Prediction of Suicide-Related Events by Analyzing Electronic Medical Records from PTSD Patients with Bipolar Disorder
Abstract
:1. Introduction
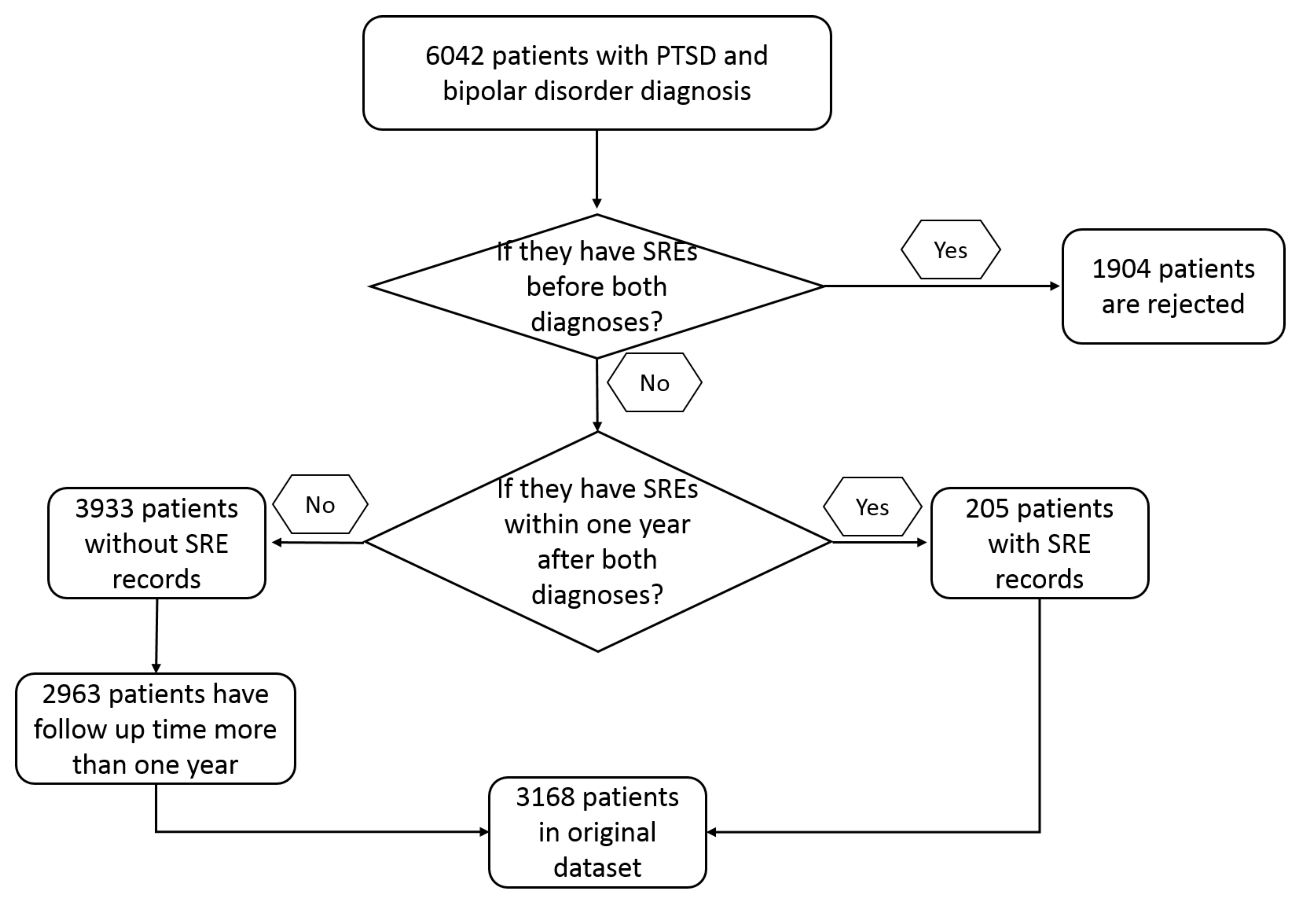
2. Materials and Methods
2.1. Data Sources
2.2. Software and Model Setup
3. Results
3.1. Model Construction and Performance
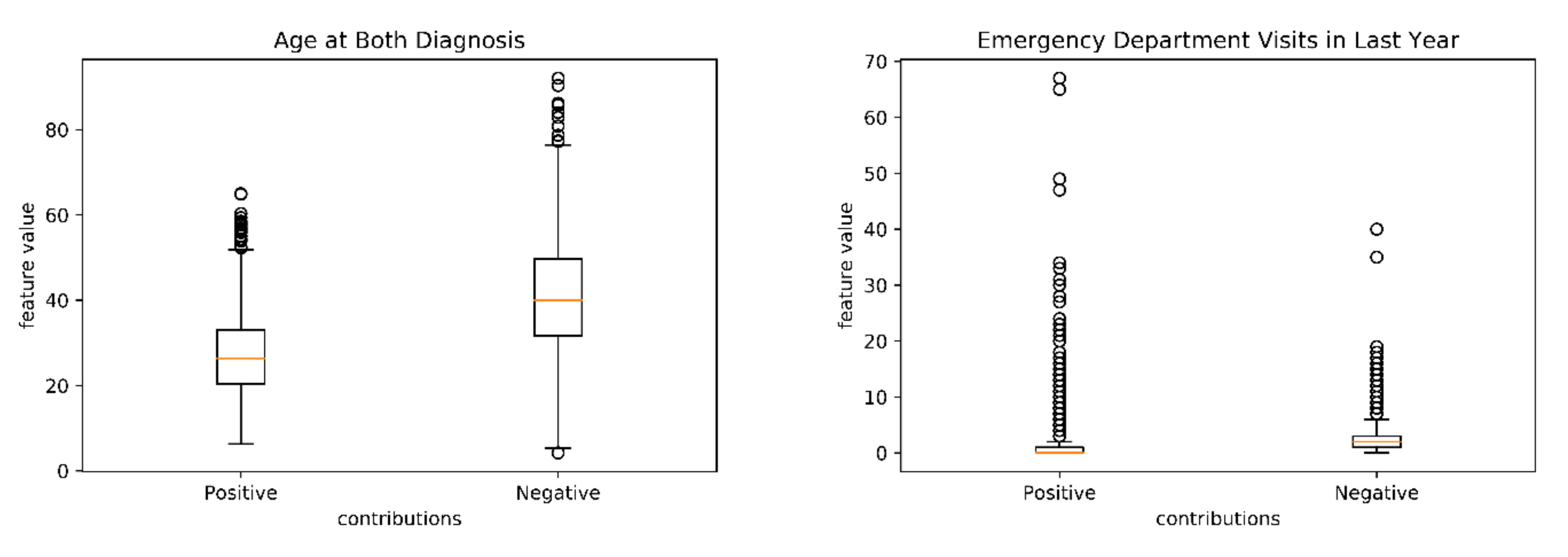
3.2. Model Decomposition and Feature Importance Analysis
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. ICD9 and ICD10 Codes for PTSD, Bipolar Disorder and Suicide Events
| ICD Code | Diagnosis Name | #Patients in 205 Patients |
|---|---|---|
| R45.851 | Suicidal ideations | 83 |
| V62.84 | Suicidal ideation | 78 |
| E950.4 | Suicide and self-inflicted poisoning by other specified drugs and medicinal substances | 9 |
| E950.3 | Suicide and self-inflicted poisoning by tranquilizers and other psychotropic agents | 7 |
| E956 | Suicide and self-inflicted injury by cutting and piercing instrument | 6 |
| E958.8 | Suicide and self-inflicted injury by other specified means | 5 |
| T43.222A | Poisoning by selective serotonin reuptake inhibitors, intentional self-harm, initial encounter | 2 |
| X78.9XXA | Intentional self-harm by unspecified sharp object, initial encounter | 2 |
| E950.0 | Suicide and self-inflicted poisoning by analgesics, antipyretics, and antirheumatics | 1 |
| E950.2 | Suicide and self-inflicted poisoning by other sedatives and hypnotics | 1 |
| T14.91XA | Suicide attempt, initial encounter | 1 |
| T39.1X2A | Poisoning by 4-Aminophenol derivatives, intentional self-harm, initial encounter | 1 |
| T40.1X2A | Poisoning by heroin, intentional self-harm, initial encounter | 1 |
| T42.1X2A | Poisoning by iminostilbenes, intentional self-harm, initial encounter | 1 |
| T42.4X2A | Poisoning by benzodiazepines, intentional self-harm, initial encounter | 1 |
| T42.4X2D | Poisoning by benzodiazepines, intentional self-harm, subsequent encounter | 0 |
| T42.6X2A | Poisoning by other antiepileptic and sedative-hypnotic drugs, intentional self-harm, initial encounter | 1 |
| T43.212A | Poisoning by selective serotonin and norepinephrine reuptake inhibitors, intentional self-harm, initial encounter | 1 |
| T46.5X2A | Poisoning by other antihypertensive drugs, intentional self-harm, initial encounter | 1 |
| T50.902D | Poisoning by unspecified drugs, medicaments and biological substances, intentional self-harm, subsequent encounter | 0 |
| T65.92XD | Toxic effect of unspecified substance, intentional self-harm, subsequent encounter | 0 |
| T71.162A | Asphyxiation due to hanging, intentional self-harm, initial encounter | 1 |
| X78.8XXA | Intentional self-harm by other sharp object, initial encounter | 1 |
| X78.9XXD | Intentional self-harm by unspecified sharp object, subsequent encounter | 0 |
| X83.8XXA | Intentional self-harm by other specified means, initial encounter | 1 |
| E950.1 | Suicide and self-inflicted poisoning by barbiturates | 0 |
| E950.5 | Suicide and self-inflicted poisoning by unspecified drug or medicinal substance | 0 |
| E950.6 | Suicide and self-inflicted poisoning by agricultural and horticultural chemical and pharmaceutical preparations other than plant foods and fertilizers | 0 |
| E950.7 | Suicide and self-inflicted poisoning by corrosive and caustic substances | 0 |
| E950.9 | Suicide and self-inflicted poisoning by other and unspecified solid and liquid substances | 0 |
| E951.0 | Suicide and self-inflicted poisoning by gas distributed by pipeline | 0 |
| E951.8 | Suicide and self-inflicted poisoning by other utility gas | 0 |
| E952.0 | Suicide and self-inflicted poisoning by motor vehicle exhaust gas | 0 |
| E952.1 | Suicide and self-inflicted poisoning by other carbon monoxide | 0 |
| E952.8 | Suicide and self-inflicted poisoning by other specified gases and vapors | 0 |
| E953.0 | Suicide and self-inflicted injury by hanging | 0 |
| E953.1 | Suicide and self-inflicted injury by suffocation by plastic bag | 0 |
| E953.8 | Suicide and self-inflicted injury by other specified means | 0 |
| E953.9 | Suicide and self-inflicted injury by unspecified means | 0 |
| E954 | Suicide and self-inflicted injury by submersion [drowning] | 0 |
| E955.0 | Suicide and self-inflicted injury by handgun | 0 |
| E955.1 | Suicide and self-inflicted injury by shotgun | 0 |
| E955.2 | Suicide and self-inflicted injury by hunting rifle | 0 |
| E955.4 | Suicide and self-inflicted injury by other and unspecified firearm | 0 |
| E955.9 | Suicide and self-inflicted injury by firearms and explosives, unspecified | 0 |
| E957.0 | Suicide and self-inflicted injuries by jumping from residential premises | 0 |
| E957.1 | Suicide and self-inflicted injuries by jumping from other man-made structures | 0 |
| E957.9 | Suicide and self-inflicted injuries by jumping from unspecified site | 0 |
| E958.0 | Suicide and self-inflicted injury by jumping or lying before moving object | 0 |
| E958.1 | Suicide and self-inflicted injury by burns, fire | 0 |
| E958.2 | Suicide and self-inflicted injury by scald | 0 |
| E958.3 | Suicide and self-inflicted injury by extremes of cold | 0 |
| E958.5 | Suicide and self-inflicted injury by crashing of motor vehicle | 0 |
| E958.6 | Suicide and self-inflicted injury by crashing of aircraft | 0 |
| E958.7 | Suicide and self-inflicted injury by caustic substances, except poisoning | 0 |
| E958.9 | Suicide and self-inflicted injury by unspecified means | 0 |
| T14.91 | Suicide attempt | 0 |
| T14.91XD | Suicide attempt, subsequent encounter | 0 |
| T14.91XS | Suicide attempt, sequela | 0 |
| T36.0X2A | Poisoning by penicillins, intentional self-harm, initial encounter | 0 |
| T36.0X2D | Poisoning by penicillins, intentional self-harm, subsequent encounter | 0 |
| T36.1X2A | Poisoning by cephalosporins and other beta-lactam antibiotics, intentional self-harm, initial encounter | 0 |
| T36.3X2A | Poisoning by macrolides, intentional self-harm, initial encounter | 0 |
| T36.4X2A | Poisoning by tetracyclines, intentional self-harm, initial encounter | 0 |
| T36.8X2A | Poisoning by other systemic antibiotics, intentional self-harm, initial encounter | 0 |
| T37.5X2A | Poisoning by antiviral drugs, intentional self-harm, initial encounter | 0 |
| T37.8X2A | Poisoning by other specified systemic anti-infectives and antiparasitics, intentional self-harm, initial encounter | 0 |
| T38.1X2A | Poisoning by thyroid hormones and substitutes, intentional self-harm, initial encounter | 0 |
| T38.2X2A | Poisoning by antithyroid drugs, intentional self-harm, initial encounter | 0 |
| T38.3X2A | Poisoning by insulin and oral hypoglycemic [antidiabetic] drugs, intentional self-harm, initial encounter | 0 |
| T38.3X2D | Poisoning by insulin and oral hypoglycemic [antidiabetic] drugs, intentional self-harm, subsequent encounter | 0 |
| T38.5X2A | Poisoning by other estrogens and progestogens, intentional self-harm, initial encounter | 0 |
| T38.892A | Poisoning by other hormones and synthetic substitutes, intentional self-harm, initial encounter | 0 |
| T39.012A | Poisoning by aspirin, intentional self-harm, initial encounter | 0 |
| T39.012D | Poisoning by aspirin, intentional self-harm, subsequent encounter | 0 |
| T39.092A | Poisoning by salicylates, intentional self-harm, initial encounter | 0 |
| T39.1X2D | Poisoning by 4-Aminophenol derivatives, intentional self-harm, subsequent encounter | 0 |
| T39.312A | Poisoning by propionic acid derivatives, intentional self-harm, initial encounter | 0 |
| T39.312D | Poisoning by propionic acid derivatives, intentional self-harm, subsequent encounter | 0 |
| T39.392A | Poisoning by other nonsteroidal anti-inflammatory drugs [NSAID], intentional self-harm, initial encounter | 0 |
| T39.4X2A | Poisoning by antirheumatics, not elsewhere classified, intentional self-harm, initial encounter | 0 |
| T39.8X2A | Poisoning by other nonopioid analgesics and antipyretics, not elsewhere classified, intentional self-harm, initial encounter | 0 |
| T39.92XA | Poisoning by unspecified nonopioid analgesic, antipyretic and antirheumatic, intentional self-harm, initial encounter | 0 |
| T40.1X2D | Poisoning by heroin, intentional self-harm, subsequent encounter | 0 |
| T40.2X2A | Poisoning by other opioids, intentional self-harm, initial encounter | 0 |
| T40.2X2D | Poisoning by other opioids, intentional self-harm, subsequent encounter | 0 |
| T40.3X2A | Poisoning by methadone, intentional self-harm, initial encounter | 0 |
| T40.4X2A | Poisoning by other synthetic narcotics, intentional self-harm, initial encounter | 0 |
| T40.5X2A | Poisoning by cocaine, intentional self-harm, initial encounter | 0 |
| T40.5X2D | Poisoning by cocaine, intentional self-harm, subsequent encounter | 0 |
| T40.602A | Poisoning by unspecified narcotics, intentional self-harm, initial encounter | 0 |
| T40.602D | Poisoning by unspecified narcotics, intentional self-harm, subsequent encounter | 0 |
| T40.7X2A | Poisoning by cannabis (derivatives), intentional self-harm, initial encounter | 0 |
| T40.8X2A | Poisoning by lysergide [LSD], intentional self-harm, initial encounter | 0 |
| T40.8X2D | Poisoning by lysergide [LSD], intentional self-harm, subsequent encounter | 0 |
| T40.992A | Poisoning by other psychodysleptics [hallucinogens], intentional self-harm, initial encounter | 0 |
| T41.292A | Poisoning by other general anesthetics, intentional self-harm, initial encounter | 0 |
| T41.3X2A | Poisoning by local anesthetics, intentional self-harm, initial encounter | 0 |
| T42.0X2A | Poisoning by hydantoin derivatives, intentional self-harm, initial encounter | 0 |
| T42.3X2A | Poisoning by barbiturates, intentional self-harm, initial encounter | 0 |
| T42.4X2S | Poisoning by benzodiazepines, intentional self-harm, sequela | 0 |
| T42.5X2A | Poisoning by mixed antiepileptics, intentional self-harm, initial encounter | 0 |
| T42.6X2 | Poisoning by other antiepileptic and sedative-hypnotic drugs, intentional self-harm | 0 |
| T42.6X2D | Poisoning by other antiepileptic and sedative-hypnotic drugs, intentional self-harm, subsequent encounter | 0 |
| T42.72XA | Poisoning by unspecified antiepileptic and sedative-hypnotic drugs, intentional self-harm, initial encounter | 0 |
| T42.8X2A | Poisoning by antiparkinsonism drugs and other central muscle-tone depressants, intentional self-harm, initial encounter | 0 |
| T43.012A | Poisoning by tricyclic antidepressants, intentional self-harm, initial encounter | 0 |
| T43.012D | Poisoning by tricyclic antidepressants, intentional self-harm, subsequent encounter | 0 |
| T43.022A | Poisoning by tetracyclic antidepressants, intentional self-harm, initial encounter | 0 |
| T43.022D | Poisoning by tetracyclic antidepressants, intentional self-harm, subsequent encounter | 0 |
| T43.1X2A | Poisoning by monoamine-oxidase-inhibitor antidepressants, intentional self-harm, initial encounter | 0 |
| T43.202A | Poisoning by unspecified antidepressants, intentional self-harm, initial encounter | 0 |
| T43.212D | Poisoning by selective serotonin and norepinephrine reuptake inhibitors, intentional self-harm, subsequent encounter | 0 |
| T43.222D | Poisoning by selective serotonin reuptake inhibitors, intentional self-harm, subsequent encounter | 0 |
| T43.292A | Poisoning by other antidepressants, intentional self-harm, initial encounter | 0 |
| T43.292D | Poisoning by other antidepressants, intentional self-harm, subsequent encounter | 0 |
| T43.3X2A | Poisoning by phenothiazine antipsychotics and neuroleptics, intentional self-harm, initial encounter | 0 |
| T43.3X2D | Poisoning by phenothiazine antipsychotics and neuroleptics, intentional self-harm, subsequent encounter | 0 |
| T43.4X2A | Poisoning by butyrophenone and thiothixene neuroleptics, intentional self-harm, initial encounter | 0 |
| T43.502A | Poisoning by unspecified antipsychotics and neuroleptics, intentional self-harm, initial encounter | 0 |
| T43.592A | Poisoning by other antipsychotics and neuroleptics, intentional self-harm, initial encounter | 0 |
| T43.592D | Poisoning by other antipsychotics and neuroleptics, intentional self-harm, subsequent encounter | 0 |
| T43.612A | Poisoning by caffeine, intentional self-harm, initial encounter | 0 |
| T43.622A | Poisoning by amphetamines, intentional self-harm, initial encounter | 0 |
| T43.622D | Poisoning by amphetamines, intentional self-harm, subsequent encounter | 0 |
| T43.632A | Poisoning by methylphenidate, intentional self-harm, initial encounter | 0 |
| T43.692A | Poisoning by other psychostimulants, intentional self-harm, initial encounter | 0 |
| T43.8X2A | Poisoning by other psychotropic drugs, intentional self-harm, initial encounter | 0 |
| T43.92XA | Poisoning by unspecified psychotropic drug, intentional self-harm, initial encounter | 0 |
| T44.1X2A | Poisoning by other parasympathomimetics [cholinergics], intentional self-harm, initial encounter | 0 |
| T44.3X2A | Poisoning by other parasympatholytics [anticholinergics and antimuscarinics] and spasmolytics, intentional self-harm, initial encounter | 0 |
| T44.4X2A | Poisoning by predominantly alpha-adrenoreceptor agonists, intentional self-harm, initial encounter | 0 |
| T44.6X2A | Poisoning by alpha-adrenoreceptor antagonists, intentional self-harm, initial encounter | 0 |
| T44.7X2A | Poisoning by beta-adrenoreceptor antagonists, intentional self-harm, initial encounter | 0 |
| T44.7X2D | Poisoning by beta-adrenoreceptor antagonists, intentional self-harm, subsequent encounter | 0 |
| T44.992A | Poisoning by other drug primarily affecting the autonomic nervous system, intentional self-harm, initial encounter | 0 |
| T45.0X2A | Poisoning by antiallergic and antiemetic drugs, intentional self-harm, initial encounter | 0 |
| T45.0X2D | Poisoning by antiallergic and antiemetic drugs, intentional self-harm, subsequent encounter | 0 |
| T45.2X2A | Poisoning by vitamins, intentional self-harm, initial encounter | 0 |
| T45.2X2D | Poisoning by vitamins, intentional self-harm, subsequent encounter | 0 |
| T45.4X2A | Poisoning by iron and its compounds, intentional self-harm, initial encounter | 0 |
| T45.512A | Poisoning by anticoagulants, intentional self-harm, initial encounter | 0 |
| T46.0X2A | Poisoning by cardiac-stimulant glycosides and drugs of similar action, intentional self-harm, initial encounter | 0 |
| T46.1X2A | Poisoning by calcium-channel blockers, intentional self-harm, initial encounter | 0 |
| T46.2X2A | Poisoning by other antidysrhythmic drugs, intentional self-harm, initial encounter | 0 |
| T46.3X2A | Poisoning by coronary vasodilators, intentional self-harm, initial encounter | 0 |
| T46.4X2A | Poisoning by angiotensin-converting-enzyme inhibitors, intentional self-harm, initial encounter | 0 |
| T46.4X2D | Poisoning by angiotensin-converting-enzyme inhibitors, intentional self-harm, subsequent encounter | 0 |
| T46.5X2D | Poisoning by other antihypertensive drugs, intentional self-harm, subsequent encounter | 0 |
| T46.6X2A | Poisoning by antihyperlipidemic and antiarteriosclerotic drugs, intentional self-harm, initial encounter | 0 |
| T46.7X2A | Poisoning by peripheral vasodilators, intentional self-harm, initial encounter | 0 |
| T46.8X2A | Poisoning by antivaricose drugs, including sclerosing agents, intentional self-harm, initial encounter | 0 |
| T47.0X2A | Poisoning by histamine H2-receptor blockers, intentional self-harm, initial encounter | 0 |
| T47.1X2A | Poisoning by other antacids and anti-gastric-secretion drugs, intentional self-harm, initial encounter | 0 |
| T47.4X2A | Poisoning by other laxatives, intentional self-harm, initial encounter | 0 |
| T47.6X2A | Poisoning by antidiarrheal drugs, intentional self-harm, initial encounter | 0 |
| T48.1X2A | Poisoning by skeletal muscle relaxants [neuromuscular blocking agents], intentional self-harm, initial encounter | 0 |
| T48.202A | Poisoning by unspecified drugs acting on muscles, intentional self-harm, initial encounter | 0 |
| T48.3X2A | Poisoning by antitussives, intentional self-harm, initial encounter | 0 |
| T48.3X2D | Poisoning by antitussives, intentional self-harm, subsequent encounter | 0 |
| T48.4X2A | Poisoning by expectorants, intentional self-harm, initial encounter | 0 |
| T48.5X2A | Poisoning by other anti-common-cold drugs, intentional self-harm, initial encounter | 0 |
| T48.6X2A | Poisoning by antiasthmatics, intentional self-harm, initial encounter | 0 |
| T49.0X2A | Poisoning by local antifungal, anti-infective and anti-inflammatory drugs, intentional self-harm, initial encounter | 0 |
| T49.6X2A | Poisoning by otorhinolaryngological drugs and preparations, intentional self-harm, initial encounter | 0 |
| T49.6X2D | Poisoning by otorhinolaryngological drugs and preparations, intentional self-harm, subsequent encounter | 0 |
| T50.2X2A | Poisoning by carbonic-anhydrase inhibitors, benzothiadiazides and other diuretics, intentional self-harm, initial encounter | 0 |
| T50.2X2D | Poisoning by carbonic-anhydrase inhibitors, benzothiadiazides and other diuretics, intentional self-harm, subsequent encounter | 0 |
| T50.3X2A | Poisoning by electrolytic, caloric and water-balance agents, intentional self-harm, initial encounter | 0 |
| T50.5X2A | Poisoning by appetite depressants, intentional self-harm, initial encounter | 0 |
| T50.6X2A | Poisoning by antidotes and chelating agents, intentional self-harm, initial encounter | 0 |
| T50.7X2A | Poisoning by analeptics and opioid receptor antagonists, intentional self-harm, initial encounter | 0 |
| T50.8X2A | Poisoning by diagnostic agents, intentional self-harm, initial encounter | 0 |
| T50.902A | Poisoning by unspecified drugs, medicaments and biological substances, intentional self-harm, initial encounter | 0 |
| T50.902S | Poisoning by unspecified drugs, medicaments and biological substances, intentional self-harm, sequela | 0 |
| T50.992A | Poisoning by other drugs, medicaments and biological substances, intentional self-harm, initial encounter | 0 |
| T50.992D | Poisoning by other drugs, medicaments and biological substances, intentional self-harm, subsequent encounter | 0 |
| T51.0X2A | Toxic effect of ethanol, intentional self-harm, initial encounter | 0 |
| T51.0X2D | Toxic effect of ethanol, intentional self-harm, subsequent encounter | 0 |
| T51.1X2A | Toxic effect of methanol, intentional self-harm, initial encounter | 0 |
| T51.2X2A | Toxic effect of 2-Propanol, intentional self-harm, initial encounter | 0 |
| T51.2X2D | Toxic effect of 2-Propanol, intentional self-harm, subsequent encounter | 0 |
| T51.2X2S | Toxic effect of 2-Propanol, intentional self-harm, sequela | 0 |
| T51.8X2A | Toxic effect of other alcohols, intentional self-harm, initial encounter | 0 |
| T51.92XA | Toxic effect of unspecified alcohol, intentional self-harm, initial encounter | 0 |
| T51.92XD | Toxic effect of unspecified alcohol, intentional self-harm, subsequent encounter | 0 |
| T52.0X2A | Toxic effect of petroleum products, intentional self-harm, initial encounter | 0 |
| T52.4X2A | Toxic effect of ketones, intentional self-harm, initial encounter | 0 |
| T52.8X2A | Toxic effect of other organic solvents, intentional self-harm, initial encounter | 0 |
| T54.0X2A | Toxic effect of phenol and phenol homologues, intentional self-harm, initial encounter | 0 |
| T54.1X2A | Toxic effect of other corrosive organic compounds, intentional self-harm, initial encounter | 0 |
| T54.2X2A | Toxic effect of corrosive acids and acid-like substances, intentional self-harm, initial encounter | 0 |
| T54.3X2A | Toxic effect of corrosive alkalis and alkali-like substances, intentional self-harm, initial encounter | 0 |
| T54.3X2D | Toxic effect of corrosive alkalis and alkali-like substances, intentional self-harm, subsequent encounter | 0 |
| T54.3X2S | Toxic effect of corrosive alkalis and alkali-like substances, intentional self-harm, sequela | 0 |
| T54.92XA | Toxic effect of unspecified corrosive substance, intentional self-harm, initial encounter | 0 |
| T54.92XS | Toxic effect of unspecified corrosive substance, intentional self-harm, sequela | 0 |
| T55.0X2A | Toxic effect of soaps, intentional self-harm, initial encounter | 0 |
| T55.1X2A | Toxic effect of detergents, intentional self-harm, initial encounter | 0 |
| T56.892A | Toxic effect of other metals, intentional self-harm, initial encounter | 0 |
| T56.892D | Toxic effect of other metals, intentional self-harm, subsequent encounter | 0 |
| T58.02XA | Toxic effect of carbon monoxide from motor vehicle exhaust, intentional self-harm, initial encounter | 0 |
| T58.92XA | Toxic effect of carbon monoxide from unspecified source, intentional self-harm, initial encounter | 0 |
| T59.892A | Toxic effect of other specified gases, fumes and vapors, intentional self-harm, initial encounter | 0 |
| T62.0X2A | Toxic effect of ingested mushrooms, intentional self-harm, initial encounter | 0 |
| T65.222D | Toxic effect of tobacco cigarettes, intentional self-harm, subsequent encounter | 0 |
| T65.222S | Toxic effect of tobacco cigarettes, intentional self-harm, sequela | 0 |
| T65.892A | Toxic effect of other specified substances, intentional self-harm, initial encounter | 0 |
| T65.892D | Toxic effect of other specified substances, intentional self-harm, subsequent encounter | 0 |
| T65.92XA | Toxic effect of unspecified substance, intentional self-harm, initial encounter | 0 |
| T65.92XS | Toxic effect of unspecified substance, intentional self-harm, sequela | 0 |
| T71.162D | Asphyxiation due to hanging, intentional self-harm, subsequent encounter | 0 |
| T71.192A | Asphyxiation due to mechanical threat to breathing due to other causes, intentional self-harm, initial encounter | 0 |
| X71.0XXS | Intentional self-harm by drowning and submersion while in bathtub, sequela | 0 |
| X71.3XXA | Intentional self-harm by drowning and submersion in natural water, initial encounter | 0 |
| X71.8XXA | Other intentional self-harm by drowning and submersion, initial encounter | 0 |
| X71.9XXA | Intentional self-harm by drowning and submersion, unspecified, initial encounter | 0 |
| X72.XXXA | Intentional self-harm by handgun discharge, initial encounter | 0 |
| X72.XXXD | Intentional self-harm by handgun discharge, subsequent encounter | 0 |
| X72.XXXS | Intentional self-harm by handgun discharge, sequela | 0 |
| X73.0XXA | Intentional self-harm by shotgun discharge, initial encounter | 0 |
| X74.01XA | Intentional self-harm by airgun, initial encounter | 0 |
| X74.8XXS | Intentional self-harm by other firearm discharge, sequela | 0 |
| X74.9XXA | Intentional self-harm by unspecified firearm discharge, initial encounter | 0 |
| X74.9XXD | Intentional self-harm by unspecified firearm discharge, subsequent encounter | 0 |
| X74.9XXS | Intentional self-harm by unspecified firearm discharge, sequela | 0 |
| X76.XXXA | Intentional self-harm by smoke, fire and flames, initial encounter | 0 |
| X76.XXXD | Intentional self-harm by smoke, fire and flames, subsequent encounter | 0 |
| X77.8XXA | Intentional self-harm by other hot objects, initial encounter | 0 |
| X78.0XXA | Intentional self-harm by sharp glass, initial encounter | 0 |
| X78.0XXD | Intentional self-harm by sharp glass, subsequent encounter | 0 |
| X78.1XXA | Intentional self-harm by knife, initial encounter | 0 |
| X78.1XXD | Intentional self-harm by knife, subsequent encounter | 0 |
| X78.8XXD | Intentional self-harm by other sharp object, subsequent encounter | 0 |
| X78.9XXS | Intentional self-harm by unspecified sharp object, sequela | 0 |
| X79.XXXA | Intentional self-harm by blunt object, initial encounter | 0 |
| X79.XXXD | Intentional self-harm by blunt object, subsequent encounter | 0 |
| X80.XXXA | Intentional self-harm by jumping from a high place, initial encounter | 0 |
| X80.XXXD | Intentional self-harm by jumping from a high place, subsequent encounter | 0 |
| X81.0XXA | Intentional self-harm by jumping or lying in front of motor vehicle, initial encounter | 0 |
| X81.8XXA | Intentional self-harm by jumping or lying in front of other moving object, initial encounter | 0 |
| X82.8XXA | Other intentional self-harm by crashing of motor vehicle, initial encounter | 0 |
| X83.2XXA | Intentional self-harm by exposure to extremes of cold, initial encounter | 0 |
| X83.8XXD | Intentional self-harm by other specified means, subsequent encounter | 0 |
| X83.8XXS | Intentional self-harm by other specified means, sequela | 0 |
Appendix B. Categories of Comorbid Diseases
| ICD9 Code | Disease Name | Category | ICD9 Code | Disease Name | Category |
|---|---|---|---|---|---|
| 291 | Alcohol-induced mental disorders | 1 | 301 (not 301.1 or 301.2) | Personality disorders (not Affective personality disorder or Schizoid personality disorder) | 6 |
| 292 | Drug-induced mental disorders | 1 | 302 | Sexual and gender identity disorders | 7 |
| 303 | Alcohol dependence syndrome | 1 | 306 | Physiological malfunction arising from mental factors | 8 |
| 304 | Drug dependence | 1 | 316 | Psychic factor w oth dis. | 8 |
| 305 (not 305.1) | Nondependent abuse of drugs (not Tobacco use disorder) | 1 | 307 | Special symptoms or syndromes not elsewhere classified | 9 |
| 295 | Schizophrenic disorders | 2 | 290 | Dementias | 10 |
| 301.2 | Schizoid personality disorder | 2 | 293 | Transient mental disorders due to conditions classified elsewhere | 10 |
| 296 | Episodic mood disorders | 3 | 294 | Persistent mental disorders due to conditions classified elsewhere | 10 |
| 298 | Depressive type psychosis | 3 | 310 | Specific nonpsychotic mental disorders due to brain damage | 10 |
| 300.4 | Dysthymic disorder | 3 | 299 | Autistic disorder-current | 11 |
| 301.1 | Affective personality disorder | 3 | 312 | Disturbance of conduct not elsewhere classified | 11 |
| 309 | Adjustment reaction | 3 | 313 | Disturbance of emotions specific to childhood and adolescence | 11 |
| 311 | Depressive disorder NEC | 3 | 314 | Hyperkinetic syndrome of childhood | 11 |
| 297 | Delusional disorders | 4 | 315 | Specific delays in development | 11 |
| 298 (but not 2980) | Other nonorganic psychoses ( not Depressive type psychosis) | 4 | 317 | Mild intellectual disabilities | 12 |
| 308 | Acute reaction to stress | 5 | 318 | Other specified intellectual disabilities | 12 |
| 300 (but not 300.4) | Anxiety, dissociative and somatoform disorders (not Dysthymic disorder) | 5 | 319 | Unspecified intellectual disabilities | 12 |
Appendix C. Distribution of All Categorical Features
| T | P | Proportion of 1 in Whole Population | Proportion of 1 with Positive Contributions | Proportion of 1 with Negative Contributions | FDR Adjusted Q Value | Direction of Effect | |
|---|---|---|---|---|---|---|---|
| Almotriptan | 18.747 | <0.001 | 0.013 | 0 | 1 | <0.001 | No SREs |
| Sertraline | 1027.936 | <0.001 | 0.117 | 0 | 0.41 | <0.001 | No SREs |
| Selegiline | 250.75 | <0.001 | 0.001 | 1 | 0 | <0.001 | SREs |
| Rotigotine | 130.307 | <0.001 | 0.009 | 0 | 1 | <0.001 | No SREs |
| Rizatriptan | 136.995 | <0.001 | 0.013 | 0.071 | 0.003 | <0.001 | SREs |
| Risperidone | 32.548 | <0.001 | 0.062 | 0.131 | 0.053 | <0.001 | SREs |
| Rasagiline | 16.996 | <0.001 | 0.014 | 0 | 1 | <0.001 | No SREs |
| Sumatriptan | 355.971 | <0.001 | 0.051 | 0.004 | 0.169 | <0.001 | No SREs |
| Quetiapine | 34.748 | <0.001 | 0.135 | 0.068 | 0.155 | <0.001 | No SREs |
| Promethazine | 130.817 | <0.001 | 0.053 | 0.008 | 0.1 | <0.001 | No SREs |
| Paroxetine | 78.278 | <0.001 | 0.032 | 0.008 | 0.065 | <0.001 | No SREs |
| Olanzapine | 174.851 | <0.001 | 0.055 | 0.184 | 0.032 | <0.001 | SREs |
| Disease Category 12 in last year | 150.338 | <0.001 | 0.011 | 0.098 | 0.004 | <0.001 | SREs |
| Mirtazapine | 113.859 | <0.001 | 0.063 | 0.013 | 0.106 | <0.001 | No SREs |
| Milnacipran | 1014.193 | <0.001 | 0.007 | 0 | 1 | <0.001 | No SREs |
| Protriptyline | 120.749 | <0.001 | 0.002 | 0 | 1 | <0.001 | No SREs |
| Tapentadol | 67.865 | <0.001 | 0.016 | 0 | 1 | <0.001 | No SREs |
| Thiothixene | 27.267 | <0.001 | 0.001 | 0.029 | 0 | <0.001 | SREs |
| Tramadol | 855.263 | <0.001 | 0.131 | 0.002 | 0.373 | <0.001 | No SREs |
| Disease Category 11 in last year | 1879.785 | <0.001 | 0.098 | 0.957 | 0.036 | <0.001 | SREs |
| Disease Category 9 in last year | 95.725 | <0.001 | 0.043 | 0.008 | 0.079 | <0.001 | No SREs |
| Disease Category 8 in last year | 44.042 | <0.001 | 0.005 | 0.042 | 0.002 | <0.001 | SREs |
| Disease Category 7 in last year | 259.428 | <0.001 | 0.012 | 0 | 1 | <0.001 | No SREs |
| Disease Category 6 in last year | 2032.077 | <0.001 | 0.066 | 0.979 | 0.022 | <0.001 | SREs |
| Disease Category 5 in last year | 152.933 | <0.001 | 0.459 | 0.992 | 0.436 | <0.001 | SREs |
| Disease Category 4 in last year | 614.247 | <0.001 | 0.034 | 0.364 | 0.014 | <0.001 | SREs |
| Disease Category 3 in last year | 13.537 | <0.001 | 0.749 | 0.906 | 0.743 | <0.001 | SREs |
| Disease Category 2 in last year | 1610.239 | <0.001 | 0.064 | 0.965 | 0.029 | <0.001 | SREs |
| Disease Category 1 in last year | 1640.843 | <0.001 | 0.234 | 1 | 0.11 | <0.001 | SREs |
| Ziprasidone | 947.034 | <0.001 | 0.037 | 0.51 | 0.014 | <0.001 | SREs |
| Vortioxetine | 903.45 | <0.001 | 0.008 | 0 | 1 | <0.001 | No SREs |
| Vilazodone | 15.103 | <0.001 | 0.006 | 0.021 | 0.004 | <0.001 | SREs |
| Trifluoperazine | 549.894 | <0.001 | 0.007 | 0 | 1 | <0.001 | No SREs |
| Trazodone | 1068.226 | <0.001 | 0.17 | 0.919 | 0.105 | <0.001 | SREs |
| Methadone | 13.736 | <0.001 | 0.024 | 0.04 | 0.017 | <0.001 | SREs |
| Meperidine | 465.207 | <0.001 | 0.022 | 0.22 | 0.006 | <0.001 | SREs |
| GENDER | 1359.169 | <0.001 | 0.237 | 0.033 | 0.624 | <0.001 | No SREs |
| Loxapine | 422.51 | <0.001 | 0.007 | 0 | 1 | <0.001 | No SREs |
| Amitriptyline | 666.435 | <0.001 | 0.047 | 0.002 | 0.259 | <0.001 | No SREs |
| Aripiprazole | 1651.206 | <0.001 | 0.088 | 0 | 0.573 | <0.001 | No SREs |
| Asenapine | 162.998 | <0.001 | 0.005 | 0.068 | 0 | <0.001 | SREs |
| Brexpiprazole | 1268.74 | <0.001 | 0.004 | 0 | 1 | <0.001 | No SREs |
| Bupropion | 148.547 | <0.001 | 0.109 | 0.013 | 0.158 | <0.001 | No SREs |
| Buspirone | 169.298 | <0.001 | 0.079 | 0.004 | 0.132 | <0.001 | No SREs |
| Cariprazine | 283.31 | <0.001 | 0.004 | 0 | 1 | <0.001 | No SREs |
| Chlorpheniramine | 251.938 | <0.001 | 0.008 | 0 | 1 | <0.001 | No SREs |
| Chlorpromazine | 72.256 | <0.001 | 0.011 | 0.054 | 0.005 | <0.001 | SREs |
| Clozapine | 208.063 | <0.001 | 0.019 | 0 | 1 | <0.001 | No SREs |
| Desipramine | 92.504 | <0.001 | 0.033 | 0 | 1 | <0.001 | No SREs |
| Desvenlafaxine | 113.145 | <0.001 | 0.007 | 0 | 0.09 | <0.001 | No SREs |
| Dihydroergotamine | 1673.55 | <0.001 | 0.008 | 0 | 1 | <0.001 | No SREs |
| Doxepin | 15.421 | <0.001 | 0.029 | 0.013 | 0.038 | <0.001 | No SREs |
| Dexmethylphenidate | 481.108 | <0.001 | 0.004 | 0 | 1 | <0.001 | No SREs |
| Fluvoxamine | 1049.281 | <0.001 | 0.006 | 0 | 1 | <0.001 | No SREs |
| Lithium | 97.967 | <0.001 | 0.075 | 0.01 | 0.109 | <0.001 | No SREs |
| Escitalopram | 14.179 | <0.001 | 0.049 | 0.024 | 0.058 | <0.001 | No SREs |
| Fentanyl | 754.886 | <0.001 | 0.188 | 0 | 0.385 | <0.001 | No SREs |
| Levomilnacipran | 462.413 | <0.001 | 0.009 | 0 | 1 | <0.001 | No SREs |
| Lamotrigine | 341.173 | <0.001 | 0.145 | 0 | 0.239 | <0.001 | No SREs |
| Flibanserin | 21.497 | <0.001 | 0.011 | 0 | 1 | <0.001 | No SREs |
| Imipramine | 863.02 | <0.001 | 0.008 | 0 | 1 | <0.001 | No SREs |
| Fluoxetine | 39.147 | <0.001 | 0.091 | 0.053 | 0.119 | <0.001 | No SREs |
| Fluphenazine | 128.321 | <0.001 | 0.016 | 0 | 1 | <0.001 | No SREs |
| Haloperidol | 450.954 | <0.001 | 0.047 | 0.32 | 0.023 | <0.001 | SREs |
| Naratriptan | 11.737 | 0.001 | 0.004 | 0.019 | 0.002 | 0.001246 | SREs |
| Amphetamine | 9.941 | 0.002 | 0.031 | 0.018 | 0.039 | 0.002455 | No SREs |
| Venlafaxine | 8.86 | 0.003 | 0.082 | 0.048 | 0.089 | 0.003627 | No SREs |
| Tranylcypromine | 8.243 | 0.004 | 0.029 | 0 | 1 | 0.004765 | No SREs |
| Disease Category 10 in last year | 4.595 | 0.032 | 0.027 | 0.035 | 0.022 | 0.037565 | SREs |
| Paliperidone | 4.379 | 0.036 | 0.007 | 0.013 | 0.005 | 0.041657 | SREs |
| Lurasidone | 4.196 | 0.041 | 0.032 | 0.023 | 0.037 | 0.046775 | No SREs |
| Zolmitriptan | 3.292 | 0.07 | 0.002 | 0.006 | 0.001 | 0.07875 | N/A |
| Ropinirole | 2.34 | 0.126 | 0.016 | 0.022 | 0.013 | 0.139808 | N/A |
| Duloxetine | 2.079 | 0.149 | 0.069 | 0.084 | 0.066 | 0.162 | N/A |
| Dextromethorphan | 2.073 | 0.15 | 0.015 | 0.01 | 0.017 | 0.162 | N/A |
| Citalopram | 2.027 | 0.155 | 0.123 | 0.094 | 0.126 | 0.165197 | N/A |
| Clomipramine | 1.945 | 0.163 | 0.004 | 0.01 | 0.003 | 0.171468 | N/A |
| Nortriptyline | 1.71 | 0.191 | 0.012 | 0.016 | 0.01 | 0.198346 | N/A |
| Perphenazine | 1.127 | 0.288 | 0.017 | 0.013 | 0.019 | 0.295291 | N/A |
| Carbamazepine | 0.301 | 0.584 | 0.03 | 0.028 | 0.032 | 0.5913 | N/A |
| Eletriptan | 0.061 | 0.804 | 0.001 | 0.003 | 0.001 | 0.804 | N/A |
References
- World Health Organization. Suicide in the World: Global Health Estimates; World Health Organisation: Genva, Switzerland, 2019. [Google Scholar]
- Drapeau, C.W.; McIntosh, J.L. USA Suicide 2018: Official Final Data. 2020; American Association of Suicidology: Washington, DC, USA, 2018. [Google Scholar]
- Hedegaard, H.; Curtin, S.C.; Warner, M. Suicide Mortality in the United States, 1999–2017; National Center for Health Statistics: Hyattsville, MD, USA, 2018. [Google Scholar]
- Curtin, S.C.; Warner, M.; Hedegaard, H. Increase in Suicide in the United States, 1999–2014. Available online: https://stacks.cdc.gov/view/cdc/39008 (accessed on 24 August 2020).
- Drapeau, C.W.; McIntosh, J.L. USA Suicide 2017: Official Final Data. 2018; American Assocation of Suicidology: Washington, DC, USA, 2017. [Google Scholar]
- Drapeau, C.W.; McIntosh, J.L. USA Suicide 2016: Official Final Data. 2017; American Association of Suicidology: Washington, DC, USA, 2016. [Google Scholar]
- Drapeau, C.W.; McIntosh, J.L. USA Suicide 2015: Official Final Data. 2016; American Association of Suicidology: Washington, DC, USA, 2015. [Google Scholar]
- Drapeau, C.W.; McIntosh, J.L. USA Suicide 2014: Official Final Data; American Association of Suicidology: Washington, DC, USA, 2015. [Google Scholar]
- Brent, D.A.; Baugher, M.; Bridge, J.; Chen, T.; Chiappetta, L. Age- and Sex-Related Risk Factors for Adolescent Suicide. J. Am. Acad. Child Adolesc. Psychiatry 1999, 38, 1497–1505. [Google Scholar] [CrossRef]
- Case, A.; Deaton, A. Suicide, Age, and Wellbeing: An Empirical Investigation; National Bureau of Economic Research: Cambridge, MA, USA, 2015. [Google Scholar]
- Large, M.; Kaneson, M.; Myles, N.; Myles, H.; Gunaratne, P.; Ryan, C. Meta-Analysis of Longitudinal Cohort Studies of Suicide Risk Assessment among Psychiatric Patients: Heterogeneity in Results and Lack of Improvement over Time. PLoS ONE 2016, 11, e0156322. [Google Scholar] [CrossRef]
- Huang, X.; Ribeiro, J.D.; Musacchio, K.M.; Franklin, J.C. Demographics as predictors of suicidal thoughts and behaviors: A meta-analysis. PLoS ONE 2017, 12, e0180793. [Google Scholar] [CrossRef] [Green Version]
- Mulder, R.T.; Newton-Howes, G.; Coid, J.W. The futility of risk prediction in psychiatry. Br. J. Psychiatry 2016, 209, 271–272. [Google Scholar] [CrossRef] [Green Version]
- Passos, I.C.; Mwangi, B.; Cao, B.; Hamilton, J.E.; Wu, M.-J.; Zhang, X.Y.; Zunta-Soares, G.B.; Quevedo, J.; Kauer-Sant’Anna, M.; Kapczinski, F.; et al. Identifying a clinical signature of suicidality among patients with mood disorders: A pilot study using a machine learning approach. J. Affect. Disord. 2016, 193, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.G.; Ribeiro, J.D.; Franklin, J.C. Predicting Risk of Suicide Attempts Over Time Through Machine Learning. Clin. Psychol. Sci. 2017, 5, 457–469. [Google Scholar] [CrossRef]
- Just, M.A.; Pan, L.; Cherkassky, V.L.; McMakin, D.L.; Cha, C.; Nock, M.K.; Brent, D. Machine learning of neural representations of suicide and emotion concepts identifies suicidal youth. Nat. Hum. Behav. 2017, 1, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanderson, M.; Bulloch, A.G.; Wang, J.; Williams, K.G.; Williamson, T.; Patten, S.B. Predicting death by suicide following an emergency department visit for parasuicide with administrative health care system data and machine learning. EClinicalMedicine 2020, 20, 100281. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.D.S.; Alencar, Á.P.; Neto, P.J.N.; Dos Santos, M.D.S.V.; Da Silva, C.G.L.; Pinheiro, S.D.F.L.; Silveira, R.T.; Bianco, B.; Junior, R.F.F.P.; De Lima, M.A.P.; et al. Risk factors for suicide in bipolar disorder: A systematic review. J. Affect. Disord. 2015, 170, 237–254. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, G.R.; Hashemi, S.J.; Akbaripour, S.; Salehi, M.; Maracy, M.R. Risk factors of suicide reattempt in patients admitted to khorshid hospital, Isfahan, Iran., 2009. Iran. J. Epidemiol. 2012, 8, 39–46. [Google Scholar]
- Grunze, H. Bipolar disorder. Neurobiol. Brain Disord. 2015, 655–673. [Google Scholar] [CrossRef]
- Martínez-Arán, A.; Vieta, E.; Torrent, C.; Sánchez-Moreno, J.; Goikolea, J.; Salamero, M.; Malhi, G.; González-Pinto, A.; Daban, C.; Alvarez-Grandi, S.; et al. Functional outcome in bipolar disorder: The role of clinical and cognitive factors. Bipolar Disord. 2007, 9, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Goikolea, J.M.; De Dios, C.; González-Pinto, A.; Montes, J.M.; Saiz-Ruiz, J.; Prieto, E.; Vieta, E. Occupational disability in bipolar disorder: Analysis of predictors of being on severe disablement benefit (PREBIS study data). Acta Psychiatr. Scand. 2012, 127, 403–411. [Google Scholar] [CrossRef]
- Cardoso, G.; Xavier, M.; Vilagut, G.; Petukhova, M.; Alonso, J.; Kessler, R.C.; Caldas-De-Almeida, J.M. Days out of role due to common physical and mental conditions in Portugal: Results from the WHO World Mental Health Survey. BJPsych Open 2017, 3, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merikangas, K.R.; Jin, R.; He, J.-P.; Kessler, R.C.; Lee, S.; Sampson, N.A.; Viana, M.C.; Andrade, L.H.; Hu, C.; Karam, E.G.; et al. Prevalence and Correlates of Bipolar Spectrum Disorder in the World Mental Health Survey Initiative. Arch. Gen. Psychiatry 2011, 68, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Merikangas, K.R.; Akiskal, H.S.; Angst, J.; Greenberg, P.E.; Hirschfeld, R.M.A.; Petukhova, M.; Kessler, R.C. Lifetime and 12-Month Prevalence of Bipolar Spectrum Disorder in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2007, 64, 543–552. [Google Scholar] [CrossRef]
- Otto, M.W.; Perlman, C.A.; Wernicke, R.; E Reese, H.; Bauer, M.S.; Pollack, M.H. Posttraumatic stress disorder in patients with bipolar disorder: A review of prevalence, correlates, and treatment strategies. Bipolar Disord. 2004, 6, 470–479. [Google Scholar] [CrossRef]
- Boylan, K.R.; Bieling, P.J.; Marriott, M.; Begin, H.; Young, L.T.; MacQueen, G.M. Impact of Comorbid Anxiety Disorders on Outcome in a Cohort of Patients With Bipolar Disorder. J. Clin. Psychiatry 2004, 65, 1106–1113. [Google Scholar] [CrossRef]
- Quarantini, L.C.; Miranda-Scippa, Â.; Nery-Fernandes, F.; Andrade-Nascimento, M.; Galvão-De-Almeida, A.; Guimarães, J.L.; Teles, C.A.; Netto, L.R.; Lira, S.B.; De Oliveira, I.R.; et al. The impact of comorbid posttraumatic stress disorder on bipolar disorder patients. J. Affect. Disord. 2010, 123, 71–76. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®); American Psychiatric Pub.: Washington, DC, USA, 2013. [Google Scholar]
- Kessler, R.C.; Sonnega, A.; Bromet, E.; Hughes, M.; Nelson, C.B. Posttraumatic Stress Disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 1995, 52, 1048–1060. [Google Scholar] [CrossRef]
- Carter, J.M.; Arentsen, T.J.; Cordova, M.J.; Ruzek, J.; Reiser, R.; Suppes, T.; Ostacher, M.J. Increased Suicidal Ideation in Patients with Co-Occurring Bipolar Disorder and Post-Traumatic Stress Disorder. Arch. Suicide Res. 2016, 21, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Dilsaver, S.C.; Benazzi, F.; Akiskal, H.S.; Akiskal, K.K. Post-traumatic stress disorder among adolescents with bipolar disorder and its relationship to suicidality. Bipolar Disord. 2007, 9, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J.; Apter, A.; Bertolote, J.; Beautrais, A.; Currier, D.; Haas, A.; Hegerl, U.; Lonnqvist, J.; Malone, K.; Marusic, A.; et al. Suicide Prevention Strategies. JAMA 2005, 294, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Zalsman, G.; Hawton, K.; Wasserman, D.; van Heeringen, K.; Arensman, E.; Sarchiapone, M.; Carli, V.; Höschl, C.; Barzilay, R.; Balazs, J.; et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatr. 2016, 3, 646–659. [Google Scholar] [CrossRef]
- Van Der Feltz-Cornelis, C.M.; Sarchiapone, M.; Postuvan, V.; Volker, D.; Roskar, S.; Grum, A.T.; Carli, V.; McDaid, D.; O’Connor, R.; Maxwell, M.; et al. Best Practice Elements of Multilevel Suicide Prevention Strategies. Crisis 2011, 32, 319–333. [Google Scholar] [CrossRef] [Green Version]
- Yip, P.S.; Caine, E.; Yousuf, S.; Chang, S.S.; Wu, K.C.C.; Chen, Y.Y. Means restriction for suicide prevention. Lancet 2012, 379, 2393–2399. [Google Scholar] [CrossRef]
- Rihmer, Z.; Belso, N.; Kiss, K. Strategies for suicide prevention. Curr. Opin. Psychiatry 2002, 15, 83–87. [Google Scholar] [CrossRef]
- Rihmer, Z. Strategies of suicide prevention: Focus on health care. J. Affect. Disord. 1996, 39, 83–91. [Google Scholar] [CrossRef]
- Cipriani, A.; Hawton, K.; Stockton, S.; Geddes, J.R. Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta-analysis. BMJ 2013, 346, f3646. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, F.K.; Fireman, B.; Simon, G.E.; Hunkeler, E.M.; Lee, J.; Revicki, D. Suicide Risk in Bipolar Disorder During Treatment With Lithium and Divalproex. JAMA 2003, 290, 1467–1473. [Google Scholar] [CrossRef] [Green Version]
- Simon, G.E.; Johnson, E.; Lawrence, J.M.; Rossom, R.C.; Ahmedani, B.; Lynch, F.L.; Beck, A.; Waitzfelder, B.E.; Ziebell, R.; Penfold, R.B.; et al. Predicting Suicide Attempts and Suicide Deaths Following Outpatient Visits Using Electronic Health Records. Am. J. Psychiatry 2018, 175, 951–960. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Kluyver, T.; Ragan-Kelley, B.; Pérez, F.; Granger, B.E.; Bussonnier, M.; Frederic, J.; Kelley, K.; Hamrick, J.B.; Grout, J.; Corlay, S.; et al. Jupyter Notebooks-a publishing format for reproducible computational workflows. ELPUB 2016. [Google Scholar] [CrossRef]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idris, I. NumPy Cookbook; Packt Publishing Ltd.: Birmingham, UK, 2012. [Google Scholar]
- McKinney, W. pandas: A foundational Python library for data analysis and statistics. Python High Perform. Sci. Comp. 2011, 14, 1–9. [Google Scholar]
- Kleinbaum, D.G.; Dietz, K.; Gail, M.; Klein, M.; Klein, M. Logistic Regression; Springer: New York, NY, USA, 2002. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Freund, Y.; Mason, L. The alternating decision tree learning algorithm. ICML 1999, 99, 124–133. [Google Scholar]
- Fukunaga, K.; Narendra, P. A Branch and Bound Algorithm for Computing k-Nearest Neighbors. IEEE Trans. Comput. 1975, 100, 750–753. [Google Scholar] [CrossRef]
- Kononenko, I. Semi-naive bayesian classifier. In Lecture Notes in Computer Science; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; pp. 206–219. [Google Scholar]
- Keerthi, S.; Shevade, S.; Bhattacharyya, C.; Murthy, K. A fast iterative nearest point algorithm for support vector machine classifier design. IEEE Trans. Neural Networks 2000, 11, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Chawla, N.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Ibrahim, N.; Din, N.C.; Ahmad, M.; Amit, N.; Ghazali, S.E.; Wahab, S.; Kadir, N.B.A.; Halim, F.W.; Halim, M.R.T.A. The role of social support and spiritual wellbeing in predicting suicidal ideation among marginalized adolescents in Malaysia. BMC Public Health 2019, 19, 553. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.N.; Ziegler, A. ranger: A Fast Implementation of Random Forests for High Dimensional Data in C++ and R. J. Stat. Softw. 2017, 77, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Ronaghan, S. The Mathematics of Decision Trees, Random Forest and Feature Importance in Scikit-Learn and Spark. 2018. Available online: https://towardsdatascience.com/the-mathematics-of-decision-trees-random-forest-and-feature-importance-in-scikit-learn-and-spark-f2861df67e3 (accessed on 15 October 2020).
- Walsh, C.G.; Ribeiro, J.D.; Franklin, J.C. Predicting suicide attempts in adolescents with longitudinal clinical data and machine learning. J. Child Psychol. Psychiatry 2018, 59, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Szuster, R.R.; Schanbacher, B.L.; McCann, S.C. Characteristics of Psychiatric Emergency Room Patients With Alcohol- or Drug-Induced Disorders. Psychiatr. Serv. 1990, 41, 1342–1345. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chiang, S.-C.; Su, L.-W.; Lin, S.-K.; Chen, C.-K. Factors Associated With Drug-Related Psychiatric Disorders and Suicide Attempts Among Illicit Drug Users in Taiwan. Subst. Use Misuse 2012, 47, 1185–1188. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Dominici, G.; Ferracuti, S.; Kotzalidis, G.D.; Serra, G.; Girardi, P.; Janiri, L.; Tatarelli, R.; et al. Suicidal Behavior and Alcohol Abuse. Int. J. Environ. Res. Public Health 2010, 7, 1392–1431. [Google Scholar] [CrossRef] [Green Version]
- Brady, J. The association between alcohol misuse and suicidal behaviour. Alcohol Alcohol. 2006, 41, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Hawton, K.; Sutton, L.; Haw, C.; Sinclair, J.; Deeks, J.J. Schizophrenia and suicide: Systematic review of risk factors. Br. J. Psychiatry 2005, 187, 9–20. [Google Scholar] [CrossRef]
- Palmer, B.A.; Pankratz, V.S.; Bostwick, J.M. The Lifetime Risk of Suicide in Schizophrenia. Arch. Gen. Psychiatry 2005, 62, 247–253. [Google Scholar] [CrossRef]
- Rokach, L.; Maimon, O. Data Mining with Decision Trees—Theory and Applications; World Scientific Pub Co Pte Ltd.: Singapore, 2007; Volume 69. [Google Scholar]
- Mago, R.; Forero, G.; Greenberg, W.M.; Gommoll, C.; Chen, C. Safety and Tolerability of Levomilnacipran ER in Major Depressive Disorder: Results from an Open-Label, 48-Week Extension Study. Clin. Drug Investig. 2013, 33, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Hansen, R.; Cheng, N.; Rahman, M.; Alatawi, Y.; Qian, J.; Peissig, P.L.; Berg, R.L.; Page, C.D. Authors’ Reply to Courtney Suggs and Colleagues’ Comment on: “Mixed Approach Retrospective Analyses of Suicide and Suicidal Ideation for Brand Compared with Generic Central Nervous System Drugs”. Drug Saf. 2018, 41, 1423–1424. [Google Scholar] [CrossRef]
- Shamseddeen, W.; Clarke, G.; Keller, M.B.; Wagner, K.D.; Birmaher, B.; Emslie, G.J.; Ryan, N.; Asarnow, J.R.; Porta, G.; Brent, D. Adjunctive Sleep Medications and Depression Outcome in the Treatment of Serotonin-Selective Reuptake Inhibitor Resistant Depression in Adolescents Study. J. Child Adolesc. Psychopharmacol. 2012, 22, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Characteristic | Suicide (Percentage) | Not Suicide (Percentage) | p Value * |
|---|---|---|---|
| N = 205 | N = 2963 | ||
| Gender | |||
| Male | 66 (32.2) | 688 (23.2) | 0.005 |
| Female | 139 (67.8) | 2275 (76.8) | |
| Lithium Use | |||
| Yes | 16 (7.8) | 221 (7.5) | 0.964 |
| Not | 189 (92.2) | 2742 (92.5) | |
| ED Visits | |||
| 10 ≤ X | 15 (7.3) | 93 (3.1) | 0.003 |
| 5 ≤ X < 10 | 28 (13.7) | 260 (8.8) | 0.026 |
| 4 | 9 (4.4) | 133 (4.5) | 0.999 |
| 3 | 19 (9.3) | 213 (7.2) | 0.334 |
| 2 | 20 (9.8) | 357 (12.0) | 0.385 |
| 1 | 43 (21.0) | 596 (20.1) | 0.836 |
| 0 | 71 (34.6) | 1311 (44.2) | 0.009 |
| Age | |||
| Mean (SD) | 35.06 (12.92) | 38.45 (13.29) | <0.001 |
| K-Nearest Neighbors | Naïve Bayes | Decision Tree | Support Vector Machine | Logistic Regression | Random Forest | |
|---|---|---|---|---|---|---|
| TP | 182 | 200.6 | 146 | 114.2 | 111.8 | 171 |
| FP | 888 | 2732.6 | 103.8 | 1238.8 | 1074.6 | 17 |
| TN | 2075 | 230.4 | 2859.2 | 1724.2 | 1888.4 | 2946 |
| FN | 23 | 4.4 | 59 | 90.8 | 93.2 | 34 |
| TPR | 0.888 | 0.979 | 0.712 | 0.557 | 0.545 | 0.834 |
| PPV | 0.17 | 0.068 | 0.585 | 0.084 | 0.094 | 0.91 |
| NPV | 0.989 | 0.981 | 0.98 | 0.95 | 0.953 | 0.989 |
| Feature | Feature Importance |
|---|---|
| Age at both diagnosed | 0.141 |
| Disease category 5 in last year | 0.081 |
| Disease category 3 in last year | 0.07 |
| Disease category 1 in last year | 0.061 |
| Trazodone | 0.055 |
| Fentanyl | 0.047 |
| Disease category 11 in last year | 0.039 |
| Emergency department visits in last year | 0.038 |
| Lamotrigine | 0.036 |
| Sertraline | 0.031 |
| Disease category 6 in last year | 0.031 |
| Disease category 2 in last year | 0.023 |
| Quetiapine | 0.023 |
| Citalopram | 0.022 |
| Bupropion | 0.021 |
| Tramadol | 0.021 |
| Fluoxetine | 0.018 |
| Aripiprazole | 0.017 |
| Haloperidol | 0.016 |
| Venlafaxine | 0.016 |
| Lithium | 0.015 |
| Duloxetine | 0.014 |
| Buspirone | 0.012 |
| GENDER | 0.012 |
| Risperidone | 0.011 |
| Disease category 4 in last year | 0.011 |
| Mirtazapine | 0.01 |
| Ziprasidone | 0.009 |
| Olanzapine | 0.009 |
| Promethazine | 0.008 |
| Escitalopram | 0.008 |
| Amphetamine | 0.006 |
| Sumatriptan | 0.006 |
| Disease category 9 in last year | 0.006 |
| Amitriptyline | 0.005 |
| Chlorpromazine | 0.005 |
| Carbamazepine | 0.005 |
| Disease category 10 in last year | 0.004 |
| Paroxetine | 0.004 |
| Methadone | 0.004 |
| Disease category 12 in last year | 0.003 |
| Dextromethorphan | 0.003 |
| Lurasidone | 0.003 |
| Meperidine | 0.003 |
| Rizatriptan | 0.003 |
| Asenapine | 0.002 |
| Doxepin | 0.002 |
| Disease category 8 in last year | 0.002 |
| Vilazodone | 0.001 |
| Perphenazine | 0.001 |
| Nortriptyline | 0.001 |
| Thiothixene | 0.001 |
| Clomipramine | 0.001 |
| Ropinirole | 0.001 |
| Paliperidone | 0.001 |
| Eletriptan | 0.001 |
| Naratriptan | 0 |
| Zolmitriptan | 0 |
| Desvenlafaxine | 0 |
| Selegiline | 0 |
| Levomilnacipran | 0 |
| Milnacipran | 0 |
| Vortioxetine | 0 |
| Dihydroergotamine | 0 |
| Imipramine | 0 |
| Desipramine | 0 |
| Tapentadol | 0 |
| Clozapine | 0 |
| Fluphenazine | 0 |
| Disease category 7 in last year | 0 |
| Trifluoperazine | 0 |
| Almotriptan | 0 |
| Rasagiline | 0 |
| Brexpiprazole | 0 |
| Chlorpheniramine | 0 |
| Cariprazine | 0 |
| Fluvoxamine | 0 |
| Loxapine | 0 |
| Rotigotine | 0 |
| Dexmethylphenidate | 0 |
| Protriptyline | 0 |
| Tranylcypromine | 0 |
| Flibanserin | 0 |
| Amoxapine | 0 |
| Frovatriptan | 0 |
| Iloperidone | 0 |
| Maprotiline | 0 |
| Phenelzine | 0 |
| Pimozide | 0 |
| Nefazodone | 0 |
| TP | FP | TN | FN | TPR | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Retrained model | 171 | 14 | 2949 | 34 | 0.834 | 0.924 | 0.988 |
| Features | T | P | Percentage of 1 in Whole Population | Percentage of 1 with Positive Contribution | Percentage of 1 with Negative Contribution | FDR Adjusted q Value | Direction of Effect |
|---|---|---|---|---|---|---|---|
| Disease category 2 in last year | 2065.444 | <0.001 | 0.064 | 0.919 | 0.017 | <0.001 | SREs |
| Disease category 11 in last year | 2239.822 | <0.001 | 0.098 | 0.996 | 0.027 | <0.001 | SREs |
| Disease category 6 in last year | 1750.073 | <0.001 | 0.066 | 0.846 | 0.021 | <0.001 | SREs |
| Disease category 1 in last year | 2193.804 | <0.001 | 0.232 | 1 | 0.068 | <0.001 | SREs |
| Trazodone | 1248.659 | <0.001 | 0.17 | 0.996 | 0.101 | <0.001 | SREs |
| Sertraline | 1205.388 | <0.001 | 0.116 | 0 | 0.459 | <0.001 | No SREs |
| GENDER | 681.776 | <0.001 | 0.237 | 0.062 | 0.463 | <0.001 | No SREs |
| Haloperidol | 489.011 | <0.001 | 0.046 | 0.32 | 0.021 | <0.001 | SREs |
| Fentanyl | 486.882 | <0.001 | 0.188 | 0.002 | 0.317 | <0.001 | No SREs |
| Aripiprazole | 428.686 | <0.001 | 0.089 | 0.003 | 0.219 | <0.001 | No SREs |
| Lamotrigine | 424.696 | <0.001 | 0.146 | 0.001 | 0.264 | <0.001 | No SREs |
| Disease category 4 in last year | 422.183 | <0.001 | 0.034 | 0.261 | 0.014 | <0.001 | SREs |
| Ziprasidone | 348.145 | <0.001 | 0.037 | 0.202 | 0.013 | <0.001 | SREs |
| Risperidone | 326.949 | <0.001 | 0.063 | 0.378 | 0.043 | <0.001 | SREs |
| Mirtazapine | 166.917 | <0.001 | 0.063 | 0.186 | 0.037 | <0.001 | SREs |
| Quetiapine | 127.63 | <0.001 | 0.135 | 0.32 | 0.109 | <0.001 | SREs |
| Venlafaxine | 119.404 | <0.001 | 0.082 | 0.214 | 0.06 | <0.001 | SREs |
| Buspirone | 111.025 | <0.001 | 0.079 | 0.024 | 0.126 | <0.001 | No SREs |
| Disease category 5 in last year | 108.425 | <0.001 | 0.46 | 0.989 | 0.443 | <0.001 | SREs |
| Duloxetine | 104.213 | <0.001 | 0.069 | 0.175 | 0.048 | <0.001 | SREs |
| Tramadol | 76.116 | <0.001 | 0.131 | 0.038 | 0.162 | <0.001 | No SREs |
| Citalopram | 63.62 | <0.001 | 0.124 | 0.233 | 0.103 | <0.001 | SREs |
| Bupropion | 18.005 | <0.001 | 0.109 | 0.066 | 0.122 | <0.001 | No SREs |
| Fluoxetine | 14.305 | <0.001 | 0.092 | 0.065 | 0.107 | <0.001 | No SREs |
| Disease category 3 in last year | 14.02 | <0.001 | 0.749 | 0.907 | 0.744 | <0.001 | SREs |
| Promethazine | 7.757 | 0.005 | 0.052 | 0.038 | 0.061 | 0.006 | No SREs |
| Lithium | 5.165 | 0.023 | 0.076 | 0.058 | 0.083 | 0.024 | No SREs |
| Olanzapine | 1.026 | 0.311 | 0.054 | 0.048 | 0.057 | 0.311 | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Guo, X.; Qi, X.; Matharu, M.; Patel, R.; Sakolsky, D.; Kirisci, L.; Silverstein, J.C.; Wang, L. Prediction of Suicide-Related Events by Analyzing Electronic Medical Records from PTSD Patients with Bipolar Disorder. Brain Sci. 2020, 10, 784. https://doi.org/10.3390/brainsci10110784
Fan P, Guo X, Qi X, Matharu M, Patel R, Sakolsky D, Kirisci L, Silverstein JC, Wang L. Prediction of Suicide-Related Events by Analyzing Electronic Medical Records from PTSD Patients with Bipolar Disorder. Brain Sciences. 2020; 10(11):784. https://doi.org/10.3390/brainsci10110784
Chicago/Turabian StyleFan, Peihao, Xiaojiang Guo, Xiguang Qi, Mallika Matharu, Ravi Patel, Dara Sakolsky, Levent Kirisci, Jonathan C. Silverstein, and Lirong Wang. 2020. "Prediction of Suicide-Related Events by Analyzing Electronic Medical Records from PTSD Patients with Bipolar Disorder" Brain Sciences 10, no. 11: 784. https://doi.org/10.3390/brainsci10110784
APA StyleFan, P., Guo, X., Qi, X., Matharu, M., Patel, R., Sakolsky, D., Kirisci, L., Silverstein, J. C., & Wang, L. (2020). Prediction of Suicide-Related Events by Analyzing Electronic Medical Records from PTSD Patients with Bipolar Disorder. Brain Sciences, 10(11), 784. https://doi.org/10.3390/brainsci10110784






