A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder
Abstract
:1. Introduction
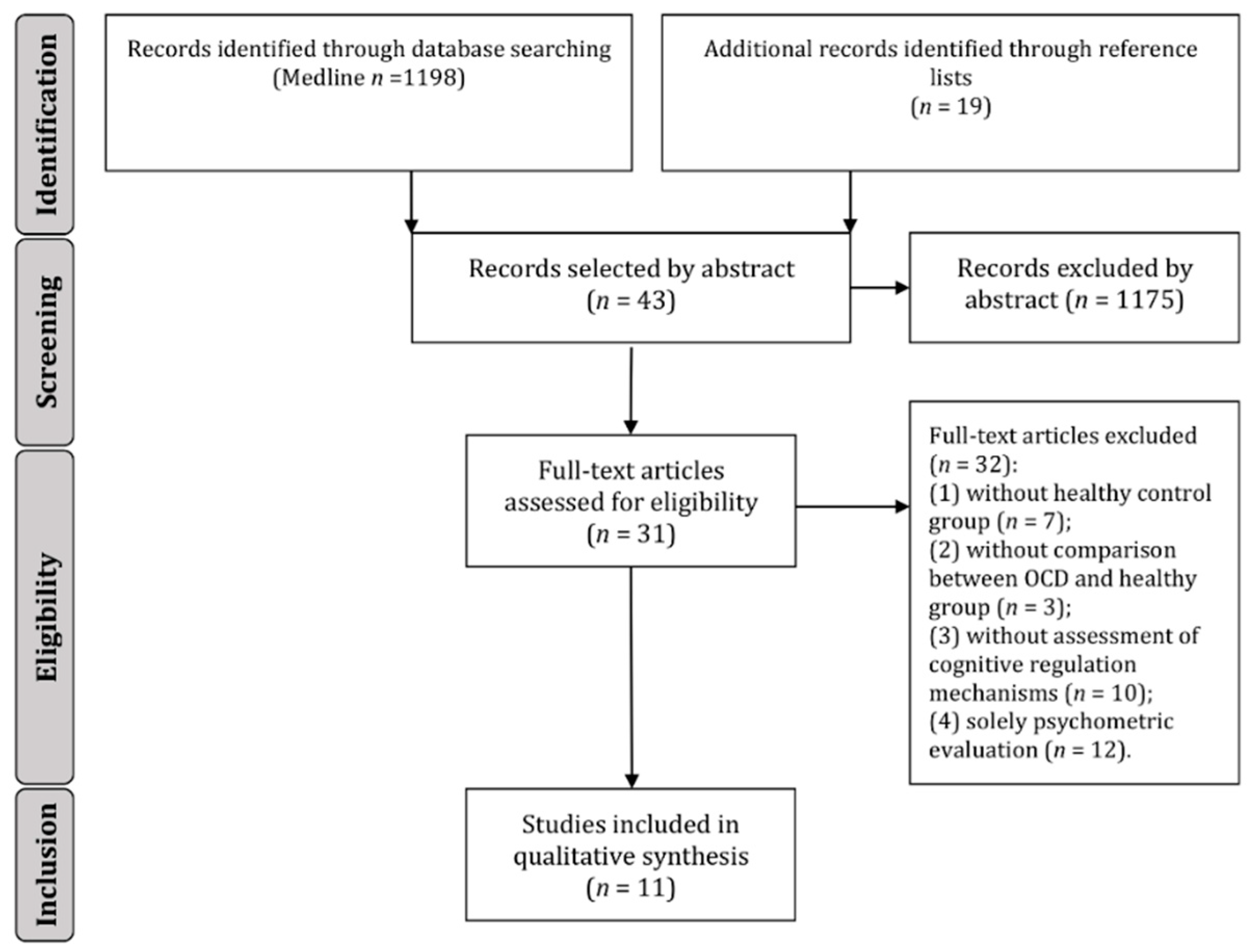
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OCD | obsessive-compulsive disorder; |
| vlPFC | ventrolateral prefrontal cortex; |
| dlPFC | dorsolateral prefrontal cortex; |
| ACC | anterior cingulate cortex; |
| Y-BOCS | Yale-Brown Obsessive Compulsive Scale; |
| DSM | Diagnostic and Statistical Manual of Mental Disorders; |
| fMRI | functional magnetic resonance imaging; |
| dmPFC | dorsomedial prefrontal cortex; |
| CBT | cognitive-behavioral therapy. |
References
- American Psychiatric Association. Diagnostic Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association Pub: Washington, DC, USA, 2013; ISBN 0890425574,9780890425572. [Google Scholar]
- Hezel, D.M.; McNally, R.J. A Theoretical Review of Cognitive Biases and Deficits in Obsessive–Compulsive Disorder. Biol. Psychol. 2016, 121, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, A.; Lee, W.H.; Leibu, E.; Laird, A.; Glahn, D.; Goodman, W.; Frangou, S. Neural correlates of affective and non-affective cognition in obsessive compulsive disorder: A meta-analysis of functional imaging studies. Eur. Psychiatry 2017, 46, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Gruner, P.; Pittenger, C. Cognitive inflexibility in Obsessive-Compulsive Disorder. Neuroscience 2017, 345, 243–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picó-Pérez, M.; Moreira, P.S.; Ferreira, V.D.M.; Radua, J.; Mataix-Cols, D.; Sousa, N.; Soriano-Mas, C.; Morgado, P. Modality-specific overlaps in brain structure and function in Obsessive-compulsive disorder: Multimodal meta-analysis of case-control MRI studies. Neurosci. Biobehav. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hallion, L.S.; Tolin, D.F.; Billingsley, A.L.; Kusmierski, S.N.; Diefenbach, G.J. “Cold” Cognitive Control and Attentional Symptoms in Anxiety: Perceptions Versus Performance. Behav. Ther. 2019, 50, 1150–1163. [Google Scholar] [CrossRef]
- Kikul, J.; Vetter, J.; Lincoln, T.M.; Exner, C. Effects of cognitive self-consciousness on visual memory in obsessive-compulsive disorder. J. Anxiety Disord. 2011, 25, 490–497. [Google Scholar] [CrossRef]
- Zetsche, U.; Rief, W.; Westermann, S.; Exner, C. Cognitive deficits are a matter of emotional context: Inflexible strategy use mediates context-specific learning impairments in OCD. Cogn. Emot. 2015, 29, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Salkovskis, P.M. Obsessional-compulsive problems: A cognitive-behavioural analysis. Behav. Res. Ther. 1985, 23, 571–583. [Google Scholar] [CrossRef]
- Rachman, S. A cognitive theory of obsessions. Behav. Res. Ther. 1997, 35, 793–802. [Google Scholar] [CrossRef]
- Frost, R.; Steketee, G.; Amir, N.; Bouvard, M.; Carmin, C.; Clark, D.A.; Cottraux, J.; Eisen, J.; Emmelkamp, P.; Foa, E.; et al. Cognitive assessment of obsessive-compulsive disorder. Behav. Res. Ther. 1997, 35, 667–681. [Google Scholar] [CrossRef]
- Najmi, S.; Reese, H.; Wilhelm, S.; Fama, J.; Beck, C.; Wegner, D.M. Learning the Futility of the Thought Suppression Enterprise in Normal Experience and in Obsessive Compulsive Disorder. Behav. Cogn. Psychother. 2010, 38, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Soriano, G.; Belloch, A. Symptom dimensions in obsessive-compulsive disorder: Differences in distress, interference, appraisals and neutralizing strategies. J. Behav. Ther. Exp. Psychiatry 2013, 44, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Morillo, C.; Belloch, A.; García-Soriano, G. Clinical obsessions in obsessive–compulsive patients and obsession-relevant intrusive thoughts in non-clinical, depressed and anxious subjects: Where are the differences? Behav. Res. Ther. 2007, 45, 1319–1333. [Google Scholar] [CrossRef]
- Purdon, C.; Rowa, K.; Antony, M.M. Thought suppression and its effects on thought frequency, appraisal and mood state in individuals with obsessive-compulsive disorder. Behav. Res. Ther. 2005, 43, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Ahern, C.; Kyrios, M.; Meyer, D. Exposure to unwanted intrusions, neutralizing and their effects on self-worth and obsessive-compulsive phenomena. J. Behav. Ther. Exp. Psychiatry 2015, 49, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Salkovskis, P.M.; Thorpe, S.J.; Wahl, K.; Wroe, A.L.; Forrester, E. Neutralizing increases discomfort associated with obsessional thoughts: An experimental study with obsessional patients. J. Abnorm. Psychol. 2003, 112, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigg, J.T. Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J. Child Psychol. Psychiatry 2017, 58, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, L.; Zalesky, A.; Fornito, A.; Mattingley, J.B. Dynamic cooperation and competition between brain systems during cognitive control. Trends Cogn. Sci. 2013, 17, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, A.L.; Hagland, P.; Radua, J.; Mataix-Cols, D.; Kvale, G.; Hansen, B.; van den Heuvel, O.A. Emotional Processing in Obsessive-Compulsive Disorder: A Systematic Review and Meta-analysis of 25 Functional Neuroimaging Studies. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Webb, T.L.; Miles, E.; Sheeran, P. Dealing with feeling: A meta-analysis of the effectiveness of strategies derived from the process model of emotion regulation. Psychol. Bull. 2012, 138, 775–808. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, S.J.; Van Der Werf, Y.D.; Mataix-Cols, D.; Trujillo, J.P.; Van Oppen, P.; Veltman, D.J.; van den Heuvel, O.A. Emotion regulation before and after transcranial magnetic stimulation in obsessive compulsive disorder. Psychol. Med. 2015, 45, 3059–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsen, A.L.L.; de Wit, S.J.J.; de Vries, F.E.E.; Cath, D.C.C.; Veltman, D.J.J.; van der Werf, Y.D.D.; Mataix-Cols, D.; Hansen, B.; Kvale, G.; van den Heuvel, O.A.A. Emotion Regulation in Obsessive-Compulsive Disorder, Unaffected Siblings, and Unrelated Healthy Control Participants. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Tolin, D.F.; Abramowitz, J.S.; Przeworski, A.; Foa, E.B. Thought suppression in obsessive-compulsive disorder. Behav. Res. Ther. 2002, 40, 1255–1274. [Google Scholar] [CrossRef]
- Tolin, D.F.; Abramowitz, J.S.; Hamlin, C.; Foa, E.B.; Synodi, D.S. Attributions for thought suppression failure in obsessive-compulsive disorder. Cognit. Ther. Res. 2002, 26, 505–517. [Google Scholar] [CrossRef]
- Janeck, A.S.; Calamari, J.E. Thought suppression in obsessive-compulsive disorder. Cognit. Ther. Res. 1999, 23, 497–509. [Google Scholar] [CrossRef]
- Najmi, S.; Riemann, B.C.; Wegner, D.M. Managing unwanted intrusive thoughts in obsessive-compulsive disorder: Relative effectiveness of suppression, focused distraction, and acceptance. Behav. Res. Ther. 2009, 47, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.; Pflugradt, E.; Stierle, C.; Exner, C. Changing disgust through imagery rescripting and cognitive reappraisal in contamination-based obsessive-compulsive disorder. J. Anxiety Disord. 2018, 54, 36–48. [Google Scholar] [CrossRef]
- Paul, S.; Simon, D.; Endrass, T.; Kathmann, N. Altered emotion regulation in obsessive-compulsive disorder as evidenced by the late positive potential. Psychol. Med. 2016, 46, 137–147. [Google Scholar] [CrossRef]
- Simon, D.; Adler, N.; Kaufmann, C.; Kathmann, N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage Clin. 2014, 4, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koçak, O.M.; Özpolat, A.Y.; Atbaşoğlu, C.; Çiçek, M. Cognitive control of a simple mental image in patients with obsessive–compulsive disorder. Brain Cogn. 2011, 76, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Ipser, J.; Taylor, P.; Alonso, P.; López-Solà, C.; Real, E.; Segalàs, C.; Roos, A.; Menchón, J.M.; Stein, D.J.; et al. Intrinsic functional and structural connectivity of emotion regulation networks in obsessive-compulsive disorder. Depress. Anxiety 2019, 36, 110–120. [Google Scholar] [CrossRef]
- Lochner, C.; Stein, D.J. Gender in obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Arch. Womens. Ment. Health 2001, 4, 19–26. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Kwong, J.S.W.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid. Based. Med. 2015, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Radua, J.; Steward, T.; Menchón, J.M.; Soriano-Mas, C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2017, 79, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilverstand, A.; Parvaz, M.A.; Goldstein, R.Z. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 2017, 151, 105–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández de la Cruz, L.; Landau, D.; Iervolino, A.C.; Santo, S.; Pertusa, A.; Singh, S.; Mataix-Cols, D. Experiential avoidance and emotion regulation difficulties in hoarding disorder. J. Anxiety Disord. 2013, 27, 204–209. [Google Scholar] [CrossRef]
- Yazici, K.U.; Yazici, I.P. Decreased theory of mind skills, increased emotion dysregulation and insight levels in adolescents diagnosed with obsessive compulsive disorder. Nord. J. Psychiatry 2019, 73, 462–469. [Google Scholar] [CrossRef]
- Goldberg, X.; Cardoner, N.; Alonso, P.; López-Solà, C.; Real, E.; Hernández-Ribas, R.; Jiménez-Murcia, S.; Subirà, M.; Segalàs, C.; Menchón, J.M.; et al. Inter-individual variability in emotion regulation: Pathways to obsessive-compulsive symptoms. J. Obsessive. Compuls. Relat. Disord. 2016, 11, 105–112. [Google Scholar] [CrossRef]
- Dörfel, D.; Lamke, J.-P.; Hummel, F.; Wagner, U.; Erk, S.; Walter, H. Common and differential neural networks of emotion regulation by Detachment, Reinterpretation, Distraction, and Expressive Suppression: A comparative fMRI investigation. Neuroimage 2014, 101, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Picó-Pérez, M.; Alonso, P.; Contreras-Rodríguez, O.; Martínez-Zalacaín, I.; López-Solà, C.; Jiménez-Murcia, S.; Verdejo-García, A.; Menchón, J.M.; Soriano-Mas, C. Dispositional use of emotion regulation strategies and resting-state cortico-limbic functional connectivity. Brain Imaging Behav. 2018, 12, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Naragon-Gainey, K.; McMahon, T.P.; Chacko, T.P. The structure of common emotion regulation strategies: A meta-analytic examination. Psychol. Bull. 2017, 143, 384–427. [Google Scholar] [CrossRef] [PubMed]
- Moodie, C.A.; Suri, G.; Goerlitz, D.S.; Mateen, M.A.; Sheppes, G.; McRae, K.; Lakhan-Pal, S.; Thiruchselvam, R.; Gross, J.J. The neural bases of cognitive emotion regulation: The roles of strategy and intensity. Cogn. Affect. Behav. Neurosci. 2020, 20, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. NeuroImage Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, J.Ö.; Naumann, E.; Holmes, E.A.; Tuschen-Caffier, B.; Samson, A.C. Emotion Regulation Strategies in Depressive and Anxiety Symptoms in Youth: A Meta-Analytic Review. J. Youth Adolesc. 2017, 46, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.L.; Milkman, K.L.; Laibson, D. Beyond Willpower: Strategies for Reducing Failures of Self-Control. Psychol. Sci. Public Interes. 2018, 19, 102–129. [Google Scholar] [CrossRef] [Green Version]
- Bluett, E.J.; Homan, K.J.; Morrison, K.L.; Levin, M.E.; Twohig, M.P. Acceptance and commitment therapy for anxiety and OCD spectrum disorders: An empirical review. J. Anxiety Disord. 2014, 28, 612–624. [Google Scholar] [CrossRef]
- Twohig, M.P.; Abramowitz, J.S.; Smith, B.M.; Fabricant, L.E.; Jacoby, R.J.; Morrison, K.L.; Bluett, E.J.; Reuman, L.; Blakey, S.M.; Ledermann, T. Adding acceptance and commitment therapy to exposure and response prevention for obsessive-compulsive disorder: A randomized controlled trial. Behav. Res. Ther. 2018, 108, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picó-Pérez, M.; Alemany-Navarro, M.; Dunsmoor, J.E.; Radua, J.; Albajes-Eizagirre, A.; Vervliet, B.; Cardoner, N.; Benet, O.; Harrison, B.J.; Soriano-Mas, C.; et al. Common and distinct neural correlates of fear extinction and cognitive reappraisal: A meta-analysis of fMRI studies. Neurosci. Biobehav. Rev. 2019, 104, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Buhle, J.T.; Silvers, J.A.; Wager, T.D.; Lopez, R.; Onyemekwu, C.; Kober, H.; Weber, J.; Ochsner, K.N. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb. Cortex 2014, 24, 2981–2990. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J.; Feusner, J. Cognitive-behavioral therapy for obsessive– compulsive disorder: Access to treatment, prediction of long-term outcome with neuroimaging. Psychol. Res. Behav. Manag. 2015, 8, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; van den Heuvel, O.A.; Simpson, H.B. Obsessive–compulsive disorder. Nat. Rev. Dis. Prim. 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, S.J.; Stein, D.J. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin. Neurosci. 2015, 17, 261–279. [Google Scholar]
- Polman, A.; Bouman, T.K.; van Hout, W.J.P.J.; de Jong, P.J.; den Boer, J.A. Processes of change in cognitive-behavioural treatment of obsessive-compulsive disorder: Current status and some future directions. Clin. Psychol. Psychother. 2009, 17. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, A.L.; van den Heuvel, O.A.; Hansen, B.; Kvale, G. Neuroimaging of psychotherapy for obsessive–compulsive disorder: A systematic review. Psychiatry Res. Neuroimaging 2015, 233, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Vandborg, S.K.J.Æ.R.; Hartmann, T.U.E.B.; Bennedsen, B.E.; Pedersen, A.D.; Eskildsen, A.; Bror, P.; Videbech, H. Do cognitive functions in obsessive—Compulsive disorder change after treatment? A systematic review and a double case report. Nord. J. Psychiatry 2012, 66, 60–67. [Google Scholar] [CrossRef]
- Wolters, L.H.; Prins, P.J.; Garst, G.J.A.; Hogendoorn, S.M.; Boer, F.; Vervoort, L.; de Haan, E. Mediating Mechanisms in Cognitive Behavioral Therapy for Childhood OCD: The Role of Dysfunctional Beliefs. Child Psychiatry Hum. Dev. 2019, 50, 173–185. [Google Scholar] [CrossRef] [Green Version]
- Polman, A.; Bouman, T.K.; van Geert, P.L.C.; de Jong, P.J.; den Boer, J.A. Dysfunctional beliefs in the process of change of cognitive treatment in obsessive compulsive checkers. Clin. Psychol. Psychother. 2011, 18, 256–273. [Google Scholar] [CrossRef]
- McLean, P.D.; Whittal, M.L.; Thordarson, D.S.; Taylor, S.; Söchting, I.; Koch, W.J.; Paterson, R.; Anderson, K.W. Cognitive versus behavior therapy in the group treatment of Obsessive-Compulsive disorder. J. Consult. Clin. Psychol. 2001, 69, 205–214. [Google Scholar] [CrossRef]
- Olatunji, B.O.; Rosenfield, D.; Tart, C.D.; Cottraux, J.; Powers, M.B.; Smits, J.A.J. Behavioral versus cognitive treatment of obsessive-compulsive disorder: An examination of outcome and mediators of change. J. Consult. Clin. Psychol. 2013, 81, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Ma, L.; Jiang, N.; Huang, R.; Li, L.; Gong, L.; He, C.; Xiao, C.; Liu, W.; Xu, S.; et al. Imbalanced functional link between reward circuits and the cognitive control system in patients with obsessive-compulsive disorder. Brain Imaging Behav. 2017, 11, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Brandl, F.; Le Houcq Corbi, Z.; Mulej Bratec, S.; Sorg, C. Cognitive reward control recruits medial and lateral frontal cortices, which are also involved in cognitive emotion regulation: A coordinate-based meta-analysis of fMRI studies. Neuroimage 2019, 200, 659–673. [Google Scholar] [CrossRef] [PubMed]

| Study | Groups | Size | Age (years) | Gender (%F|%M) | Diagnosis | Y-BOCS | Treatment | Psychometrics | Task | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Janeck et al., [28] | OCD | 31 | 31.9 ± 10.2 | 39|61 | DSM-IV | 22.0 ± 6.3 | 48% medicated | − | Suppression of negative thought | ↑overall frequency and distress from negative thought; ↑number of participants with negative thought after suppression. |
| Healthy | 32 | 31.2 ± 13.5 | 66|34 | − | − | |||||
| Tolin et al., [26] | OCD | 15 | 29.6 ± 9.9 | 50|50 | DSM-IV | 23.8 ± 5.4 | 67% medicated; 73% CBT | − | Suppression of neutral thought | ↑frequency of target thought during suppression; ↑frequency and time thinking about target thought overall. |
| Healthy | 14 | 26.9 ± 6.5 | 43|57 | − | − | |||||
| OCD | 15 | 25.8 ± 10.1 | 36|64 | DSM-IV | 24.2 ± 5.3 | 75% medicated; 75% CBT | − | Suppression of neutral thought | ↓detection time for words related to target thought versus non-related words and non-words during suppression. | |
| Healthy | 13 | 25.5 ± 6.0 | 61|39 | − | − | |||||
| Tolin et al., [27] | OCD | 17 | 29.6 ± 9.9 | 50|50 | DSM-IV | 23.8 ± 5.4 | 67% medicated; 73% CBT | − | Suppression of neutral thought | ↑frequency of target thought during suppression; ↑internal meaning (weakness/uncontrollable thoughts) of suppression failure. |
| Healthy | 8 | 25.1 ± 4.8 | 37|63 | − | − | |||||
| Najmi et al., [29] | OCD | 20 | 29.0 ± 12.0 | 55|45 | DSM-IV | obsessions 11.1 ± 3.1; compulsions 10.7 ± 4.7 | 95% medicated | − | Suppression, focused distraction, or acceptance of intrusive thoughts | ↑distress during all conditions; ↑intrusive thoughts after and during suppression; ↑distress after versus during suppression; ↑distress after suppression versus focused distraction and acceptance; ↑intrusive thoughts after suppression versus acceptance; ↓distress after versus during acceptance. |
| Healthy | 20 | 30.0 ± 9.0 | 65|35 | obsessions 1.5 ± 2.0; compulsions 1.0 ± 1.7 | − | |||||
| Fink et al., [30] | OCD contamination/cleaning | 30 | 33.3 ± 11.4 | 59|41 | DSM-IV | 23.0 ± 6.1 | 60% medicated | ↓ERQ reappraisal and ↑ERQ suppression | Mental imagery rescripting or cognitive reappraisal of disgust-inducing pictures | ↑disgust ratings before the task. |
| Healthy | 30 | 32.8 ± 11.9 | 59|41 | − | − |
| Study | Groups | Size | Age (years) | Gender (%F|%M) | Diagnosis | Y-BOCS | Treatment | Psychometrics | Technique | Task | Behavioral Results | Brain Activity Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Koçak et al., [33] | OCD | 12 | 27.0 ± 5.8 | 50|50 | DSM-IV | 20.2 ± 6.2 | 66% medicated | − | fMRI | Maintenance, suppression, or manipulation of a mental image. | ↑performance score during suppression. | ↓activity in R inferior parietal lobe, R posterior cingulate, and R superior frontal gyrus for all conditions. |
| Healthy | 12 | 25.1 ± 3.32 | 50|50 | − | − | |||||||
| Simon et al., [32] | OCD | 21 | 33.1 ± 10.8 | 62|38 | DSM-IV | 21.2 ± 6.8 | Medication-free; 33% CBT | − | fMRI | Appraisal or distraction of OCD-related, aversive, or neutral pictures. | − | ↓activity in L amygdala, L dorsal anterior cingulate cortex, L insula, L postcentral gyrus, and R anterior cerebellum during distraction for OCD-related pictures. |
| Healthy | 21 | 33.1 ± 10.1 | 62|38 | − | − | |||||||
| Paul et al., [31] | OCD | 24 | 31.7 ± 9.1 | 54|46 | DSM-IV | 22.2 ± 4.1 | 37% medicated; 37% CBT | ↓ERQ reappraisal; ↓CERQ positive refocusing; ↑CERQ catastrophizing. | EEG | Cognitive reappraisal or cognitive distraction of neutral, aversive, and OCD-related pictures. | ↓arousal for aversive pictures after reappraisal compared to distraction. | Unchanged Late Positive Potential amplitude during reappraisal and distraction (↓healthy). |
| Healthy | 24 | 31.2 ± 8.2 | 54|46 | − | − | |||||||
| de Wit et al., [24]; Thorsen et al., [25] | OCD | 43 | 37.6 ± 10.0 | 51|49 | DSM-IV | 21.6 ± 6.1 | Unmedicated for ≥ 4 weeks | ↓ERQ reappraisal. | fMRI | Cognitive reappraisal of fearful and OCD-related pictures. | ↑distress reduction during OCD-related reappraisal. | Fear reappraisal: ↓activity in R superior temporal gyrus and L middle frontal gyrus, and ↓functional connectivity in L posterior insula and R amygdala; OCD-related reappraisal: ↑activity in R superior frontal gyrus ad R lingual gyrus (uncorrected results). |
| Healthy | 38 | 39.0 ± 11.3 | 53|47 | 0.0 ± 0.0 | − | |||||||
| Maria Picó-Pérez et al., [34] | OCD | 73 | 37.7 ± 10.2 | 41|59 | DSM-IV | 22.1 ± 6.3 | 92% medicated | ↓ERQ reappraisal and ↑ERQ suppression. | fMRI | − | − | Negative correlation between L amygdala–L posterior insula functional connectivity and reappraisal score in controls but not OCD. |
| Healthy | 42 | 39.4 ± 9.8 | 48|52 | − | − |
| Study | Definition of Appropriate and Focused Question | Selection of Cases and Controls from Comparable Populations | Use of Same Exclusion Criteria for Cases and Controls | Participation Rate for Cases and Controls | Comparison of Similarities/Differences between Participants and non-Participants | Definition and Differentiation between Cases and Controls | Clear Definition That Controls Are Not Cases | Selection of Measures to Prevent Knowledge of Primary Exposure from Influencing Case Ascertainment | Standard, Valid, and Reliable Measurement of Exposure Status | Identification and Accountability (Design/Analysis) of Potential Confounders | Description of Confidence Intervals |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Janeck et al., [28] | Well covered | Poorly addressed | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Not addressed | Well covered |
| Tolin et al., [26] | Well covered | Poorly addressed | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Not addressed | Poorly addressed |
| Tolin et al., [27] | Well covered | Poorly addressed | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Poorly addressed | Poorly addressed |
| Najmi et al., [29] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Adequately addressed | Poorly addressed |
| Koçak et al., [33] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Not addressed | Poorly addressed |
| Simon et al., [32] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Well covered | Adequately addressed |
| de Wit et al., [24] | Adequately addressed | Well covered | Well covered | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Not addressed | Poorly addressed |
| Paul et al., [31] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Well covered | Adequately addressed |
| Fink et al., [30] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Not addressed | Adequately addressed |
| Thorsen et al., [25] | Well covered | Well covered | Well covered | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Well covered | Adequately addressed |
| Maria Picó-Pérez et al., [34] | Well covered | Well covered | Adequately addressed | Not addressed | Not addressed | Well covered | Well covered | Not applicable | Not applicable | Well covered | Adequately addressed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, S.; Pêgo, J.M.; Morgado, P. A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder. Brain Sci. 2020, 10, 797. https://doi.org/10.3390/brainsci10110797
Ferreira S, Pêgo JM, Morgado P. A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder. Brain Sciences. 2020; 10(11):797. https://doi.org/10.3390/brainsci10110797
Chicago/Turabian StyleFerreira, Sónia, José Miguel Pêgo, and Pedro Morgado. 2020. "A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder" Brain Sciences 10, no. 11: 797. https://doi.org/10.3390/brainsci10110797
APA StyleFerreira, S., Pêgo, J. M., & Morgado, P. (2020). A Systematic Review of Behavioral, Physiological, and Neurobiological Cognitive Regulation Alterations in Obsessive-Compulsive Disorder. Brain Sciences, 10(11), 797. https://doi.org/10.3390/brainsci10110797