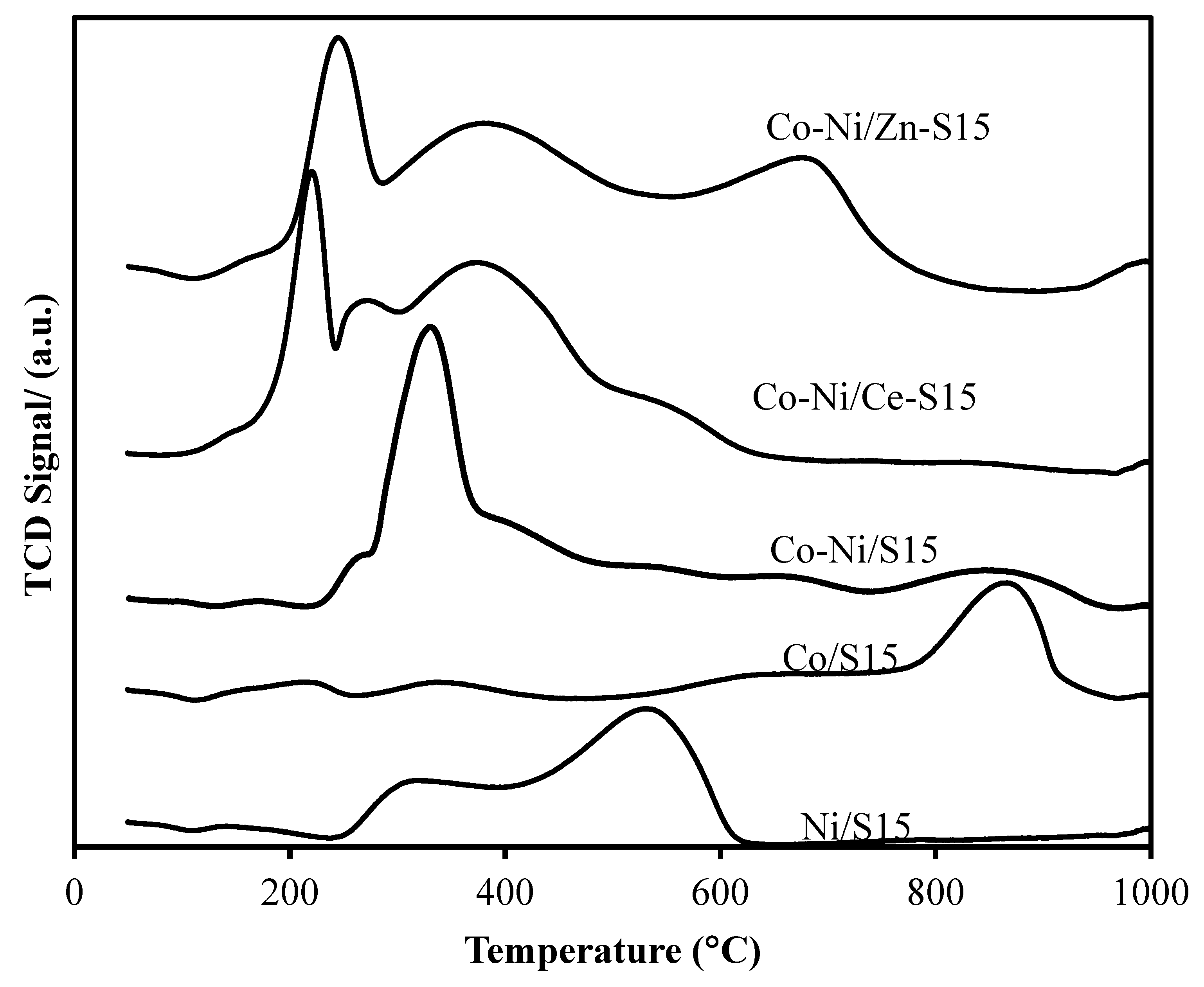
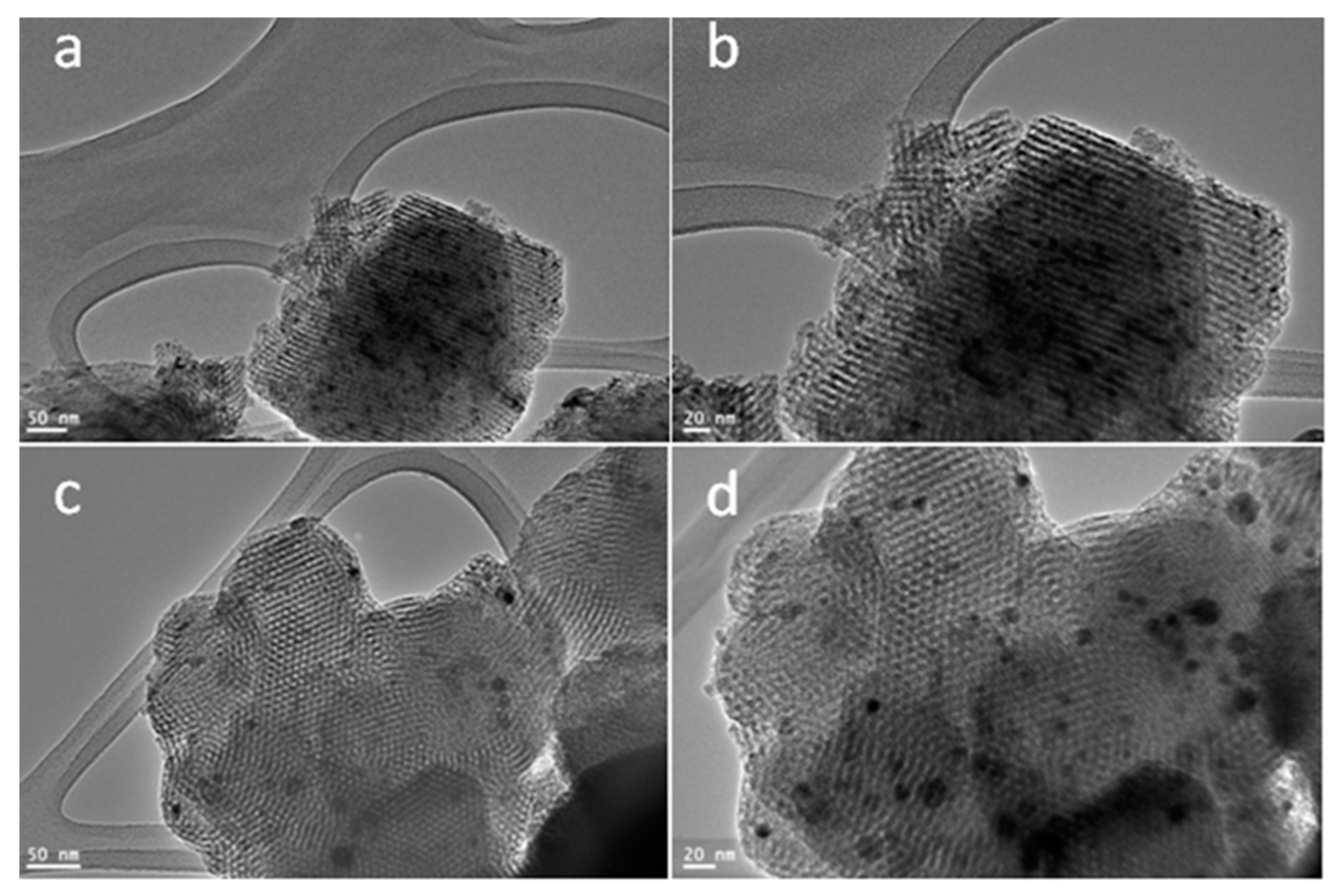
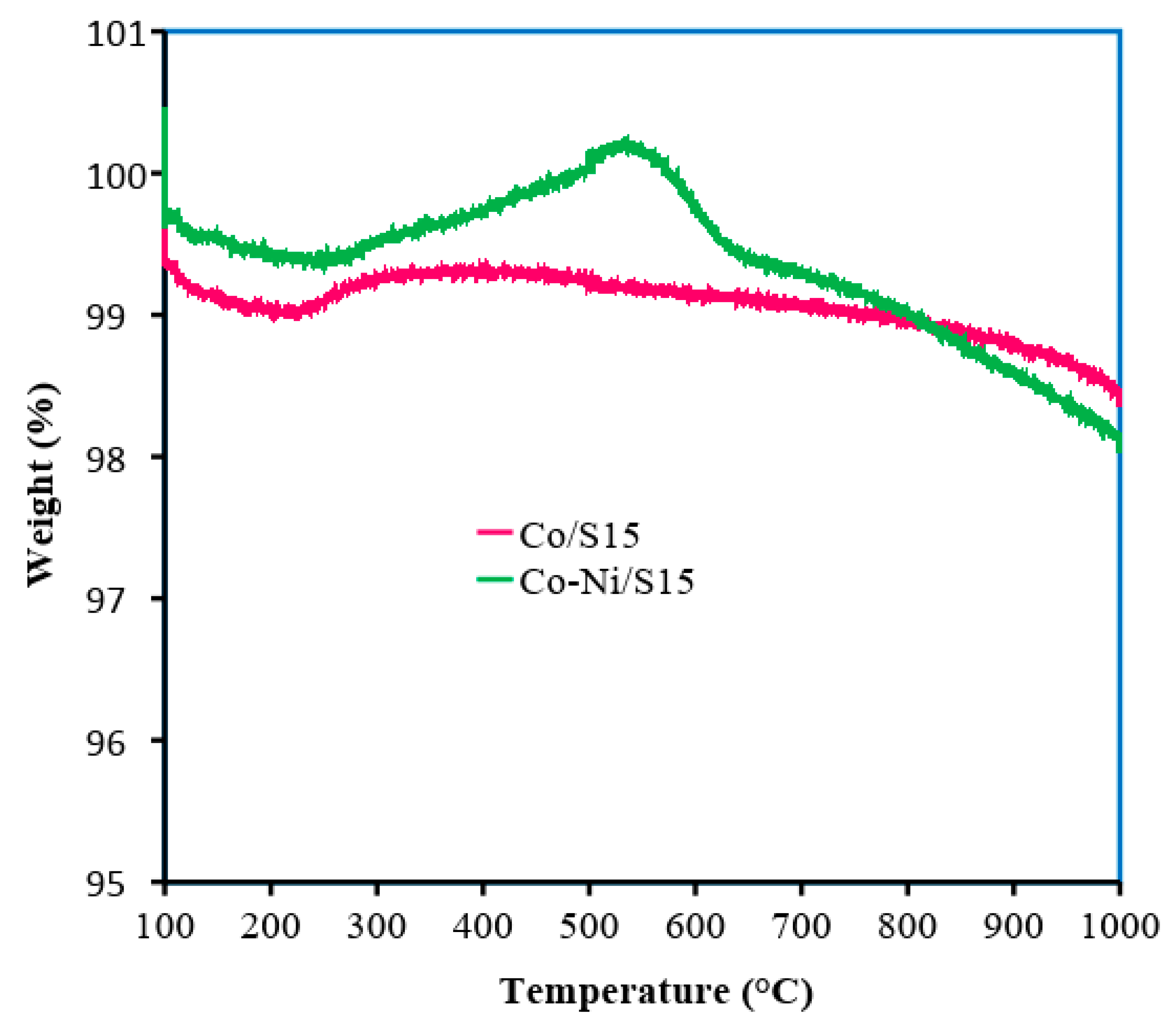
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abasaeed, A.E.; Al-Fatesh, A.S.; Naeem, M.A.; Ibrahim, A.A.; Fakeeha, A.H. Catalytic performance of CeO2 and ZrO2 supported Co catalysts for hydrogen production via dry reforming of methane. Int. J. Hydrogen Energy 2015, 40, 6818–6826. [Google Scholar] [CrossRef]
- Amin, A.; Epling, W.; Croiset, E. Reaction and Deactivation Rates of Methane Catalytic Cracking over Nickel. Ind. Eng. Chem. Res. 2011, 50, 12460–12470. [Google Scholar] [CrossRef]
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 1–17. [Google Scholar] [CrossRef]
- Benguerba, Y.; Dehimi, L.; Virginie, M.; Dumas, C.; Ernst, B. Modelling of methane dry reforming over Ni/Al2O3 catalyst in a fixed-bed catalytic reactor. React. Kinet. Mech. Catal. 2015, 114, 109–119. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Albarazi, A.; Beaunier, P.; Da Costa, P. Hydrogen and syngas production by methane dry reforming on SBA-15 supported nickel catalysts: On the effect of promotion by Ce0.75Zr0.25O2 mixed oxide. Int. J. Hydrogen Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. The Effect of Sc Promoter on the Performance of Co/TiO2–P25 Catalyst in Dry Reforming of Methane. Bull. Korean Chem. Soc. 2015, 36, 2081–2088. [Google Scholar] [CrossRef]
- Fakeeha, A.; Naeem, M.; Abasaeed, A.; Ibrahim, A.; Khan, W.; Alfatesh, A. Coke suppression by holmium promoter in dry reforming of methane. J. Chem. Soc. Pak. 2016, 38, 388–397. [Google Scholar]
- Al-Fatesh, A.S.A.; Fakeeha, A.H.; Abasaeed, A.E. Effects of Selected Promoters on Ni/Y-Al2O3 Catalyst Performance in Methane Dry Reforming. Chin. J. Catal. 2011, 32, 1604–1609. [Google Scholar] [CrossRef]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Synthesis, characterization and catalytic performances of Ce-SBA-15 supported nickel catalysts for methane dry reforming to hydrogen and syngas. Int. J. Hydrogen Energy 2012, 37, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatesh, A.S. Promotional effect of Gd over Ni/Y2O3 catalyst used in dry reforming of CH4 for H2 production. Int. J. Hydrogen Energy 2017, 42, 18805–18816. [Google Scholar] [CrossRef]
- De Araujo, G.C.; De Lima, S.M.; Assaf, J.M.; Pena, M.A.; Fierro, J.L.G.; Do Carmo Rangel, M. Catalytic Evaluation of Perovskite-type Oxide LaNi1−xRuxO3 in Methane Dry Reforming. Catal. Today 2008, 133–135, 129–135. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Liu, Z.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.-D.; Zhou, Y.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C–H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef]
- Rivero-Mendoza, D.; Stanley, J.; Scott, J.; Aguey-Zinsou, K.-F. An Alumina-Supported Ni-La-Based Catalyst for Producing Synthetic Natural Gas. Catalysts 2016, 6, 170. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Wang, N.; Shen, K.; Huang, L.; Yu, X.; Qian, W.; Chu, W. Facile Route for Synthesizing Ordered Mesoporous Ni–Ce–Al Oxide Materials and Their Catalytic Performance for Methane Dry Reforming to Hydrogen and Syngas. ACS Catal. 2013, 3, 1638–1651. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Naeem, M.A.; Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E. Production of Synthesis Gas via Dry Reforming of Methane over Co-Based Catalysts: Effect on H2/CO Ratio and Carbon Deposition. Chem. Eng. Technol. 2015, 38, 1397–1405. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; González, M.G.; Montes, M. Characterization of Ni/SiO2 and Ni/Li-SiO2 catalysts for methane dry reforming. Catal. Today 2005, 107–108, 856–862. [Google Scholar] [CrossRef]
- Wu, H.; La Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.; Liotta, L. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef] [Green Version]
- Chiou, J.Y.Z.; Yang, S.-Y.; Lai, C.-L.; Kung, H.-Y.; Tang, C.-W.; Wang, C.-B. Steam Reforming of Ethanol over CoMg/SBA-15 Catalysts. Mod. Res. Catal. 2013, 2, 9. [Google Scholar] [CrossRef]
- Ozkara-Aydınoglu, S.; Ozensoy, E.; Aksoylu, A.E. The effect of impregnation strategy on methane dry reforming activity of Ce promoted Pt/ZrO2. Int. J. Hydrogen Energy 2009, 34, 9711–9722. [Google Scholar] [CrossRef]
- Lovell, E.; Scott, J.; Amal, R. Ni-SiO2 Catalysts for the Carbon Dioxide Reforming of Methane: Varying Support Properties by Flame Spray Pyrolysis. Molecules 2015, 20, 4594–4609. [Google Scholar] [CrossRef]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.-S. Manganese promoting effects on the Co–Ce–Zr–Ox nano catalysts for methane dry reforming with carbon dioxide to hydrogen and carbon monoxide. Chem. Eng. J. 2011, 170, 457–463. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Wang, Y.; Chu, W.; Liu, M. A comparison study on methane dry reforming with carbon dioxide over LaNiO3 perovskite catalysts supported on mesoporous SBA-15, MCM-41 and silica carrier. Catal. Today 2013, 212, 98–107. [Google Scholar] [CrossRef]
- Courson, C.; Udron, L.; Świerczyński, D.; Petit, C.; Kiennemann, A. Hydrogen production from biomass gasification on nickel catalysts: Tests for dry reforming of methane. Catal. Today 2002, 76, 75–86. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Effect of NiAl2O4 Formation on Ni/Al2O3 Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2508–2516. [Google Scholar] [CrossRef]
- Shanmugam, V.; Zapf, R.; Neuberg, S.; Hessel, V.; Kolb, G. Effect of ceria and zirconia promotors on Ni/SBA-15 catalysts for coking and sintering resistant steam reforming of propylene glycol in microreactors. Appl. Catal. B Environ. 2017, 203, 859–869. [Google Scholar] [CrossRef]
- Phan, B.M.Q.; Ha, Q.L.M.; Le, N.P.; Ngo, P.T.; Nguyen, T.H.; Dang, T.T.; Nguyen, L.H.; Nguyen, D.A.; Luu, L.C. Influences of Various Supports, γ-Al2O3, CeO2, and SBA-15 on HDO Performance of NiMo Catalyst. Catal. Lett. 2015, 145, 662–667. [Google Scholar] [CrossRef]
- Jabbour, K.; El Hassan, N.; Davidson, A.; Casale, S.; Massiani, P. Factors affecting the long-term stability of mesoporous nickel-based catalysts in combined steam and dry reforming of methane. Catal. Sci. Technol. 2016, 6, 4616–4631. [Google Scholar] [CrossRef]
- Vizcaino, A.J.; Carrero, A.; Calles, J.A. Comparison of ethanol steam reforming using Co and Ni catalysts supported on SBA-15 modified by Ca and Mg. Fuel Process. Technol. 2016, 146, 99–109. [Google Scholar] [CrossRef]
- Abee, M.W.; York, S.C.; Cox, D.F. CO2 adsorption on α-Cr2O3 (101̄2) Surfaces. J. Phys. Chem. B 2001, 105, 7755–7761. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Sc promoted and aerogel confined Ni catalysts for coking-resistant dry reforming of methane. RSC Adv. 2017, 7, 4735–4745. [Google Scholar] [CrossRef] [Green Version]
- Konsolakis, M.; Ioakimidis, Z.; Kraia, T.; Marnellos, G.E. Hydrogen Production by Ethanol Steam Reforming (ESR) over CeO2 Supported Transition Metal (Fe, Co, Ni, Cu) Catalysts: Insight into the Structure-Activity Relationship. Catalysts 2016, 6, 39. [Google Scholar] [CrossRef]
- Zhong, S.; Liang, L.; Liu, M.; Liu, B.; Sun, J. DMF and mesoporous Zn/SBA-15 as synergistic catalysts for the cycloaddition of CO2 to propylene oxide. J. CO2 Util. 2015, 9, 58–65. [Google Scholar] [CrossRef]




| Catalyst | Surface Area (m2/g) |
|---|---|
| S15 | 819 |
| Ni/S15 | 547 |
| Co/S15 | 532 |
| Co–Ni/S15 | 549 |
| Co–Ni/Ce–S15 | 551 |
| Co–Ni/Zn–S15 | 548 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Ibrahim, A.; Amin, A.; S. Al-Fatesh, A.; Siva Kumar, N.; Olajide Kasim, S.; S. Al-Awadi, A.; M. El-Toni, A.; Elhag Abasaeed, A.; H. Fakeeha, A. Nanosized Ni/SBA-15 Catalysts for CO2 Reforming of CH4. Appl. Sci. 2019, 9, 1926. https://doi.org/10.3390/app9091926
A. Ibrahim A, Amin A, S. Al-Fatesh A, Siva Kumar N, Olajide Kasim S, S. Al-Awadi A, M. El-Toni A, Elhag Abasaeed A, H. Fakeeha A. Nanosized Ni/SBA-15 Catalysts for CO2 Reforming of CH4. Applied Sciences. 2019; 9(9):1926. https://doi.org/10.3390/app9091926
Chicago/Turabian StyleA. Ibrahim, Ahmed, Ashraf Amin, Ahmed S. Al-Fatesh, Nadavala Siva Kumar, Samsudeen Olajide Kasim, Abdulrhman S. Al-Awadi, Ahmed M. El-Toni, Ahmed Elhag Abasaeed, and Anis H. Fakeeha. 2019. "Nanosized Ni/SBA-15 Catalysts for CO2 Reforming of CH4" Applied Sciences 9, no. 9: 1926. https://doi.org/10.3390/app9091926







