Bioenergy Crisis in Coronavirus Diseases?
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Coronavirus COVID-19 (SARS-CoV-2)|Johns Hopkins ABX Guide. Available online: https://www.hopkinsguides.com/hopkins/.//view/Johns_Hopkins_ABX_Guide/540747/all/Coronavirus_COVID_19__SARS_CoV_2_?refer=true (accessed on 11 April 2020).
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cureus|Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Available online: https://www.cureus.com/articles/29414-neurological-complications-of-coronavirus-disease-covid-19-encephalopathy (accessed on 29 March 2020).
- Poston, J.T.; Patel, B.K.; Davis, A.M. Management of Critically Ill Adults with COVID-19. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020. [Google Scholar] [CrossRef]
- Poyiadji, N.; Shahin, G.; Noujaim, D.; Stone, M.; Patel, S.; Griffith, B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology 2020, 201187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hack, C.E.; Zeerleder, S. The endothelium in sepsis: Source of and a target for inflammation. Crit. Care Med. 2001, 29, S21–S27. [Google Scholar] [CrossRef] [Green Version]
- Heming, N.; Mazeraud, A.; Verdonk, F.; Bozza, F.A.; Chrétien, F.; Sharshar, T. Neuroanatomy of sepsis-associated encephalopathy. Crit. Care 2017, 21, 65. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, A.M.; Zappasodi, F.; Pompeo, F.D.; Merla, A. Simultaneous functional near-infrared spectroscopy and electroencephalography for monitoring of human brain activity and oxygenation: A review. Neurophotonics 2017, 4, 041411. [Google Scholar] [CrossRef]
- Lee, I.; Hüttemann, M. Energy crisis: The role of oxidative phosphorylation in acute inflammation and sepsis. Biochim. Biophys. Acta 2014, 1842, 1579–1586. [Google Scholar] [CrossRef] [Green Version]
- Du, F.; Zhu, X.-H.; Zhang, Y.; Friedman, M.; Zhang, N.; Ugurbil, K.; Chen, W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc. Natl. Acad. Sci. USA 2008, 105, 6409–6414. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first Case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, M.; Dutta, A. Computational Modeling of the Photon Transport, Tissue Heating, and Cytochrome C Oxidase Absorption during Transcranial Near-Infrared Stimulation. Brain Sci. 2019, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domercq, M.; Vazquez, N.; Matute, C. Neurotransmitter signaling in the pathophysiology of microglia. Front. Cell. Neurosci. 2013, 7, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, C.; Fordsmann, J.C.; Jensen, S.H.; Gesslein, B.; Lønstrup, M.; Hald, B.O.; Zambach, S.A.; Brodin, B.; Lauritzen, M.J. Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proc. Natl. Acad. Sci. USA 2018, 115, E5796–E5804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Georgia, M.A.; Deogaonkar, A. Multimodal monitoring in the neurological intensive care unit. Neurologist 2005, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Busl, K.M.; Bleck, T.P.; Varelas, P.N. Neurocritical Care Outcomes, Research, and Technology: A Review. JAMA Neurol. 2019, 76, 612–618. [Google Scholar] [CrossRef]
- Wurfel, M.M. Genetic insights into sepsis: What have we learned and how will it help? Curr. Pharm. Des. 2008, 14, 1900–1911. [Google Scholar] [CrossRef]
- Nies, V.J.M.; Sancar, G.; Liu, W.; van Zutphen, T.; Struik, D.; Yu, R.T.; Atkins, A.R.; Evans, R.M.; Jonker, J.W.; Downes, M.R. Fibroblast Growth Factor Signaling in Metabolic Regulation. Front. Endocrinol. 2016, 6, 193. [Google Scholar] [CrossRef] [Green Version]
- Senyilmaz, D.; Teleman, A.A. Chicken or the egg: Warburg effect and mitochondrial dysfunction. F1000Prime Rep. 2015, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-W.; Jiang, X.; Zhang, Y.; Wang, J.; Xie, J.; Wang, Y.-Q.; Li, Y.-H. FGF21 Protects Against Hypoxia Injury Through Inducing HSP72 in Cerebral Microvascular Endothelial Cells. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, E.K.; Benson, C.A.; Narla, S.T.; Dimitri, A.; Chuye, L.E.B.; Dhiman, S.; Harikrishnan, K.; Elahi, S.; Freedman, D.; Brennand, K.J.; et al. Cerebral organoids reveal early cortical maldevelopment in schizophrenia-computational anatomy and genomics, role of FGFR1. Transl. Psychiatry 2017, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, H. The Effects of Immune Factor, TNFα, on Human Fetal Neural Development and Schizophrenia in a Cerebral Organoid Model. Master’s Thesis, State University of New York at Buffalo, Buffalo, NY, USA, 2019. [Google Scholar]
- Balestra, G.M.; Legrand, M.; Ince, C. Microcirculation and mitochondria in sepsis: Getting out of breath. Curr. Opin. Anaesthesiol. 2009, 22, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011, 107, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banaji, M.; Mallet, A.; Elwell, C.E.; Nicholls, P.; Tachtsidis, I.; Smith, M.; Cooper, C.E. Modelling of mitochondrial oxygen consumption and NIRS detection of cytochrome oxidase redox state. Adv. Exp. Med. Biol. 2010, 662, 285–291. [Google Scholar]
- Yamane, K.; Indalao, I.L.; Chida, J.; Yamamoto, Y.; Hanawa, M.; Kido, H. Diisopropylamine Dichloroacetate, a Novel Pyruvate Dehydrogenase Kinase 4 Inhibitor, as a Potential Therapeutic Agent for Metabolic Disorders and Multiorgan Failure in Severe Influenza. PLoS ONE 2014, 9, e98032. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Yang, Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell. Mol. Immunol. 2016, 13, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020, 38, 379–381. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T. Organoids for Drug Discovery and Personalized Medicine. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 447–462. [Google Scholar] [CrossRef]
- Ormel, P.R.; Vieira de Sá, R.; van Bodegraven, E.J.; Karst, H.; Harschnitz, O.; Sneeboer, M.A.M.; Johansen, L.E.; van Dijk, R.E.; Scheefhals, N.; Berdenis van Berlekom, A.; et al. Microglia innately develop within cerebral organoids. Nat. Commun. 2018, 9, 4167. [Google Scholar] [CrossRef]
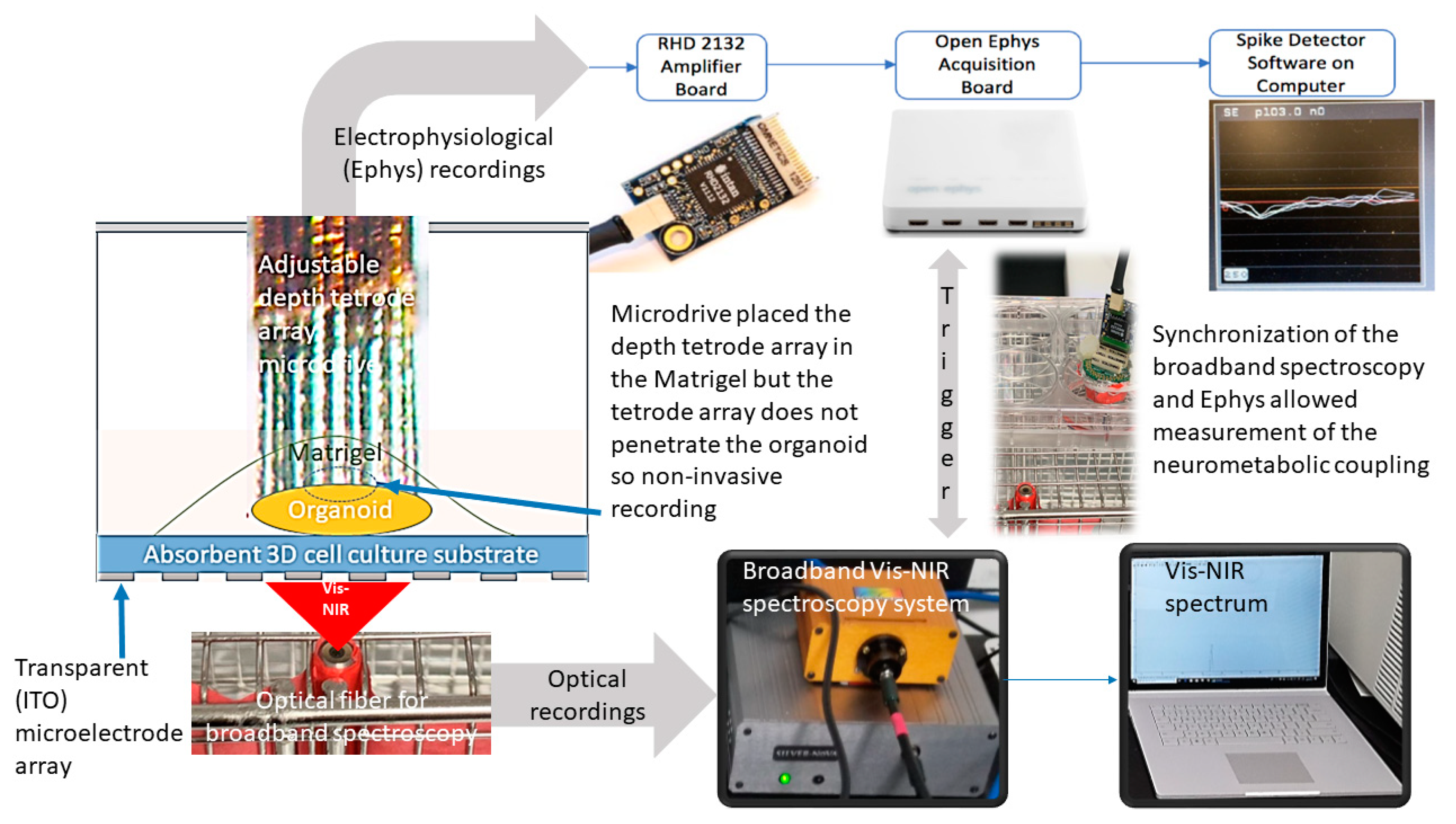
- Bhattacharya, M.; Freedman, D.; Stachowiak, E.; Stachowiak, M.; Dutta, A. Development of Bidirectional ‘Mini-Brain’ Computer Interface (mBCI) to Modulate Functional Neural Circuits—Stimulation and Recording from a Cerebral Organoid. Available online: https://www.researchgate.net/publication/329103836_Development_of_bidirectional_'mini-Brain'_computer_interface_mBCI_to_modulate_functional_neural_circuits_-_stimulation_and_recording_from_a_cerebral_organoid (accessed on 30 March 2020).
- Siegle, J.H.; López, A.C.; Patel, Y.A.; Abramov, K.; Ohayon, S.; Voigts, J. Open Ephys: An open-source, plugin-based platform for multichannel electrophysiology. J. Neural Eng. 2017, 14, 045003. [Google Scholar] [CrossRef]
- Hollis, V.S.; Palacios-Callender, M.; Springett, R.J.; Delpy, D.T.; Moncada, S. Monitoring cytochrome redox changes in the mitochondria of intact cells using multi-wavelength visible light spectroscopy. Biochim. Biophys. Acta 2003, 1607, 191–202. [Google Scholar] [CrossRef] [PubMed]
- de Roever, I.; Bale, G.; Cooper, R.J.; Tachtsidis, I. Functional NIRS Measurement of Cytochrome-C-Oxidase Demonstrates a More Brain-Specific Marker of Frontal Lobe Activation Compared to the Haemoglobins. Adv. Exp. Med. Biol. 2017, 977, 141–147. [Google Scholar]
- Hotchkiss, R.S.; Moldawer, L.L.; Opal, S.M.; Reinhart, K.; Turnbull, I.R.; Vincent, J.-L. Sepsis and septic shock. Nat. Rev. Dis. Primer 2016, 2, 16045. [Google Scholar] [CrossRef] [Green Version]
- Othman, M.; Bhattacharya, M.; Møller, K.; Kjeldsen, S.; Grand, J.; Kjaergaard, J.; Dutta, A.; Kondziella, D. Resting-state NIRS-EEG in unresponsive patients with acute brain injury: A proof-of-concept study. Neurocrit. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Sordillo, L.A.; Rodríguez-Contreras, A.; Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophotonics 2016, 9, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Faigle, R.; Sutter, R.; Kaplan, P.W. The electroencephalography of encephalopathy in patients with endocrine and metabolic disorders. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2013, 30, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutta, A.; Das, A.; Kondziella, D.; Stachowiak, M.K. Bioenergy Crisis in Coronavirus Diseases? Brain Sci. 2020, 10, 277. https://doi.org/10.3390/brainsci10050277
Dutta A, Das A, Kondziella D, Stachowiak MK. Bioenergy Crisis in Coronavirus Diseases? Brain Sciences. 2020; 10(5):277. https://doi.org/10.3390/brainsci10050277
Chicago/Turabian StyleDutta, Anirban, Abhijit Das, Daniel Kondziella, and Michal K. Stachowiak. 2020. "Bioenergy Crisis in Coronavirus Diseases?" Brain Sciences 10, no. 5: 277. https://doi.org/10.3390/brainsci10050277
APA StyleDutta, A., Das, A., Kondziella, D., & Stachowiak, M. K. (2020). Bioenergy Crisis in Coronavirus Diseases? Brain Sciences, 10(5), 277. https://doi.org/10.3390/brainsci10050277






