Participatory Art Activities Increase Salivary Oxytocin Secretion of ASD Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics Statement
2.3. Visual Art-Based Participatory Art Workshop
2.4. Assessment
2.5. Saliva Collection and Analysis
2.6. Statistical Analysis
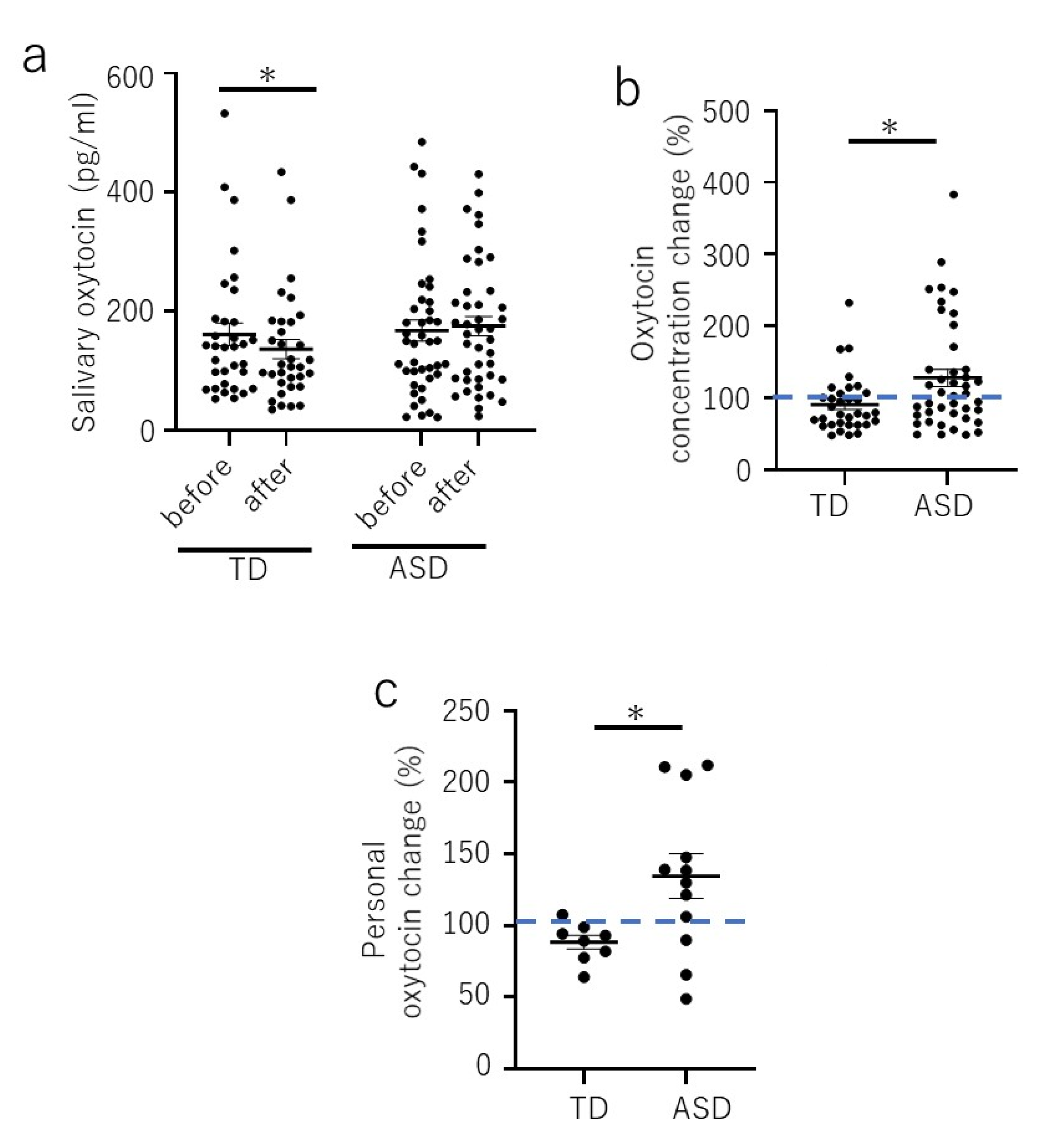
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcin, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Geschwind, D.H. Advances in autism. Annu. Rev. Med. 2009, 60, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Hellhammer, D.H.; Wust, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Joels, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef]
- Taylor, J.L.; Corbett, B.A. A review of rhythm and responsiveness of cortisol in individuals with autism spectrum disorders. Psychoneuroendocrinology 2014, 49, 207–228. [Google Scholar] [CrossRef] [Green Version]
- Shirtcliff, E.A.; Essex, M.J. Concurrent and Longitudinal Associations of Basal and Diurnal Cortisol with Mental Health Symptoms in Early Adolescence. Dev. Psychobiol. 2008, 50, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Modi, M.E.; Young, L.J. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm. Behav. 2012, 61, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Tabbaa, M.; Paedae, B.; Liu, Y.; Wang, Z. Neuropeptide Regulation of Social Attachment: The Prairie Vole Model. Compr. Physiol. 2016, 7, 81–104. [Google Scholar] [CrossRef] [Green Version]
- Feldman, R. Oxytocin and social affiliation in humans. Horm. Behav. 2012, 61, 380–391. [Google Scholar] [CrossRef]
- Feldman, R.; Gordon, I.; Schneiderman, I.; Weisman, O.; Zagoory-Sharon, O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology 2010, 35, 1133–1141. [Google Scholar] [CrossRef]
- Andari, E.; Hurlemann, R.; Young, L.J. A Precision Medicine Approach to Oxytocin Trials. Curr. Top. Behav. Neurosci. 2018, 35, 559–590. [Google Scholar] [CrossRef]
- Domes, G.; Heinrichs, M.; Michel, A.; Berger, C.; Herpertz, S.C. Oxytocin improves “mind-reading” in humans. Biol. Psychiatry 2007, 61, 731–733. [Google Scholar] [CrossRef]
- Savaskan, E.; Ehrhardt, R.; Schulz, A.; Walter, M.; Schachinger, H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 2008, 33, 368–374. [Google Scholar] [CrossRef]
- Yamasue, H.; Domes, G. Oxytocin and Autism Spectrum Disorders. Curr. Top. Behav. Neurosci. 2018, 35, 449–465. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Zak, P.J.; Stanton, A.A.; Ahmadi, S. Oxytocin increases generosity in humans. PLoS ONE 2007, 2, e1128. [Google Scholar] [CrossRef]
- Young, L.J.; Barrett, C.E. Neuroscience. Can oxytocin treat autism? Science 2015, 347, 825–826. [Google Scholar] [CrossRef] [Green Version]
- Gulliver, D.; Werry, E.; Reekie, T.A.; Katte, T.A.; Jorgensen, W.; Kassiou, M. Targeting the Oxytocin System: New Pharmacotherapeutic Approaches. Trends Pharmacol. Sci. 2019, 40, 22–37. [Google Scholar] [CrossRef]
- Fancourt, D.; Finn, S. What is the Evidence on the Role of the Arts in Improving Health and Well-Being? A Scoping Review; (Health Evidence Network (HEN) Synthesis Report 67); WHO Regional Office for Europe: Copenhagen, Denmark, 2019. [Google Scholar]
- Hacking, S.; Secker, J.; Kent, L.; Shenton, J.; Spandler, H. Mental health and arts participation: The state of the art in England. J. R Soc. Promot. Health 2006, 126, 121–127. [Google Scholar] [CrossRef]
- Bone, T.A. Art and Mental Health Recovery: Evaluating the Impact of a Community-Based Participatory Arts Program through Artist Voices. Community Ment Health J. 2018, 54, 1180–1188. [Google Scholar] [CrossRef]
- Hacking, S.; Secker, J.; Spandler, H.; Kent, L.; Shenton, J. Evaluating the impact of participatory art projects for people with mental health needs. Health Soc. Care Community 2008, 16, 638–648. [Google Scholar] [CrossRef]
- Stickley, T.; Wright, N.; Slade, M. The art of recovery: Outcomes from participatory arts activities for people using mental health services. J. Ment. Health 2018, 27, 367–373. [Google Scholar] [CrossRef]
- Bickerdike, L.; Booth, A.; Wilson, P.M.; Farley, K.; Wright, K. Social prescribing: Less rhetoric and more reality. A systematic review of the evidence. BMJ Open 2017, 7, e013384. [Google Scholar] [CrossRef]
- Toma, M.; Morris, J.; Kelly, C.; Jinal-Snape, D. The Impact of Art Attendance and Participation on Health and Wellbeing: Systematic Literature Review (Work Package 1); Glasgow Centre for Population Health: Glasgow, UK, 2014. [Google Scholar]
- Hatakenaka, Y.; Fernell, E.; Sakaguchi, M.; Ninomiya, H.; Fukunaga, I.; Gillberg, C. ESSENCE-Q-a first clinical validation study of a new screening questionnaire for young children with suspected neurodevelopmental problems in south Japan. Neuropsychiatr. Dis. Treat. 2016, 12, 1739–1746. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, S.; Yuhi, T.; Furuhara, K.; Ohta, S.; Shimizu, Y.; Higashida, H. Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: A pilot study. Front. Psychiatry 2015, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- MacLean, E.L.; Gesquiere, L.R.; Gee, N.; Levy, K.; Martin, W.L.; Carter, C.S. Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods 2018, 293, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Yuhi, T.; Ise, K.; Iwashina, K.; Terao, N.; Yoshioka, S.; Shomura, K.; Maehara, T.; Yazaki, A.; Koichi, K.; Furuhara, K.; et al. Sex Differences in Salivary Oxytocin and Cortisol Concentration Changes during Cooking in a Small Group. Behav. Sci. 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Kumazaki, H.; Warren, Z.; Corbett, B.A.; Yoshikawa, Y.; Matsumoto, Y.; Higashida, H.; Yuhi, T.; Ikeda, T.; Ishiguro, H.; Kikuchi, M. Android Robot-Mediated Mock Job Interview Sessions for Young Adults with Autism Spectrum Disorder: A Pilot Study. Front. Psychiatry 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Nishizato, M.; Fujisawa, T.X.; Kosaka, H.; Tomoda, A. Developmental changes in social attention and oxytocin levels in infants and children. Sci. Rep. UK 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Guillon, Q.; Hadjikhani, N.; Baduel, S.; Roge, B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neurosci. Biobehav. Rev. 2014, 42, 279–297. [Google Scholar] [CrossRef]
- Case-Smith, J.; Weaver, L.L.; Fristad, M.A. A systematic review of sensory processing interventions for children with autism spectrum disorders. Autism 2015, 19, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Schladt, T.M.; Nordmann, G.C.; Emilius, R.; Kudielka, B.M.; de Jong, T.R.; Neumann, I.D. Choir versus Solo Singing: Effects on Mood, and Salivary Oxytocin and Cortisol Concentrations. Front. Hum. Neurosci. 2017, 11, 430. [Google Scholar] [CrossRef] [Green Version]
- Jong, T.R.; Menon, R.; Bludau, A.; Grund, T.; Biermeier, V.; Klampfl, S.M.; Jurek, B.; Bosch, O.J.; Hellhammer, J.; Neumann, I.D. Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: The Regensburg Oxytocin Challenge (ROC) study. Psychoneuroendocrinology 2015, 62, 381–388. [Google Scholar] [CrossRef]
- Yuhi, T.; Kyuta, H.; Mori, H.A.; Murakami, C.; Furuhara, K.; Okuno, M.; Takahashi, M.; Fuji, D.; Higashida, H. Salivary Oxytocin Concentration Changes during a Group Drumming Intervention for Maltreated School Children. Brain Sci. 2017, 7, 152. [Google Scholar] [CrossRef]

| TD | ASD | |
|---|---|---|
| Numbers | 8 (male: 4, female: 4) | 12 (male: 11, female: 1) |
| Age | 96–119 months | 113–162 months |
| mean: 107, SD: 6.9 | mean: 135, SD: 16.7 | |
| ESSENCE-Q | Yes: 0.4, Maybe/A Little: 0.1/0.3 | Yes: 2.83, Maybe/A Little: 1.58/2.83 |
| Education | regular class | regular or special needs classes or special education support classes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, S.; Komagome, A.; Iguchi-Sherry, A.; Nagasaka, A.; Yuhi, T.; Higashida, H.; Rooksby, M.; Kikuchi, M.; Arai, O.; Minami, K.; et al. Participatory Art Activities Increase Salivary Oxytocin Secretion of ASD Children. Brain Sci. 2020, 10, 680. https://doi.org/10.3390/brainsci10100680
Tanaka S, Komagome A, Iguchi-Sherry A, Nagasaka A, Yuhi T, Higashida H, Rooksby M, Kikuchi M, Arai O, Minami K, et al. Participatory Art Activities Increase Salivary Oxytocin Secretion of ASD Children. Brain Sciences. 2020; 10(10):680. https://doi.org/10.3390/brainsci10100680
Chicago/Turabian StyleTanaka, Sanae, Aiko Komagome, Aya Iguchi-Sherry, Akiko Nagasaka, Teruko Yuhi, Haruhiro Higashida, Maki Rooksby, Mitsuru Kikuchi, Oko Arai, Kana Minami, and et al. 2020. "Participatory Art Activities Increase Salivary Oxytocin Secretion of ASD Children" Brain Sciences 10, no. 10: 680. https://doi.org/10.3390/brainsci10100680
APA StyleTanaka, S., Komagome, A., Iguchi-Sherry, A., Nagasaka, A., Yuhi, T., Higashida, H., Rooksby, M., Kikuchi, M., Arai, O., Minami, K., Tsuji, T., & Tsuji, C. (2020). Participatory Art Activities Increase Salivary Oxytocin Secretion of ASD Children. Brain Sciences, 10(10), 680. https://doi.org/10.3390/brainsci10100680






