Atrial Fibrillation on Patients with Vascular Dementia: A Fundamental Target for Correct Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Viticchi, G.; Falsetti, L.; Buratti, L.; Sajeva, G.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Framingham Risk Score and the Risk of Progression from Mild Cognitive Impairment to Dementia. J. Alzheimer’s Dis. 2017, 59, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Viticchi, G.; Altamura, C.; Luzzi, S.; Balucani, C.; Vernieri, F. Cerebrovascular Assessment for the Risk Prediction of Alzheimer’s Disease. J. Alzheimer’s Dis. 2012, 32, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Viticchi, G.; Falsetti, L.; Buratti, L.; Luzzi, S.; Bartolini, M.; Acciarri, M.C.; Provinciali, L.; Silvestrini, M. Metabolic syndrome and cerebrovascular impairment in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2015, 30, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.-C.; Hart, R.G.; Koudstaal, P.J.; Lane, D.A.; Lip, G.Y. Atrial Fibrillation and Cognitive Function: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.-C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef]
- Dietzel, J.; Haeusler, K.G.; Endres, M. Does atrial fibrillation cause cognitive decline and dementia? Europace 2017, 20, 408–419. [Google Scholar] [CrossRef]
- Falsetti, L.; Viticchi, G.; Buratti, L.; Grigioni, F.; Capucci, A.; Silvestrini, M. Interactions between Atrial Fibrillation, Cardiovascular Risk Factors, and ApoE Genotype in Promoting Cognitive Decline in Patients with Alzheimer’s Disease: A Prospective Cohort Study. J. Alzheimer’s Dis. 2018, 62, 713–725. [Google Scholar] [CrossRef]
- De Bruijn, R.F.A.G.; Heeringa, J.; Wolters, F.J.; Franco, O.H.; Stricker, B.H.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Association Between Atrial Fibrillation and Dementia in the General Population. JAMA Neurol. 2015, 72, 1288–1294. [Google Scholar] [CrossRef]
- Chen, L.Y.; Lopez, F.L.; Gottesman, R.F.; Huxley, R.R.; Agarwal, S.K.; Loehr, L.; Mosley, T.; Alonso, A. Atrial fibrillation and cognitive decline-the role of subclinical cerebral infarcts: The atherosclerosis risk in communities study. Stroke 2014, 45, 2568–2574. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Liu, D.; Gupta, D.K.; Pechman, K.R.; Watchmaker, J.M.; Gordon, E.A.; Rane, S.; Bell, S.P.; Mendes, L.A.; Davis, L.T.; et al. Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 2017, 89, 2327–2334. [Google Scholar] [CrossRef]
- Madhavan, M.; Hu, T.Y.; Gersh, B.J.; Roger, V.L.; Killian, J.; Weston, S.A.; Graff-Radford, J.; Asirvatham, S.J.; Chamberlain, A.M. Efficacy of Warfarin Anticoagulation and Incident Dementia in a Community-Based Cohort of Atrial Fibrillation. Mayo Clin. Proc. 2018, 93, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Friberg, L.; Rosenqvist, M. Less dementia with oral anticoagulation in atrial fibrillation. Eur. Heart J. 2017, 39, 453–460. [Google Scholar] [CrossRef]
- Moffitt, P.; Park, H.; O’Connell, J.; Lane, D.A.; Quinn, T.J. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: Systematic review. Age Ageing 2016, 45, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Alfonso, F. Atrial fibrillation in the elderly. J. Geriatr. Cardiol. 2019, 16, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Fuster, V.; Rydén, L.E.; Cannom, D.S.; Crijns, H.J.; Curtis, A.B.; Ellenbogen, K.A.; Halperin, J.L.; Le Heuzey, J.-Y.; Kay, G.N.; Lowe, J.E.; et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients With Atrial Fibrillation-Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). Eur. Heart J. 2006, 27, 1979–2030. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castellà, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Freedman, B.; Potpara, T.S.; Lip, G.Y.H. Stroke prevention in atrial fibrillation. Lancet 2016, 388, 806–817. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef]
- Subic, A.; Cermakova, P.; Religa, D.; Han, S.; Von Euler, M.; Kåreholt, I.; Johnell, K.; Fastbom, J.; Bognandi, L.; Winblad, B.; et al. Treatment of Atrial Fibrillation in Patients with Dementia: A Cohort Study from the Swedish Dementia Registry. J. Alzheimer’s Dis. 2018, 61, 1119–1128. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial fibrillation as an independent risk factor for stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Odutayo, A.; Wong, C.X.; Hsiao, A.J.; Hopewell, S.; Altman, U.G.; Emdin, C. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ 2016, 354, i4482. [Google Scholar] [CrossRef] [PubMed]
- Rivard, L.; Khairy, P.; Talajic, M.; Tardif, J.-C.; Nattel, S.; Bherer, L.; Black, S.; Healey, J.S.; Lanthier, S.; Andrade, J.; et al. Blinded Randomized Trial of Anticoagulation to Prevent Ischemic Stroke and Neurocognitive Impairment in Atrial Fibrillation (BRAIN-AF): Methods and Design. Can. J. Cardiol. 2019, 35, 1069–1077. [Google Scholar] [CrossRef]
- Harada, M.; Van Wagoner, D.R.; Nattel, S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015, 79, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.; Tait, C.; Scott, J.; Rumley, A.; Lowe, G.D.O.; Stott, D.J. Dementia in subjects with atrial fibrillation: Hemostatic function and the role of anticoagulation. J. Thromb. Haemost. 2004, 2, 1873–1878. [Google Scholar] [CrossRef]
- Marengoni, A.; Qiu, C.; Winblad, B.; Fratiglioni, L. Atrial fibrillation, stroke and dementia in the very old: A population-based study. Neurobiol. Aging 2011, 32, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Rastas, S.; Verkkoniemi, A.; Polvikoski, T.; Juva, K.; Niinisto, L.; Mattila, K.; Lansimies, E.; Pirttila, T.; Sulkava, R. Atrial Fibrillation, Stroke, and Cognition: A Longitudinal Population-Based Study of People Aged 85 and Older. Stroke 2007, 38, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, V.; Woller, S.C.; Stevens, S.; May, H.; Bair, T.L.; Anderson, J.L.; Crandall, B.G.; Day, J.D.; Johanning, K.; Long, Y.; et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm. 2014, 11, 2206–2213. [Google Scholar] [CrossRef]
- Friberg, L.; Andersson, T.; Rosenqvist, M. Less dementia and stroke in low-risk patients with atrial fibrillation taking oral anticoagulation. Eur. Heart J. 2019, 40, 2327–2335. [Google Scholar] [CrossRef]
- Rash, A.; Downes, T.; Portner, R.; Yeo, W.W.; Morgan, N.; Channer, K.S. A randomized controlled trial of warfarin versus aspirin for strokeprevention in octogenarians with atrial fibrillation (WASPO). Age Ageing 2007, 36, 151–156. [Google Scholar] [CrossRef]
- Subic, A.; Cermakova, P.; Norrving, B.; Winblad, B.; Von Euler, M.; Kramberger, M.G.; Eriksdotter, M.; Garcia-Ptacek, S. Management of acute ischaemic stroke in patients with dementia. J. Intern. Med. 2017, 281, 348–364. [Google Scholar] [CrossRef]
- Andreotti, F.; Rocca, B.; Husted, S.; Ajjan, R.A.; Berg, J.T.; Cattaneo, M.; Collet, J.-P.; De Caterina, R.; Fox, K.A.; Halvorsen, S.; et al. Antithrombotic therapy in the elderly: Expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2015, 36, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Mant, J.; Hobbs, F.D.; Fletcher, K.; Roalfe, A.; Fitzmaurice, D.; Lip, G.Y.; Murray, E.; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the BirminghamAtrial Fibrillation Treatment of the Aged Study, BAFTA): A randomised controlled trial. Lancet 2007, 370, 493–503. [Google Scholar] [CrossRef]
- Albers, G.W.; Dalen, J.E.; Laupacis, A.; Manning, W.J.; Petersen, P.; Singer, D.E. Antithrombotic Therapy in Atrial Fibrillation. Chest 2001, 119, 194S–206S. [Google Scholar] [CrossRef]
- Tavassoli, N.; the REAL.FR Group; Perrin, A.; Berard, E.; Gillette, S.; Vellas, B.; Rolland, Y. Factors Associated with Undertreatment of Atrial Fibrillation in Geriatric Outpatients with Alzheimer Disease. Am. J. Cardiovasc. Drugs 2013, 13, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Jankowska-Polanska, B.; Katarzyna, L.; Lidia, A.; Joanna, J.; Dudek, K.; Izabella, U. Cognitive function and adher-ence to anticoagulation treatment in patients with atrial fibrillation. J. Geriatr. Cardiol. 2016, 13, 559–565. [Google Scholar]
| Overall (n = 1705) | No-DEM (n = 1468) | VAD (n = 193) | P | |
|---|---|---|---|---|
| General characteristics | ||||
| Age (mean ± SD) | 77.7 ± 10.9 | 76.6 ± 11.2 | 84.3 ± 6.85 | 0.000 |
| Male sex (n, %) | 862 (50.6%) | 770 (52.4%) | 77 (39.8%) | 0.0001 |
| Main outcomes | ||||
| TF (n, %) | 199 (11.6%) | 164 (11.2%) | 25 (12.9%) | 0.464 |
| Stroke/TIA (n, %) | 211 (12.4%) | 158 (10.7%) | 47 (24.3%) | 0.0001 |
| MB (n, %) | 146 (8.6%) | 130 (8.9%) | 9 (4.7%) | 0.048 |
| Comorbidities | ||||
| Hypertension (n, %) | 838 (47.8%) | 728 (49.6%) | 89 (4.6%) | 0.364 |
| T2DM (n, %) | 284 (16.6%) | 256 (17.4%) | 22 (11.4%) | 0.035 |
| CAD (n, %) | 657 (38.5%) | 567 (38.6%) | 78 (40.4%) | 0.631 |
| CHF (n, %) | 693 (40.6%) | 589 (40.1%) | 82 (42.4%) | 0.529 |
| PAD (n, %) | 153 (8.9%) | 138 (9.4%) | 13 (6.7%) | 0.226 |
| Previous stroke/TIA (n, %) | 300 (17.1%) | 110 (7.5%) | 166 (86.0%) | 0.0001 |
| CHD (n, %) | 46 (2.7%) | 41 (2.8%) | 4 (2.1%) | 0.562 |
| CKD (n, %) | 283 (16.6%) | 250 (17.0%) | 24 (12.4%) | 0.106 |
| Chronic anemia (n, %) | 135 (7.91%) | 114 (7.8%) | 14 (7.2%) | 0.802 |
| Active cancer (n, %) | 279 (16.4%) | 246 (16.7%) | 28 (14.5%) | 0.429 |
| COPD (n, %) | 412 (24.2%) | 369 (25.1%) | 35 (18.1%) | 0.033 |
| Risk scores | ||||
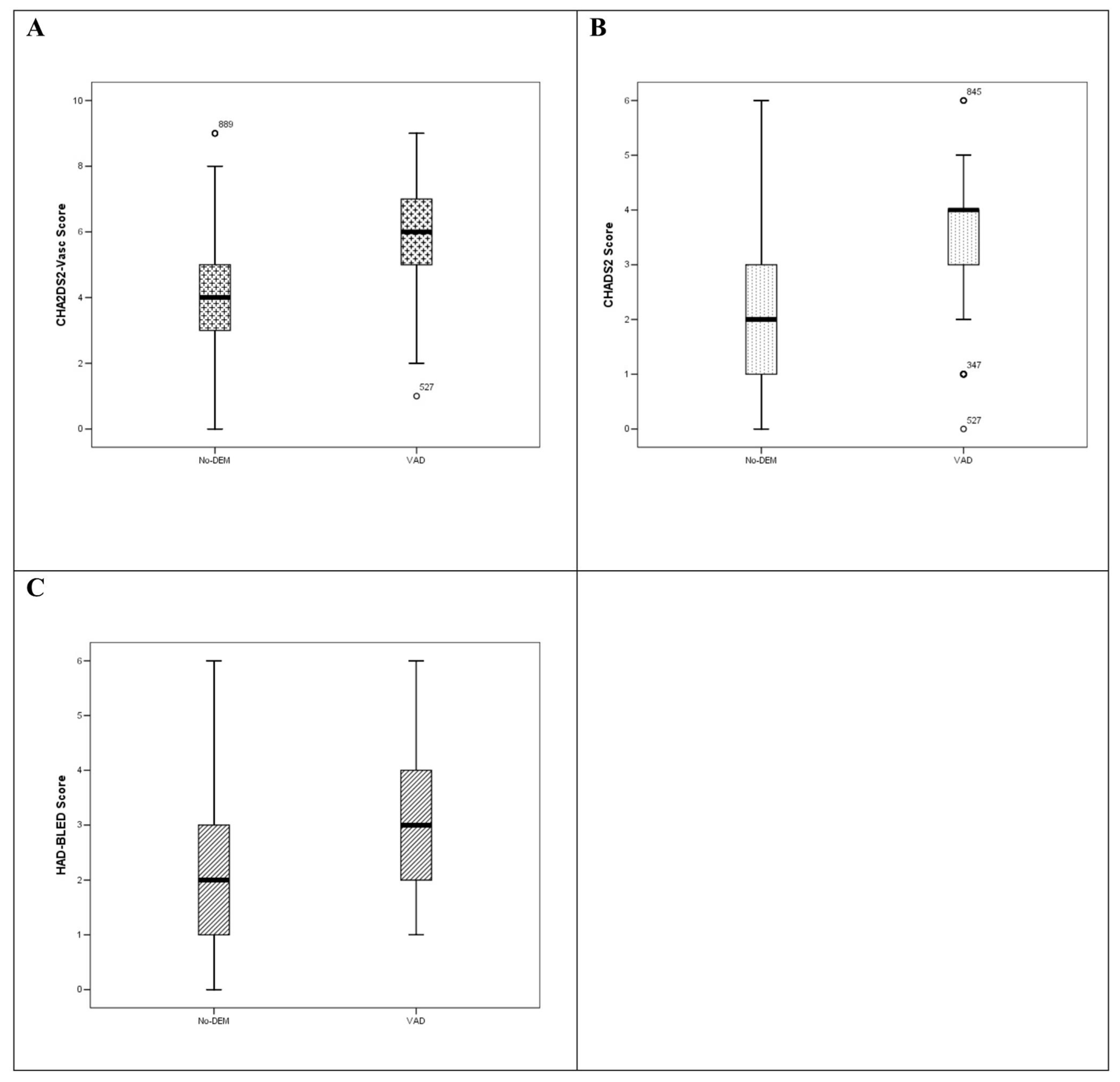
| CHA2DS2VASc (mean, ±SD) | 3.93 ± 1.8 | 3.68 ± 1.7 | 5.69 ± 1.4 | 0.0001 |
| CHADS2 (mean, ±SD) | 2.11 ± 1.4 | 1.89 ± 1.3 | 3.65 ± 1.2 | 0.0001 |
| HAS-BLED (mean, ±SD) | 2.21 ± 1.1 | 2.11 ± 1.1 | 2.93 ± 1.0 | 0.0001 |
| Treatment | ||||
| Anticoagulants (n, %) | 1043 (61.2%) | 882 (60.1%) | 135 (69.9%) | 0.008 |
| Antiplatelet drugs (n, %) | 640 (37.5%) | 550 (37.4%) | 78 (40.4%) | 0.427 |
| Dependent Variable | Mean (I) | Mean (J) | Mean Difference (I−J) | SE | P |
|---|---|---|---|---|---|
| CHA2DS2-Vasc | No-DEM | VAD | −2.009 | 0.130 | 0.0001 |
| CHADS2 | No-DEM | VAD | −1.763 | 0.097 | 0.0001 |
| HAS-BLED | No-DEM | VAD | −0.820 | 0.083 | 0.0001 |
| P | OR | 95% CI | ||
|---|---|---|---|---|
| Lower | Upper | |||
| No dementia (Ref.) | 0.001 | |||
| VAD | 0.001 | 2.282 | 1.569 | 3.319 |
| CHA2DS2VASc ≥ 2 | 0.005 | 2.711 | 1.353 | 5.432 |
| No anticoagulant at admission | 0.001 | 1.745 | 1.257 | 2.424 |
| COPD | 0.028 | 0.756 | 0.447 | 0.956 |
| Constant | 0.000 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viticchi, G.; Falsetti, L.; Burattini, M.; Zaccone, V.; Buratti, L.; Bartolini, M.; Moroncini, G.; Silvestrini, M. Atrial Fibrillation on Patients with Vascular Dementia: A Fundamental Target for Correct Management. Brain Sci. 2020, 10, 420. https://doi.org/10.3390/brainsci10070420
Viticchi G, Falsetti L, Burattini M, Zaccone V, Buratti L, Bartolini M, Moroncini G, Silvestrini M. Atrial Fibrillation on Patients with Vascular Dementia: A Fundamental Target for Correct Management. Brain Sciences. 2020; 10(7):420. https://doi.org/10.3390/brainsci10070420
Chicago/Turabian StyleViticchi, Giovanna, Lorenzo Falsetti, Marco Burattini, Vincenzo Zaccone, Laura Buratti, Marco Bartolini, Gianluca Moroncini, and Mauro Silvestrini. 2020. "Atrial Fibrillation on Patients with Vascular Dementia: A Fundamental Target for Correct Management" Brain Sciences 10, no. 7: 420. https://doi.org/10.3390/brainsci10070420
APA StyleViticchi, G., Falsetti, L., Burattini, M., Zaccone, V., Buratti, L., Bartolini, M., Moroncini, G., & Silvestrini, M. (2020). Atrial Fibrillation on Patients with Vascular Dementia: A Fundamental Target for Correct Management. Brain Sciences, 10(7), 420. https://doi.org/10.3390/brainsci10070420






